钙化焙烧-酸浸工艺提取低冰镍中有价金属
来源期刊:中国有色金属学报(英文版)2019年第10期
论文作者:耿淑华 李光石 赵勇 程红伟 陆一 鲁雄刚 许茜
文章页码:2202 - 2212
关键词:低冰镍;钙化焙烧;CaO;铁酸盐;针铁矿工艺
Key words:low nickel matte; calcified roasting; CaO; ferrite; goethite process
摘 要:利用钙化焙烧-酸浸工艺,以CaO为焙烧添加剂从低冰镍中高效提取有价金属。研究焙烧温度、焙烧时间、添加剂CaO、H2SO4浓度和浸出液固比对有价金属提取率的影响。研究结果表明:在最佳条件下,Ni、Cu、Co和Fe的提取率分别为 94.2%、98.1%、92.2%和89.3%。在随后的针铁矿法除铁工艺中,99.6%的Fe从浸出液中以针铁矿的形式分离。阐明CaO的焙烧行为与机理,焙烧过程中CaO与Fe2O3优先反应,抑制有色金属铁氧体的形成,使得金属氧化物(CuO和NixCu1-xO)在高温焙烧过程中能保持稳定,最终通过稀硫酸溶液浸出实现有价金属的高效提取。
Abstract: A calcified roasting-acid leaching process was developed as a highly effective method for the extraction of valuable metals from low nickel matte in the presence of CaO additive. The influences of process parameters on the metal extraction were studied, including the roasting temperature, roasting time, addition of CaO, H2SO4 concentration and liquid-solid ratio. Under the optimum condition, 94.2% of Ni, 98.1% of Cu, 92.2% of Co and 89.3% of Fe were recovered. Additionally, 99.6% of Fe was removed from the leachate as goethite by a subsequent goethite iron precipitation process. The behavior and mechanism of CaO additive in the roasting process was clarified. The role of CaO is to prevent the formation of nonferrous metal ferrite phases by a preferential reaction with Fe2O3 during the roasting process. The metal oxides (CuO and NixCu1-xO) remained stable during high-temperature roasting and were subsequently efficiently leached using a sulfuric acid solution.
Trans. Nonferrous Met. Soc. China 29(2019) 2202-2212
Shu-hua GENG, Guang-shi LI, Yong ZHAO, Hong-wei CHENG, Yi LU, Xiong-gang LU, Qian XU
State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China
Received 2 January 2019; accepted 11 August 2019
Abstract: A calcified roasting-acid leaching process was developed as a highly effective method for the extraction of valuable metals from low nickel matte in the presence of CaO additive. The influences of process parameters on the metal extraction were studied, including the roasting temperature, roasting time, addition of CaO, H2SO4 concentration and liquid-solid ratio. Under the optimum condition, 94.2% of Ni, 98.1% of Cu, 92.2% of Co and 89.3% of Fe were recovered. Additionally, 99.6% of Fe was removed from the leachate as goethite by a subsequent goethite iron precipitation process. The behavior and mechanism of CaO additive in the roasting process was clarified. The role of CaO is to prevent the formation of nonferrous metal ferrite phases by a preferential reaction with Fe2O3 during the roasting process. The metal oxides (CuO and NixCu1-xO) remained stable during high-temperature roasting and were subsequently efficiently leached using a sulfuric acid solution.
Key words: low nickel matte; calcified roasting; CaO; ferrite; goethite process
1 Introduction
Nickel (Ni) is naturally present as two principal ore types: approximately 60% in sulfides and 40% in laterites [1]. Most of nickel production is derived from sulfide ores [2]. The main reason is that the laterite ores require more extensive and complex treatment for nickel extraction [3,4]. Compared to sulfides, laterite processing is more expensive. With the increasing discrepancy between the growing industrial demands and the continuous diminution of nickel sulfide ores, the search for alternative methods for extraction of nickel from complex sulfide ores has become an important research topic [5]. Conventionally, the floating-smelting- converting process, which has a high efficiency and low cost is used to treat sulfide ores [6-8]. Recently, recovery of Ni, Cu, and Co from low nickel matte has been studied via hydrometallurgical processes such as atmospheric pressure acid leaching, ammonia leaching, oxygen pressure acid leaching and oxidative leaching [9,10]. Among these processes, only the oxygen pressure acid leaching method could achieve a high recovery of metals. However, this approach is inhibited by several challenges such as the requirement for specialized facilities and complex operation [11].
The combination of pyrometallurgy and hydrometallurgy processes which is associated with a lower energy consumption and reduced pollution, is gradually becoming a research hotspot of metallurgy [12]. Among the alternative processes, additives roasting followed by acid leaching process is an important technique in the recovery of many nonferrous metals from minerals or concentrates [13,14]. As sodium roasting may cause corrosion of equipment and environment pollution, it is gradually replaced by calcified roasting that is used for vanadium extraction from its ore [15-17]. The treatment of sulfide ores via calcified roasting was reported for the extraction of copper from flotation Cu concentrates, which can achieve a high recovery of Cu and is environmentally friendly [18-21]. The calcification roasting followed by water leaching process has been used for the recovery of Ni and Mo from carbonaceous shale ore by researchers at Central South University, China [22,23].
In this study, our research focused on calcified roasting of low nickel matte in the presence of CaO followed by acid leaching to extract valuable metal components. The optimum parameters for the calcified roasting-sulfate acid leaching technique for a high recovery of Ni, Co and Cu were determined. The effects of the roasting time, roasting temperature, the addition of CaO, H2SO4 concentration and liquid-solid ratio on the extraction of metals were also investigated. The role of CaO in promoting the calcified roasting of sulfide ores was demonstrated. This study is also applicable to the efficient extraction and separation of valuable metals in sulfides materials.
2 Experimental
2.1 Materials
The low nickel matte samples used in this study was from Jinchuan Co., Ltd., in Gansu Province, China. The composition was analyzed by X-ray fluorescence (XRF) and the results are shown in Table 1. The concentrations of Ni, Cu and iron (Fe) in low nickel matte were 27.27, 16.21, and 29.90 wt.%, respectively, while the Co content was very low at only 0.5 wt.%. X-ray diffraction (XRD) pattern shown in Fig. 1 combined with SEM image and EDS analysis results (Fig. 2) revealed that Ni existed as pentlandite ((Fe,Ni)9S8) and trevorite (Ni3Fe). Almost all the Cu existed as chalcocite (Cu2S), and Fe exited as (Fe,Ni)9S8, Ni3Fe and magnetite (Fe3O4). The diffraction peak of Co-based phase was not detected by XRD due to the low content of Co. As observed in Fig. 2, there were some holes on the low nickel matte which are attributable to the shrinkage cavities formed during the solidification process.
Table 1 Chemical composition of low nickel matte (wt.%)

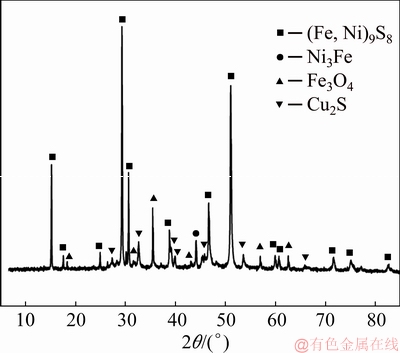
Fig. 1 XRD pattern of low nickel matte
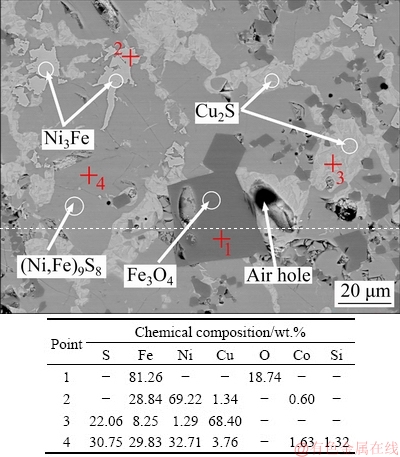
Fig. 2 SEM image and EDS analysis results of low nickel matte
All chemical reagents used in this study were analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., China.
2.2 Methods
2.2.1 Calcified roasting and acid leaching
The low nickel matte was ground and passed through a mesh of sieve size 200#, and the average particle size of the sieved powder was less than 75 μm. Then the low nickel matte powder was dried at 105 °C for 2 h. The experimental procedure is illustrated in Fig. 3. After 10 g of low nickel matte was thoroughly mixed with a certain amount of CaO, the mixture was transferred into a 100 mL crucible. The crucible was then placed into a shaft furnace containing a quartz reaction tube after the furnace had been preheated to the designated temperature in an air atmosphere. The exhaust gases (SO2 and SO3) were absorbed by the alkali solution. After cooling to room temperature, the roasted sample was removed from the crucible and then leached in dilute sulfuric acid solution at 85 °C to extract valuable metals. After 1 h of leaching, the leaching residue was removed by filtration. The residue was washed using distilled water and heated at 90 °C for 24 h in a muff furnace in preparation for XRD analysis. The leachate was prepared for Inductively Coupled Plasma- Atomic Emission Spectroscopy (ICP-AES) analysis to evaluate the concentration of Ni, Co, Cu, and Fe.

Fig. 3 Flow diagram of calcified roasting-acid leaching process
The leaching solution was then filtered and transferred to a 1000 mL volumetric flask. The extraction (η) of Ni, Cu, Fe, and Co was calculated according to the following equation:
 (1)
(1)
where C and V are the metal content (mg/L) and the volume of the filtered leaching solution (L), respectively. The variable m is the mass of low nickel matte used in the experiments (g), and w is the mass fraction of a given metal in the low nickel matte. Parallel experiments were conducted three times, and the average extraction was obtained to ensure the reproducibility of the results.
The removal of iron from the leaching solution was performed in a 2 L water-heated reaction kettle. This solution was first diluted to a concentration of less than 1 g/L of iron, added to the reaction kettle at 85 °C, and stirred at a rate of 80 r/min for 60 min. The pH of the solution was adjusted by slowly adding NH3·H2O to maintain a value of 3.4-3.6. The goethite residue was collected for further recovery and the filter liquor was transferred to a 500 mL volumetric flask for ICP-AES determination.
2.2.2 Characterization
The chemical compositions of the samples were determined by XRF (XRF-1800, Shimadzu, Kyoto, Japan). The content of Ni, Cu, Co, and Fe in the leaching solution was determined by ICP-AES (Perkin-Elmer 7300DV, USA). The pH value of the solution was measured using a pH meter (pH, Shanghai INESA, PHS-3C). Thermal analysis was performed using thermogravimetric analysis (TG) combined with differential scanning calorimetry (DSC) (NETZSCH STA 449F3 Jupiter, Germany). The phases in low nickel matte and roasted products were characterized by XRD (Bruker AXS, D8 Advance, Cu Kα radiation, 40 kV, 40 mA). Microscopic observations and analysis of the samples were conducted using SEM (HITACHI SU-1500, HITACHI, Japan). The infrared spectrum of the leach residue was obtained using Fourier transform infrared spectroscopy (FTIR) (Nicolet Avatrar370) in a wave range from 400 to 4000 cm-1 using the KBr pellet technique.
3 Results and discussion
3.1 Behavior of low nickel matte and CaO during roasting
To investigate the roasting process and the effect of the roasting temperature on the extraction of the metals, a series of roasting experiments were performed at 500-1000 °C for 2 h with the addition of CaO. For comparison, the experiments were also conducted without CaO addition (blank sample) under the same conditions. Figure 4 represents the TG-DSC curves of low nickel matte and its mixture with CaO at a CaO to low nickel mate mass ratio of 2:1 and a heating rate of 10 °C /min in an air atmosphere. In Fig. 4(a), three exothermic peaks are observed at 530, 660 and 745 °C in the DSC curve of the blank sample, due to the sulfation process of metal sulfides. In addition, an endothermic peak is presented at 770 °C, which resulted from the decomposition of NiSO4 [24]. With the addition of CaO, the DSC curve exhibited three exothermic peaks at 550, 660 and 745 °C that were similar to those of the blank sample and attributable to the sulfation process of the metal sulfides. However, the endothermic peak at 770 °C was not observed because the mixture with CaO reacts with metal sulfate to form a more stable CaSO4.
As shown in Fig. 4(b), obvious differences were observed in the TG curves of low nickel matte with and without CaO addition. The mass of the blank sample increased at 237 °C, indicating that the sulfide began to react with oxygen to form sulfate. This was followed by an increase in mass with temperature. At a temperature of 675 °C, the mass of the blank sample had a maximum increase of 16.0%. Subsequently, the mass decreased rapidly with an increase in temperature. These phenomena are related to the sulfation process of metal sulfides and the decomposition process of sulfates respectively, which are consistent with the DSC results. With CaO addition, temperature at the start of the reaction was 367 °C, which was higher than that of the blank sample. This is because CaO may envelop the low nickel matte particles thereby preventing oxygen from readily getting into contact with the low nickel matte. With the increase in temperature, the mass of the mixture sample continuously increased, indicating that most of the SO2 and SO3 produced by the decomposition of sulfates can be absorbed by CaO.

Fig. 4 DSC (a) and TG (b) curves of low nickel matte and mixture with addition of CaO
To investigate the effect of roasting temperature on the extraction of metals, a series of roasting experiments were performed at 500-1000 °C for 2 h with low nickel matte and a mixture with the addition of CaO. The experimental results are presented in Fig. 5. It is clearly shown that the extraction of the metals was strongly influenced by the roasting temperature and also by the CaO addition. When roasting was performed without CaO addition, the extraction rate of Cu, Fe, and Co decreased quickly as the temperature increased to 1000 °C, while the extraction of Ni increased initially when the temperature was below 700 °C, followed by a sharp decrease as the temperature continued to increase. This phenomenon is possibly attributed to the formation of ferrites (MeFe2O4, Me=Cu, Co or Ni). When roasting was performed at 500 °C, Cu, Fe, and Co were already sulfated or oxidized. As the temperature increased, these elements gradually formed ferrites which are insoluble in the acid. However, a portion of nickel still existed in the form of sulfide (NiS) at 500 °C, which led to a slight increase in the rate of nickel extraction because the sulfide was gradually oxidized as the temperature approached to 700 °C. After the NiS was completely oxidized, the extraction rate started to decrease due to the formation of NiFe2O4. However, with the addition of CaO, a substantially different phenomenon was observed. As shown in Fig. 5(b), the extraction rate of all the metals increased slightly when the temperature was lower than 700 °C, this was followed by a significant decrease in the temperature range of 700-800 °C, and a rapid increase as the temperature increased to 1000 °C. The decrease in the extraction rate was attributed to the formation of a large amount of ferrites at 700-800 °C, which could be effectively suppressed by CaO at higher temperatures [24, 25], leading to an increase in the rate of metal extraction.

Fig. 5 Effect of roasting temperature on extraction of metals without (a) and with (b) addition of CaO
Figure 6(a) represents the XRD patterns of low nickel matte obtained after roasting at different temperatures. The main products after roasting at 500 °C were Fe2O3, CuSO4, NiS, and NiSO4. As the temperature increased to 700 °C, the peaks of NiS disappeared. Considering that the peaks of NiSO4 disappeared at 800 °C and the TG-DSC curves shown in the preceding figure, this implies that the decomposition temperature of NiS is approximately 740 °C. Moreover, the peaks of MeFe2O4 (Me=Ni, Cu and Co) started to appear at 600 °C and the peaks of oxides and sulfides gradually disappeared as the temperature increased. Finally, only the ferrites were left in the roasting products.
Based on this analysis, the possible chemical reactions during the roasting process are expressed as reactions (2)-(9) [26,27]:
4(Fe,Ni)9S8+29O2→18Fe2O3+4NiO+32NiS (2)
NiS+2O2→NiSO4 (3)
2NiSO4→2NiO+2SO2↑+O2↑ (4)
NiSO4→NiO+SO3↑ (5)
2Cu2S+5O2→2CuO+2CuSO4 (6)
CuSO4→CuO+SO3↑ (7)
2CuSO4→2CuO+2SO2↑+O2↑ (8)
MeO+Fe2O3→MeFe2O4 (Me=Ni, Cu, Co) (9)
Based on these reactions, SO2 and SO3 may be produced due to the decomposition of sulfate. As the temperature increased, more ferrite was formed, which led to a decrease in the rate of metal extraction. This phenomenon is consistent with the metal extraction results shown in Fig. 5(a).
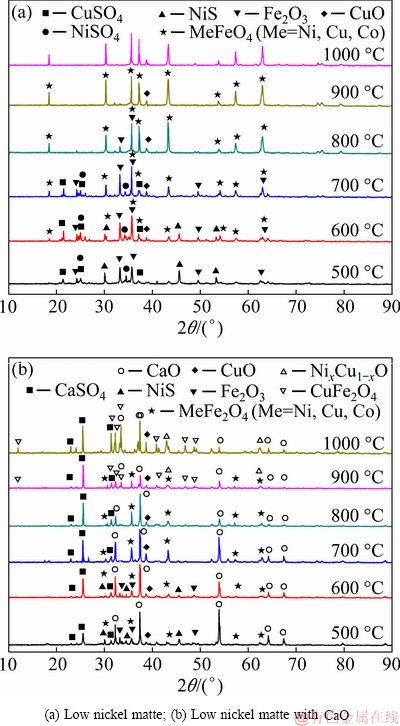
Fig. 6 XRD patterns of products after roasting at different temperatures
As shown in Fig. 6(b), the main phases of the mixture with CaO addition were CaO, Fe2O3, NiS, CuO, CaSO4 at 500 °C, and minor MeFe2O4 (Me=Ni, Cu and Co). The peak intensity of NiS decreased and disappeared at 700 °C, similar to the case of low nickel matte. However, compared to Fig. 6(a), there is an obvious difference in that the MeFe2O4 peak decreased in intensity at 900 °C and then disappeared at 1000 °C. CaFe2O4 appeared at 900 °C and increased at 1000 °C. The phases were CaFe2O4, CuO, NixCu1-xO and minor CaSO4 and CaO at 1000 °C. The main chemical reactions can be expressed by equations (10)-(15):
4(Fe,Ni)9S8+29O2→18Fe2O3+4NiO+32NiS (10)
NiS+2O2+CaO→NiO+CaSO4 (11)
2Cu2S+5O2+2CaO→4CuO+2CaSO4 (12)
MeO+Fe2O3→MeFe2O4 (Me=Ni, Cu, Co) (13)
MeFe2O4+nCaO→nCaO·Fe2O3+MeO
(Me=Ni, Cu, Co; n=1, 2) (14)
xNiO+(1-x)CuO=NixCu1-xO (15)
The experimental results showed that CaO could inhibit the formation of ferrite at a higher temperature, resulting in the presence of metal oxides in the calcined product. Therefore, it is feasible to transfer the metal components in the calcined product to the solution using an acid leaching process. The phase diagram of CaO-Fe2O3 at high temperatures (over 900 °C) is shown in Fig. 7. It is evident that, in the case of excessive CaO, increasing the roasting temperature promotes the formation of calcium ferrite (CaO·Fe2O3 and 2CaO· Fe2O3) [28], thereby effectively inhibiting the formation of ferrite.

Fig. 7 Phase diagram of CaO-Fe2O3 system [28]
3.2 Effect of roasting temperature on leaching yields of metals
Based on the aforementioned experimental results and analysis, subsequent experiments were performed at 800-1100 °C with low nickel matte with CaO addition. The experimental results for the effect of roasting temperature on the extraction process are presented in Fig. 8. In this figure, it is observed that a significant improvement in the extraction of Ni, Cu, Co and Fe occurred as the temperature increased from 800 to 1100 °C. The results revealed that when the temperature increased to 1100 °C, the extractions of Ni, Cu, Co, and Fe reached high levels of 94.5%, 98.1%, 92.2%, and 90.2%.
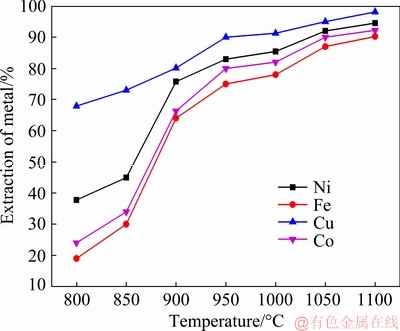
Fig. 8 Effect of roasting temperature on extraction of metals (mass ratio of CaO to low nickel matte 2:1, roasting time 2 h, H2SO4 concentration 2 mol/L, liquid-solid ratio 25 mL/g)
The XRD patterns of the residues are shown in Fig. 9. From this figure, it is evident that the phases of the leach residue were CaSO4 and MeFe2O4 (Me=Ni, Cu, Co) at 800 °C. As the temperature increased, the magnitude of the peaks of MeFe2O4 (Me=Ni, Cu, Co) gradually decreased. At 1100 °C, a ferrite peak was not observed, indicating that ferrite formation was completely inhibited at this temperature. This is also consistent with the results of the leaching rate experiments.
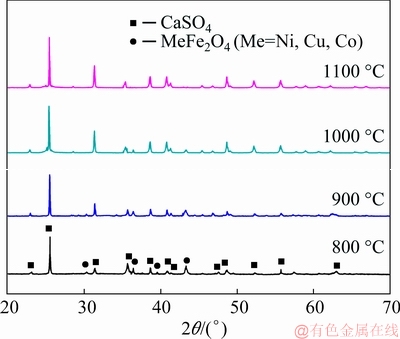
Fig. 9 XRD patterns of leaching residues at different roasting temperatures (mass ratio of CaO to low nickel matte 2:1, roasting time 2 h, H2SO4 concentration 2 mol/L, liquid-solid ratio 25 mL/g)
Figure 10 shows the SEM images of low nickel matte roasted with the addition of CaO at different temperatures for 2 h. Compared with low nickel matte raw material (Fig. 10(e)), a porous morphology is observed for the product roasted at 800 °C, due to the diffusion of SO2 and SO3 during the roasting process. As the temperature increased to 900 °C, the surface of the product became very smooth and dense. A further increase of the temperature caused the roasted products to be sintered, with a smooth and dense surface.
3.3 Effect of roasting time on leaching yield of metals
The effect of roasting time on the extraction of the metals was investigated. As shown in Fig. 11, the extraction rate of the metals increased rapidly with an increase of the roasting time. When the time was more than 2 h, the extraction rate remained almost constant as the roasting time continued to increase. This indicates that the leaching process was almost completed after 2 h. Therefore, 2 h was chosen as the optimum roasting time for subsequent experiments.
3.4 Effect of CaO addition on leaching yield of metals
Figure 12 shows the relationship between metal extraction and the amount of CaO added at 1100 °C for 2 h. The extraction rates of Fe, Co, Ni and Cu increased with the increased amount of CaO addition when the mass ratio of CaO to low nickel matte was lower than 1:1, which remained almost constant with the continued addition of CaO. Therefore, the optimum mass ratio of CaO to low nickel matte is 1:1. Compared with the blank experiment, the increase of the leaching rate of Cu was approximately 250% due to the addition of CaO, while that of Ni and Co was almost 900%.
3.5 Effect of H2SO4 concentration on leaching yield of metals
Figure 13 presents the effect of H2SO4 concentration on the extraction rate of the metals. It is evident from Fig. 13 that the extraction rate of the metals increased significantly with the increase of the concentration of sulfuric acid. When this concentration reached 1 mol/L, the increase in the concentration of sulfuric acid had little effect on the extraction rate. Therefore, the concentration of sulfuric acid selected for leaching was 1 mol/L.
3.6 Effect of liquid-solid ratio on leaching yield of metals
As shown in Fig. 14, the effect of liquid-solid ratio on the sulfuric acid leaching of the roasted products was investigated. The results showed that the leaching yields of metals increased significantly and then remained at a certain level with the increasing of liquid-solid ratio. Thus, a liquid-solid ratio of 20 mL/g was chosen as the optimal parameter from the technological and scientific point of view.

Fig. 10 SEM images of low nickel matte mixed with CaO after roasting at different temperatures

Fig. 11 Effect of roasting time on extraction of metals (roasting temperature 1100 °C, mass ratio of CaO to low nickel matte 2:1; H2SO4 concentration 2 mol/L, liquid-solid ratio 25 mL/g)
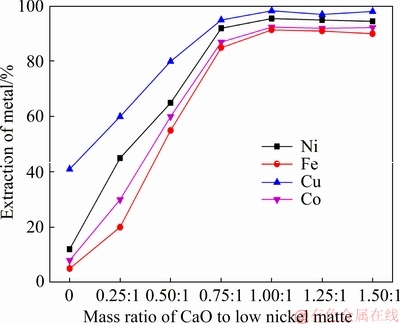
Fig. 12 Effect of CaO addition on extraction of metals (roasting temperature 1100 °C, roasting time 2 h; H2SO4 concentration 2 mol/L, liquid-solid ratio 25 mL/g)
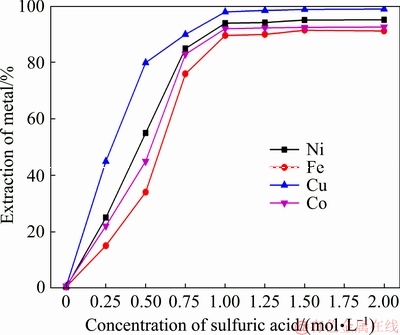
Fig. 13 Effect of concentration of sulfuric acid on extraction of metals (mass ratio of CaO to low nickel matte 1:1, roasting temperature 1100 °C, roasting time 2 h; liquid-solid ratio 25 mL/g)

Fig. 14 Effect of liquid-solid ratio on extraction of metals (mass ratio of CaO to low nickel matte 1:1, roasting temperature 1100 °C, roasting time 2 h, H2SO4 concentration 1 mol/L)
Hence, it was determined that the optimum conditions for calcified roasting were as follows: mass ratio of CaO to low nickel matte of 1:1, calcium roasting at 1100 °C for 2 h, 1 mol/L dilute H2SO4 leaching at 85 °C for 60 min with a liquid-solid ratio of 20 mL/g. Under the optimum parameters, the extractions of Ni, Cu, Co, and Fe were 94.2%, 98.1%, 92.2%, and 89.3% respectively.
3.7 Iron removal process
Based on the preceding experimental results, after calcification roasting-acid leaching, metal components including Ni, Cu, and Co can be successfully transferred to the leachate. However, Fe is also transferred to the leachate during the process. The results of current studies indicate that when the iron content in the leachate is more than 20 mg/L, it has a significant impact on the subsequent refining process, thereby reducing the efficiency of electrolysis and adversely affecting electrolytic deposition [29,30]. Therefore, an iron- removal step (goethite process) was utilized to remove iron from the solution.
The essential aspect of the goethite process maintained a low concentration of ferric iron (<1 g/L) in the reactor during precipitation. This requirement can be achieved by either reducing all ferric ions to their ferrous state (V.M. method) or by adding a concentrated pressure leaching solution into a large precipitation vessel at the same rate as the goethite precipitation (E.Z. method) [31,32]. Considering that the main iron ions in the leaching solution are trivalent and the concentration is not high, the E.Z. method was chosen. The reaction equation can be written as reaction (16):
Fe3++2H2O=FeOOH+3H+ (16)
The precipitation process generates an acid, which inhibits the hydrolysis of Fe3+. Therefore, the choice of an appropriate pH is an important aspect of the iron removal process. The effect of pH on the concentration of Fe and the metal loss rate after the iron removal process is shown in Fig. 15. The concentration of Fe3+ in the iron removal solution decreased sharply with an increase of pH while the loss rates of Ni, Cu, and Co increased. This is because the iron removal process of goethite is an acid-making process and increasing the pH contributes to the hydrolysis of Fe3+ to form goethite. The target metal ions (Ni2+, Cu2+, Co2+) in the solution were also partially hydrolyzed and precipitated together with goethite. In addition, porous colloidal Fe(OH)3 oxide in the solution increases the adsorption of metal ions and causes a loss of the target metal elements. Therefore, a high pH accelerates the loss of the target metal due to hydrolysis. Considering that the pH should be adjusted during the iron removal process to approximately 3.5, 2 mol/L diluted ammonia was added at a fixed rate to adjust the pH within the range of 3.4-3.6. The removal experiments were performed at 90 °C and the duration time was 2 h.

Fig. 15 Effect of pH on concentration of Fe and metal loss rate after iron removal process (85 °C, removal time 2 h)
The XRD patterns of the residue obtained after the iron-removal process are shown in Fig. 16(a). It is observed that the goethite residue consists of a single goethite phase that can be used as a mineral material in the smelting of steel after further treatment [31]. The FTIR spectrum of the goethite residue is shown in Fig. 16(b), in which the bands at 3434 and 3256 cm-1 are the stretching modes of hydroxyls of goethite water adsorbed on the surface, due to moisture adsorbed onto KBr. The bands near 1000 cm-1 are the characteristic absorption peaks of goethite which are associated with the bending vibration of the Fe—OH group on the surface of the mineral. The peaks at 890, 742, and 536 cm-1 are associated with the bending modes of Fe—OH—Fe [32]. In both XRD pattern and FTIR spectrum, the peaks are symmetric and sharp. This indicates that goethite is well-crystallized during the precipitation process. Thus, the residue could be easily filtered out.
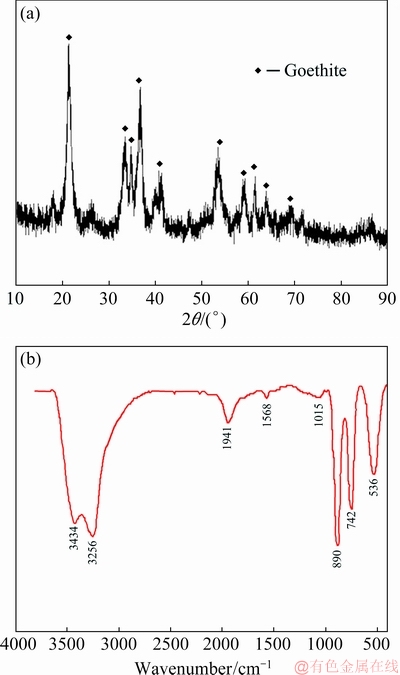
Fig. 16 XRD pattern (a) and FTIR spectrum (b) of residue after iron-removal process
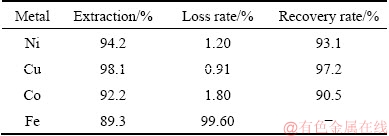
A summary of the extracted metals after the iron-removal process is shown in Table 2. The results indicate that 99.6% of Fe can be removed via the goethite process. Although 1.20% of Ni, 0.91% of Cu and 1.8% of Co may be lost, due to the adsorption and co-precipitation with iron particles in the goethite process, high recovery rates of Ni 93.1%, Cu 97.2%, and Co 90.5% can be achieved in the iron liquid. In the final solution, the iron concentration is as low as 3.84 mg/L, which meets the requirement of the electrolytic refining process.
Table 2 Extraction of metals after leaching process, metal loss during iron-removal process and total recovery ratio of metals
4 Conclusions
(1) With the oxidation of metal sulfides during the roasting process, CaO reacted with SO2 or SO3 gas that was generated, resulting in the formation of CaSO4. In addition, CaO can preferentially react with Fe2O3, leading to the formation of a high-temperature stable calcium ferrite phase (CaO·Fe2O3 or 2CaO·Fe2O3). Thus, the nonferrous metal oxide phases can be stably maintained during roasting and the generation of a nonferrous metal ferrite can be inhibited. Therefore, the valuable metal oxides (CuO and NixCu1-xO) can be transferred to the solution in the subsequent acid leaching process and can be easily dissolved by H2SO4 solution, which results in a high leaching rate of valuable metals (Ni, Cu, Co).
(2) Under optimum experimental conditions (1:1 mass ratio of CaO to low nickel matte; 2 h calcium roasting at 1100 °C; 1 mol/L dilute H2SO4 leaching at 85 °C for 1 h with a liquid-solid ratio of 20 mL/g), the extraction efficiencies of Ni, Cu, Co and Fe were 94.2%, 98.1%, 92.2% and 89.3%, respectively. The increase of the leaching rate of Cu was approximately 250% due to the addition of CaO, while that of Ni and Co was almost 900%.
(3) Iron was effectively precipitated in the form of goethite using a process with a carefully controlled temperature and pH. Approximately 99.6% of Fe could be effectively removed from the leaching solution after the iron-removal step. Although a small amount of Ni, Cu, and Co (1.20% of Ni, 0.91% of Cu and 1.80% of Co) were lost, the final recoveries of Ni, Cu, and Co reached 93.1%, 97.2%, and 90.5%, respectively.
References
[1] MUDD G M. Nickel sulfide versus laterite: The hard sustainability challenge remains [C]// 48th Annual Conference of Metallurgists. Sudbury, Ontario, Canada: Canadian Metallurgical Society, 2009: 23-26.
[2] CHEN Sheng-li, GUO Xue-yi, SHI Wen-tang, LI Dong. Extraction of valuable metals from low-grade nickeliferous laterite ore by reduction roasting-ammonia leaching method [J]. Journal of Central South University, 2010, 17(4): 765-769.
[3] LU Chang-yuan, LU Xiong-gang, ZOU Xing-li, CHENG Hong-wei, XU Qian. Current situation and utilization technology of nickel ore in China [J]. Chinese Journal of Nature, 2015, 37(4): 269-277. (in Chinese)
[4] LIU Zhi-guo, SUN Ti-chang, WANG Xiao-ping, GAO En-xia. Generation process of FeS and its inhibition mechanism on iron mineral reduction in selective direct reduction of laterite nickel ore [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(9): 901-906.
[5] WATLING H R. The bioleaching of nickel-copper sulfides [J]. Hydrometallurgy, 2008, 91(1): 70-88.
[6] XU Cong, CHENG Hong-wei, LI Guang-shi, LU Xiong-gang, ZOU Xing-li, XU Qian, Extraction of metals from complex sulfide nickel concentrates by low-temperature chlorination roasting and water leaching [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(4): 377-385.
[7] WARNER A E M, DIAZ C M, DALVI A D, MACKEY P J, TARASOV A V, JONES R T. JOM world nonferrous smelter survey Part IV: Nickel: sulfide [J]. JOM, 2007, 59(4): 58-72.
[8] CHEN Guang-ju, GAO Jian-ming, ZHANG Mei, GUO Min. Efficient and selective recovery of Ni, Cu, and Co from low nickel matte via a hydrometallurgical process [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(3): 249-256.
[9] LIU Yan. Influence of different pretreatment methods on extracting of nickel matte by sulfate roasting [J]. Non-Ferrous Mining and Metallurgy, 2011, 27(5): 23-25. (in Chinese)
[10] YIN Fei, WANG Zhen-wen, WANG Chen-yan, JIANG Pei-hai. Research on pressure leaching process for low nickel matte [J]. Mining & Metallurgy, 2009, 18(4): 35-37. (in Chinese)
[11] ZHAI Xiu-jing, WU Qiang, FU Yan, MA Lin-zhi, FAN Chuan-lin, LI Nai-jun. Leaching of nickel laterite ore assisted by microwave technique [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 77-81.
[12] WANG Ming-yu, WANG Xue-wen, SHEN Ji-feng, WU Ri-na. Extraction of vanadium from stone coal by modified salt-roasting process [J]. Journal of Central South University, 2011, 18(6): 1940-1944.
[13] LI Chang-lin, ZHOU Xiang-yang, WANG Hui, ZHANG Tai-kang, LI Jie, OU Xing, JIANG Xiao-duo. Effect of oxidation on vanadium extraction from stone coal with calcified roasting [J]. Journal of Central South University (Science and Technology), 2011, 42(1): 7-10. (in Chinese)
[14] CAI Zhen-lei, ZHANG Yi-min, LIU Tao, HUANG Jing. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology [J]. Minerals, 2016, 6(2): 26-39.
[15] WANG Bo, LIU Tao, ZHANG Yi-min, HUANG Jing. Effect of CaF2 /CaO composite additive on roasting of vanadium-bearing stone coal and acid leaching kinetics [J]. Minerals, 2017, 7(3): 43-56.
[16] LI Chang-lin, ZHOU Xiang-yang, WANG Hui, ZHANG Tai-kang, LI Jie, OU Xing, JIANG Xiao-duo. Effect of oxidation on vanadium extraction from stone coal with calcified roasting [J]. Journal of Central South University (Science and Technology), 2011, 42(1): 7-10.
[17] HAUNG H H, BARTLETT R W. Oxidation kinetics of a lime-copper concentrate pellet [J]. Metallurgical Transactions B, 1976, 7(3): 369-374.
[18] HUA Yi-xin, CAI Chao-jun, CUI Yan. Microwave-enhanced roasting of copper sulfide concentrates in the presence of CaCO3 [J]. Separation and Purification Technology, 2006, 50(1): 22-29.
[19] RIVEROS G, MARIN T, PUGA C. Lime-concentrate roasting studies-effect of activated limestone [J]. Minerals Engineering, 2004 17(3): 469-471.
[20] LIAO Ya-long, ZHOU Juan, HUANG Fei-rong, WANG Yi-yang. Leaching kinetics of calcification roasting calcinate from multimetallic sulfide copper concentrate containing high content of lead and iron [J]. Separation and Purification Technology, 2015, 149: 190-196.
[21] WANG Ming-yu, WANG Xue-wen. Extraction of molybdenum and nickel from carbonaceous shale by oxidation roasting, sulphation roasting and water leaching [J]. Hydrometallurgy, 2010, 102(1): 50-54.
[22] WANG Xue-wen, PENG Jun, WANG Ming-yu, YE Pu-hong, XIAO Yuan. The role of CaO in the extraction of Ni and Mo from carbonaceous shale by calcification roasting, sulphation roasting and water leaching [J]. International Journal of Mineral Processing, 2011, 100(3): 130-135.
[23] YU Da-wei, UTIGARD T A. TG/DTA study on the oxidation of nickel concentrate [J]. Thermochimica Acta, 2012, 533: 56-65.
[24] DUNN J G. The oxidation of sulphide minerals [J]. Thermochimica Acta, 1997, 300(1): 127-139.
[25] SHI Li-hua, WANG Xue-wen, WANG Ming-yu, PENG Jun, XIAO Cai-xia. Extraction of molybdenum from high-impurity ferromolybdenum by roasting with Na2CO3 and CaO and leaching with water [J]. Hydrometallurgy, 2011, 108(3): 214-219.
[26] DUNN J G, KELLY C E. A TG/MS and DTA study of the oxidation of pentlandite [J]. Journal of Thermal Analysis, 1980, 18(1): 147-154.
[27] PRASAD S, PANDEY B D. Alternative processes for treatment of chalcopyrite—A review [J]. Minerals Engineering, 1998, 11(8): 763-781.
[28] CHEN Shu-jiang, TIAN Feng-ren, LI Guo-hua, ZHANG Yun. Phase diagram analysis and its application [M]. 1st ed. Beijing: Metallurgical Industry Press, 2007: 131. (in Chinese)
[29] CHANG Yong-feng, ZHAI Xiu-jing, LI Bin-chuan, FU-Yan. Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate [J]. Hydrometallurgy, 2010, 101(1): 84-87.
[30] HAN Hai-sheng, SUN Wei, HU Yue-hua, TANG Hong-hu, YUE Tong. Magnetic separation of iron precipitate from nickel sulfate solution by magnetic seeding [J]. Hydrometallurgy, 2015, 156: 182-187.
[31] YUE Tong, HAN Hai-sheng, SUN Wei, HU Yue-hua, CHEN Pan, LIU Run-qing. Low-pH mediated goethite precipitation and nickel loss in nickel hydrometallurgy [J]. Hydrometallurgy, 2016, 165: 238-243.
[32] PRASAD P S R, SHIVA PRASAD K, KRISHNA CHAITANYA V, BABU E V S S K, SREEDHAR B, RAMANA MURTHY S. In situ FTIR study on the dehydration of natural goethite [J]. Journal of Asian Earth Sciences, 2006, 27(4): 503-511.
耿淑华,李光石,赵 勇,程红伟,陆 一,鲁雄刚,许 茜
上海大学 材料科学与工程学院 省部共建高品质特殊钢冶金与制备国家重点实验室,上海 200444
摘 要:利用钙化焙烧-酸浸工艺,以CaO为焙烧添加剂从低冰镍中高效提取有价金属。研究焙烧温度、焙烧时间、添加剂CaO、H2SO4浓度和浸出液固比对有价金属提取率的影响。研究结果表明:在最佳条件下,Ni、Cu、Co和Fe的提取率分别为 94.2%、98.1%、92.2%和89.3%。在随后的针铁矿法除铁工艺中,99.6%的Fe从浸出液中以针铁矿的形式分离。阐明CaO的焙烧行为与机理,焙烧过程中CaO与Fe2O3优先反应,抑制有色金属铁氧体的形成,使得金属氧化物(CuO和NixCu1-xO)在高温焙烧过程中能保持稳定,最终通过稀硫酸溶液浸出实现有价金属的高效提取。
关键词:低冰镍;钙化焙烧;CaO;铁酸盐;针铁矿工艺
(Edited by Xiang-qun LI)
Foundation item: Projects (U1860203, U1860108, 51574164) supported by the National Natural Science Foundation of China
Corresponding author: Xiong-gang LU; Tel: +86-21-66136527; E-mail: luxg@shu.edu.cn
DOI: 10.1016/S1003-6326(19)65126-5

