固溶原子、析出相和变形对Al-Sc-Zr合金组织和性能的定量影响
来源期刊:中国有色金属学报(英文版)2019年第5期
论文作者:管仁国 金红梅 Wensen JIANG 王祥 王宇翔 李征 张俭 Huinan LIU
文章页码:907 - 918
关键词:Al-Sc-Zr 合金;热处理;冷变形;力学性能;导电率
Key words:Al-Sc-Zr alloy; thermal treatment; cold deformation; mechanical properties; conductivity
摘 要:为了研究固溶原子、析出相粒子和冷变形对Al-Sc-Zr合金组织和性能的影响,对连续流变挤压制备的Al-Sc-Zr合金进行形变热处理,采用电导率、强度、显微硬度测试以及光学显微镜、TEM、STEM对合金在不同处理状态下的显微组织和性能进行研究。建立固溶原子、析出相粒子和冷变形对合金导电率定量贡献的数学模型。结果表明:固溶、时效和冷变形都能使合金的强度得到显著的提升,而固溶原子、沉淀粒子和冷变形对Al合金导电率影响值分别是10.5% (IACS)、2.3% (IACS)和0.5% (IACS)。时效和冷变形处理是获得高强高导铝合金导线的关键。
Abstract: In order to investigate the effects of solid solution atoms, precipitated particles and cold deformation on the microstructures and properties of Al-Sc-Zr alloys, the Al-Sc-Zr alloys prepared by continuous rheo-extrusion were treated by thermomechanical treatment, analyzed for conductivity and mechanical properties by tensile and microhardness testing, and characterized using optical microscope, TEM and STEM. A mathematical model was established to quantitatively characterize the contribution of solid solution atoms, precipitates and cold deformation to the conductivity of the alloy. The results show that the strength of Al alloy can be significantly improved by solid solution, aging and cold deformation, and the quantitative impacts of solution atoms, precipitates and cold deformation on the conductivity of Al alloy are 10.5% (IACS), 2.3% (IACS) and 0.5% (IACS), respectively. Aging and cold deformation treatments are the keys to obtain high-strength and high-conductivity aluminum alloy wires.
Trans. Nonferrous Met. Soc. China 29(2019) 907-918
Ren-guo GUAN1,2, Hong-mei JIN2, Wensen JIANG3, Xiang WANG2, Yu-xiang WANG2, Zheng LI1, Jian ZHANG2, Huinan LIU3,4
1. Center of Advanced Lubrication and Seal Materials, State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China;
2. School of Material Science and Engineering, Northeastern University, Shenyang 110819, China;
3. Materials Science and Engineering Program, University of California at Riverside, 900 University Avenue, Riverside, California 92521, USA;
4. Department of Bioengineering, University of California at Riverside, 900 University Avenue, Riverside, California 92521, USA
Received 17 March 2018; accepted 14 January 2019
Abstract: In order to investigate the effects of solid solution atoms, precipitated particles and cold deformation on the microstructures and properties of Al-Sc-Zr alloys, the Al-Sc-Zr alloys prepared by continuous rheo-extrusion were treated by thermomechanical treatment, analyzed for conductivity and mechanical properties by tensile and microhardness testing, and characterized using optical microscope, TEM and STEM. A mathematical model was established to quantitatively characterize the contribution of solid solution atoms, precipitates and cold deformation to the conductivity of the alloy. The results show that the strength of Al alloy can be significantly improved by solid solution, aging and cold deformation, and the quantitative impacts of solution atoms, precipitates and cold deformation on the conductivity of Al alloy are 10.5% (IACS), 2.3% (IACS) and 0.5% (IACS), respectively. Aging and cold deformation treatments are the keys to obtain high-strength and high-conductivity aluminum alloy wires.
Key words: Al-Sc-Zr alloy; thermal treatment; cold deformation; mechanical properties; conductivity
1 Introduction
Sc and Zr elements are distinguished precipitation strengthening elements due to nano Al3Sc and Al3Zr particles formed in aluminum (Al) matrix [1-3]. These particles can also pin grain boundaries and inhibit grain coarsening, which improves high-temperature stability of the Al alloys [4-6]. Al-Sc-Zr alloys have thus been extensively explored in recent years for their attractive properties, including good mechanical property, conductivity and high-temperature stability [7]. Al-Sc-Zr alloys are one of the most promising classes of lightweight materials as an alternative to high density iron-based alloys in automobile industry and aerospace field [8,9].
Processing technology and microstructures are the key factors that influence the properties of alloys [10,11]. COSTA et al [12] reported that the hardness of the solid-solution-treated Al-0.5Sc (wt.%) alloy was decreased considerably compared with that of the directly aged alloy. The reason is that the decreased density of the vacancies induced by solid solution atoms contributed to a lower diffusion rate and thus hindered a finer dispersion of Al3Sc particles. DU [13] reported that the hardness of Al-0.1Zr (wt.%) alloy after aging at 250 °C for 60 h increased from HV 35 to HV 90, and creep resistance increased significantly. The reason was that the nano-scale Al3Sc precipitated in grain or at grain boundary and dispersed uniformly in the alloy matrix. KEITH et al [14] found that precipitation hardening commenced at 325 °C in Al-0.1Sc-0.1Zr (at.%) alloy, and the alloy achieved peak micro-hardness at 400 °C. In this temperature range, the strength and the conductivity of Al-0.1Sc-0.1Zr (at.%) alloy increased with increasing mean radius (R<2 nm) and volume fractions of the precipitates. VLACH et al [15] noted that cold deformation could improve the hardness of the Al-Sc-Zr alloys and have no substantial effect on the size and distribution of Al3(Sc,Zr) precipitates. ZHOU [16] reported that both strength and conductivity of Al-0.2Sc-0.04Zr (wt.%) alloy were improved by cold deformation and aging treatment, and the increase of strength should be associated with the formation of finer Al3(Sc,Zr) precipitates while the improvement of conductivity should be resulted from the closure of microvoids.
Up to now, the quantitative and systematic investigations of the effects of solid solution atoms and precipitated particles as well as cold deformation on the microstructures and properties of Al-Sc-Zr alloys are rare. Therefore, the objective of this study was to determine and systematically quantify the specific contribution of each factor to the mechanical property and conductivity of Al-Sc-Zr alloys.
2 Experimental
2.1 Materials preparation
The raw materials for experiment were pure aluminum, Al-Sc master alloy (scandium content: 2.12 wt.%) and Al-Zr master alloy (zirconium content: 4.6 wt.%). The KGPS50-2 type medium frequency induction heating equipment was used for smelting. Al-xSc-0.2Zr (wt.%) alloys with different Sc contents were prepared (x=0.1, 0.25, 0.3, 0.35, 0.4, respectively). All the raw materials of pure aluminum, Al-Sc master alloy and Al-Zr master alloy were preheated in a RJ2-75-6 type resistance furnace at 200 °C for 30 min for desiccation. Then, the pure aluminum was melted to 720 °C in the medium frequency induction heating equipment. Subsequently, Al-Sc master alloy and Al-Zr master alloy with a proper proportion were added into the melt. The alloys were heated to 720 °C again and kept for 10 min. After drossing, the alloy melt was poured into DZJ-300 continuous rheo-extrusion machine for wire preparation. The Al-xSc-0.2Zr (wt.%) alloys with the diameter of 10 mm were prepared.
Solid solution and aging experiments were performed in a DFH-13-4 type aging furnace with a temperature control accuracy of ±2 °C. The solid solution temperature was set at 630 °C and solid solution time was controlled from 6 to 72 h. All the alloys were immediately water quenched into room temperature, and then aged at 380 °C for different time from 1 to 120 h. After thermal treatment, the alloys were drawn at room temperature at a Y112M-4 5 m chain type drawbench, and the drawing velocity was set at 0.05 m/s. The alloys were drawn from 10 to 3.0 mm.
2.2 Microstructures and properties characterization
Metallographic observation was performed on an Olympus DS×500 optical digital microscope. The samples of the alloys were ground by sandpaper and polished, and then etched by the solid solution of 4.0% HF + 96% H2O. Subsequently, microstructures of samples were observed and metallographic photographs were taken. JEM-2010 (UHR) transmission electron microscope (TEM) was used to observe the size, morphology and distribution of precipitates in the inner and grain boundaries of the alloys, and the crystal structure of the precipitated phase was determined. The alloy samples were ground to a thickness of 60-70 μm and stamped into a wafer of 3 mm in diameter. Then, they were thinned in an electro polisher. Finally, TEM images were taken on a JEM-2010 (UHR) TEM.
The tensile test at room temperature was carried out on the CMT5105 microcomputer controlled electronic universal testing machine with a tensile speed of 5 mm/min. The hardness test was performed on the HXD-1000TM/LCD Vickers hardness tester. The load was 49 N (5 kg) and the holding time was 15 s. A PC36C type low resistance ohm measuring instrument was used to measure the resistance. The working current of the instrument was from 100 μA to 10 A, and the error range was ±0.01 μΩ. The conductivity K of the alloy at 20 °C was calculated by following equation:
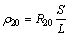 (1)
(1)
 (2)
(2)
where K20 is conductivity (% IACS), ρ20 is resistivity (Ω·mm2/m), S is the cross-section area of the conductor (mm2), L is the length of the conductor (m), and R20 is the resistance (Ω).
2.3 Mathematical modeling
The conductivity k1 of Al-0.35Sc-0.2Zr (wt.%) alloy after solid solution at a certain temperature was detected. The quantitative contribution of solid solution atoms to the conductivity of the alloy is |Δks|=|k1-k0|, where k0 is the conductivity of pure Al. In the aging process, the precipitation of particles and the dissolution of solid solution atoms was a dynamic process. In order to obtain the quantitative contribution of precipitates to the conductivity of the alloy aged at a certain temperature, the equilibrium state that the precipitation of particles was equal to the dissolution of solid solution atoms was investigated. Then, the critical solid solubility (only saturated solid solution atoms and no precipitates exist in the matrix of the Al-Sc-Zr alloys) under the equilibrium state was determined. In this situation, the relative conductivity kcs was detected. The precipitates contribution to the conductivity of the alloy is |Δkp|=|k2-kcs|, where k2 is the conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy after solid solution at a certain temperature and aging at a certain temperature. After cold deformation, the conductivity k3 of Al-0.35Sc-0.2Zr (wt.%) alloy was detected. The quantitative contribution of cold deformation to the conductivity of the alloy is |Δkε|=|k3-k2|.
3 Results and discussion
3.1 Effects of thermal treatment on microstructures and properties of Al-Sc-Zr alloys
Figure 1 shows microstructures at longitudinal section of rheo-extruded Al-0.35Sc-0.2Zr (wt.%) alloy before and after solid solution. The longitudinal microstructures of Al-0.35Sc-0.2Zr (wt.%) alloy were composed of equiaxed grains. The average grain size of the alloy was 80 μm. The grain size of the alloy increased with solid solution time prolonging. When the solid solution time increased to 72 h, the average grain size reached 890 μm. The main driving force of grain growth is the difference of interfacial energy between grains before and after growth. The grain boundary atoms gradually migrated from the convex side to the concave side of grain boundaries, and some grains were gradually swallowed.
Figure 2 shows the longitudinal TEM micro- structures of Al-0.35Sc-0.2Zr (wt.%) alloy with different solid solution time. As shown in Fig. 2(a), there were some precipitates in the alloy before solid solution, and the distribution of precipitates in aluminum matrix was uneven. There were also many dislocations in the Al-0.35Sc-0.2Zr (wt.%) alloy, and the dislocations tangled to form high dislocation density region near the particles. When the solid solution time was 12 h, the dislocations migration occurred. The original vacancies in the alloy also moved (because the activation energy of the vacancy is less than that of the dislocation). The dislocations and original vacancies annihilated in the interface, and the amount of dislocations decreased in the alloy. Due to the redissolution of precipitates (Fig. 2(b)), new vacancies were produced and retained during subsequent quenching. When the solid solution time was further extended, the dislocation density decreased greatly, and the precipitates dissolved into the Al matrix further, as shown in Figs. 2(c) and (d).
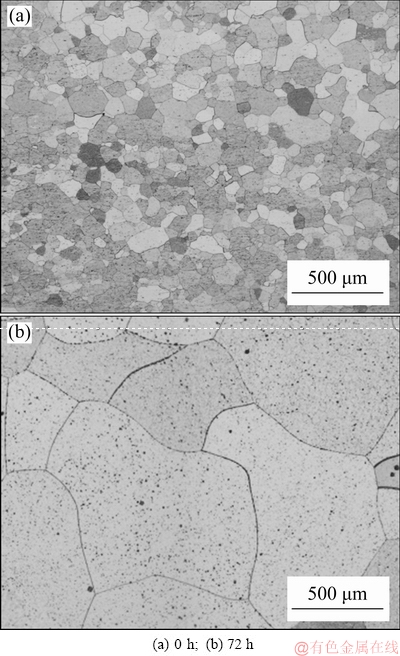
Fig. 1 Microstructures of Al-0.35Sc-0.2Zr (wt.%) alloy at longitudinal section with different solid solution time
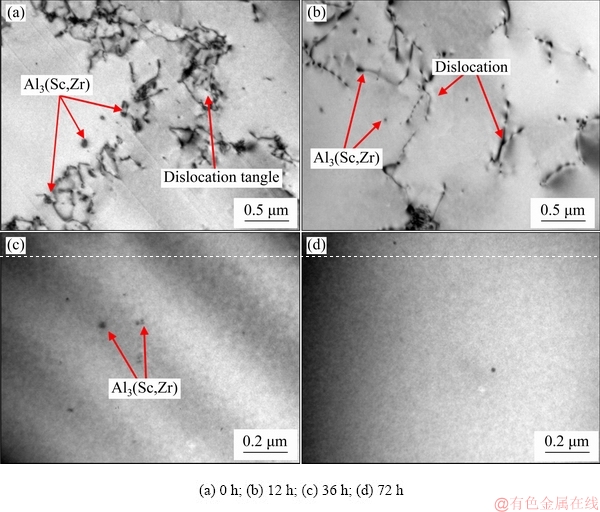
Fig. 2 TEM images of Al-0.35Sc-0.2Zr (wt.%) alloy with different solid solution time
Figure 3 shows the change of conductivity and tensile strength of Al-0.35Sc-0.2Zr (wt.%) alloy with solid solution time. As can be seen, with the increase of solid solution time, the conductivity of alloy increased from 52.6% (IACS) to 53.5% (IACS). The tensile strength of alloy decreased from 107.4 to 76.3 MPa correspondingly. In the solid solution process, on one hand, precipitates containing Sc and Zr atoms dissolved into aluminum matrix, thus inducing the lattice distortion [17], which improved scattering effect of the electron wave. On the other hand, solid solution led to a decrease in dislocation density, which reduced scattering effect of the electron wave. The dislocations were more influential on the conductivity than solid solution atoms. Therefore, with the solid solution time prolonging, the dislocation density reduced, and the conductivity of Al-Sc-Zr alloys gradually increased. As for tensile strength, on one hand, with the increase of solid solution time, the Sc and Zr atoms were gradually dissolved into the aluminum matrix (Fig. 2), resulting in the increase of tensile strength. On the other hand, the dislocation density in the alloy decreased, the precipitates disappeared, and the grain size increased. All these changes led to the reduction of tensile strength of the alloy. The second factor was the dominant one.
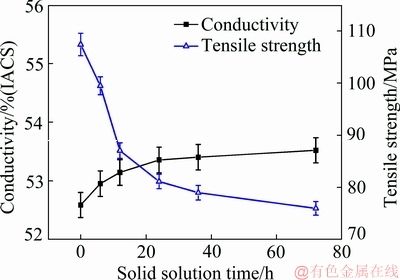
Fig. 3 Effect of solid solution time on conductivity and tensile strength of Al-0.35Sc-0.2Zr (wt.%) alloy (Solid solution temperature: 630 °C)
The conductivity of pure Al (k0) is 64% (IACS), and the conductivity (k1) of Al-0.35Sc-0.2Zr (wt.%) alloy after solid solution at 630 °C for 72 h was 53.5% (IACS). So, the quantitative contribution of solid solution atoms to the conductivity of the alloy is |Δks|=10.5% (IACS).
Figure 4 shows the longitudinal microstructures of Al-0.35Sc-0.2Zr (wt.%) alloy after solid solution at 630 °C for 72 h and aging at 380 °C for different time. As can be seen, the aging time has no obvious effect on grain size and morphology. Figure 5 shows the STEM photographs and diffraction pattern as well as EDS result of alloy with different aging time. The precipitates mainly contain Al, Sc and Zr (Fig. 5(e)). Figure 5(f) shows a diffraction pattern produced by the overlapping of the phase in the corresponding position of Fig. 5(e) and the surrounding α(Al) matrix. The diffraction spot diameters of spherical precipitates are R11=68.975 and R12=68.835, respectively, and the intersection angle between R11 and R12 is 90°. The diffraction spots can be calibrated according to [100]β axis of Al3(Sc,Zr) phase. So, it can be determined that the precipitates are Al3(Sc,Zr) whose structure is similar to L12 type [18]. Al3(Sc,Zr) and α(Al) matrix are coherent structures. The phase relationship is crystal band axis 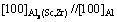 and lattice plane
and lattice plane 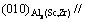 (0
(0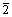 0)Al. The solubility of Zr and Sc atoms in the Al matrix changes as a function of temperature [19]. During aging process, the Sc and Zr atoms in the Al matrix were gradually precipitated. Because the diffusion rate of Sc atom in the Al matrix is larger than that of Zr atom, Al3Sc phase first form. Then, Zr atoms enrich around Al3Sc phase and replace a part of Sc atoms, and hence Al3(Sc,Zr) phases form [20-22]. Al3(Sc,Zr) precipitates have core of Al3Sc surrounded by Zr-rich shell. L12 superlattice is clearly observed in this shell [23,24]. The growth and dissolution of the precipitate are slow because of low diffusion rate of Zr [25]. So, the size of Al3(Sc,Zr) precipitates remains stable during the aging as the precipitate has a higher thermal stability.
0)Al. The solubility of Zr and Sc atoms in the Al matrix changes as a function of temperature [19]. During aging process, the Sc and Zr atoms in the Al matrix were gradually precipitated. Because the diffusion rate of Sc atom in the Al matrix is larger than that of Zr atom, Al3Sc phase first form. Then, Zr atoms enrich around Al3Sc phase and replace a part of Sc atoms, and hence Al3(Sc,Zr) phases form [20-22]. Al3(Sc,Zr) precipitates have core of Al3Sc surrounded by Zr-rich shell. L12 superlattice is clearly observed in this shell [23,24]. The growth and dissolution of the precipitate are slow because of low diffusion rate of Zr [25]. So, the size of Al3(Sc,Zr) precipitates remains stable during the aging as the precipitate has a higher thermal stability.
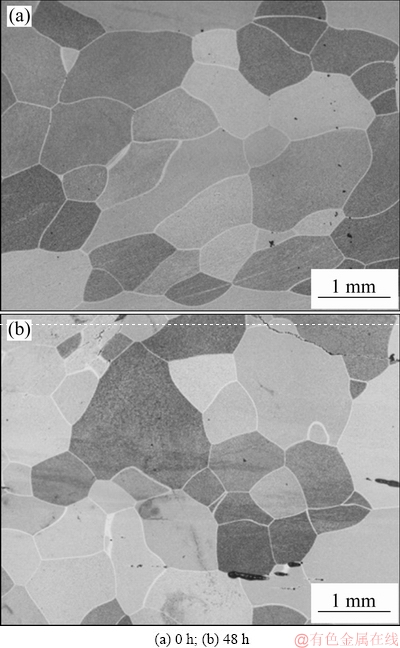
Fig. 4 Microstructures of Al-0.35Sc-0.2Zr (wt.%) alloy at longitudinal section with different aging time
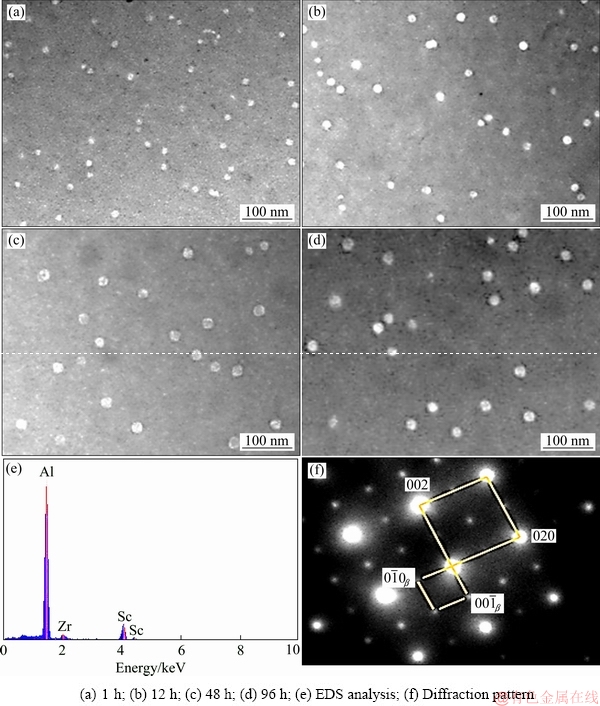
Fig. 5 STEM photographs, diffraction pattern and EDS result of Al-0.35Sc-0.2Zr (wt.%) alloy with different aging time
Figure 6 shows the hardness and conductivity variation of Al-0.35Sc-0.2Zr (wt.%) alloy with aging time after solid solution at 630 °C for 72 h. With the increase of aging time, the hardness and the conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy increased. The hardness of Al-0.35Sc-0.2Zr (wt.%) alloy reached the maximum value of HV 55.8 after aging for 24 h. Then, the hardness of Al-0.35Sc-0.2Zr (wt.%) alloy decreased slightly due to over-aging accompanied by the coarsing of precipitated particles and the decrease of particle dispersity. But, the Al3(Sc,Zr) precipitates remained coherent and homogeneous, and their size did not change obviously (Fig. 5). The hardness of the alloy remained at the high value with the prolonging of aging time. The conductivity curve can be divided into three stages, namely, 0-1 h, 1-48 h and after 48 h. The trend of the conductivity curve of Al-0.35Sc-0.2Zr (wt.%) alloy in early two stages of precipitation process is consistent with that of MARTIN et al [26]. It can be seen that the electrical conductivity increases linearly in the first stage as the formation of Sc-rich clusters and Al3Sc (L12). The second stage is segregation of Zr atoms to Al3Sc/α(Al) matrix interfaces and formation of Zr-enriched shell of these dispersoids. The conductivity of Al alloy containing Al3Sc is higher than that of Al alloy containing Al3Zr [27]. Therefore, the increasing rate of the first stage of the alloy conductivity curve is higher than that of the second stage. The conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy achieved peak value of 60.6% (IACS) after aging for 48 h. After that, the electrical conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy was almost stable with the further increase of aging time.
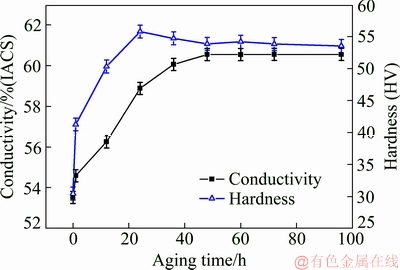
Fig. 6 Effect of aging time on hardness and conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy aged at 380 °C
In order to obtain the quantitative contribution of precipitates to the conductivity of the alloy aged at 380 °C, according to the mathematical model, a series of Al-Sc-Zr alloys was investigated.
Figure 7 shows the effect of aging time on the conductivities of Al-Sc-Zr alloys aged at 380 °C. The trend of the conductivity with aging time of the Al-Sc-Zr alloys with different compositions was basically the same. The lower the content of Sc is, the higher the initial conductivity of the alloy is. There may be two reasons for the difference of initial conductivity. As electrical resistivity has its origin in the scattering of free electrons by phonons and lattice defects, one is that with the increase of Sc content, the concentration of solid solution atoms increases and the conductivity decreases. The other is significantly enhanced concentration of Sc in the vicinity of defects for Al-Sc-Zr alloy before aging [26], so the Al-Sc-Zr alloy containing more Sc content has lower conductivity. The conductivities increase with the increase of aging time. After aging for 48 h, the peak conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy was achieved. When the aging time was further prolonged, the conductivity of alloy tended to be stable.
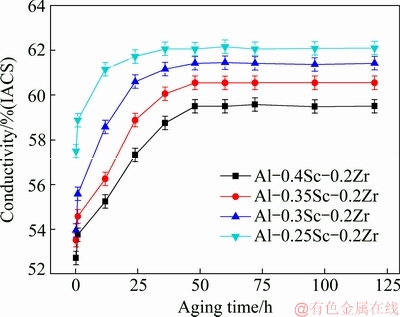
Fig. 7 Effect of aging time on conductivities of Al-Sc-Zr alloys aged at 380 °C
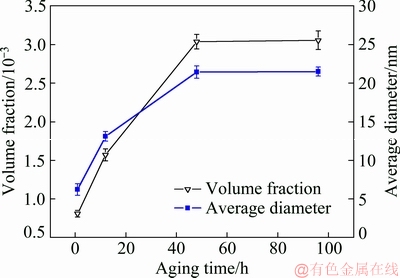
Fig. 8 Effect of aging time on volume fraction and average diameter of precipitates in Al-0.35Sc-0.2Zr (wt.%) alloy aged at 380 °C
Figure 8 shows the aging time-dependent variations of volume fraction and diameter of precipitates in Al-0.35Sc-0.2Zr (wt.%) alloy aged at 380 °C. The average diameter and volume fraction of the Al3(Sc,Zr) phase increase with the increase of aging time. After aging for 48 h, the average diameter and volume fraction of Al3(Sc, Zr) phase in the alloy did not change any more. This indicates that the precipitation and dissolution of the solid solution atoms reached a dynamic equilibrium after aging at 380 °C for 48 h. So, for the Al-Sc-Zr alloys aged at 380 °C, the dynamic equilibrium time between precipitation and dissolution of the solid solution atoms was 48 h.
By comparing Fig. 7 and Fig. 9, it can be found that the electrical conductivity curve of Al-0.05Sc-0.05Zr (wt.%) alloy with the aging time in Fig. 9 is different from those of other Al-Sc-Zr alloys in Fig. 7. For Al-0.05Sc-0.05Zr (wt.%) alloy, the electrical conductivity does not change significantly with aging time which is equal to the conductivity of Al-0.05Sc-0.05Zr (wt.%) alloy after solid solution, and this indicates that Al3(Sc,Zr) phase does not precipitate during aging. This suggests that the content of Sc and Zr atoms at 380 °C is close to 0.05 wt.%, which can be referred to as critical solid solubility. In this case, only saturated solid solution atoms exist and no precipitates form in the matrix of the Al-Sc-Zr alloys. The corresponding conductivity is 62.9% (IACS), so it can be regarded as kcs, which is the conductivity of Al-Sc-Zr alloys at critical solid solubility. The corresponding conductivity reduction induced by precipitates is calculated as follows:
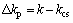 (3)
(3)
where k is tested conductivity.
The |Δkp| values induced by precipitates of Al-Sc-Zr alloys aged at 380 °C for 48 h are given in Table 1.
Figure 10 shows the STEM photographs of Al-Sc-Zr alloys after aging at 380 °C for 48 h. Table 2 gives the volume fractions and diameters of the precipitates after aging at 380 °C for 48 h. The average diameter and volume fraction of the Al3(Sc,Zr) in Al-0.25Sc-0.2Zr (wt.%) alloy after aging at 380 °C for 48 h are 12 nm and 0.84×10-3, respectively. The average diameter and volume fraction of the Al3(Sc,Zr) in Al-0.3Sc-0.2Zr (wt.%) alloy after aging at 380 °C for 48 h are 16 nm and 1.5×10-3, respectively. The average diameter and volume fraction of the Al3(Sc,Zr) in Al-0.35Sc-0.2Zr (wt.%) alloy after aging at 380 °C for 48 h are 21 nm and 3.2×10-3, respectively. The average diameter and volume fraction of the Al3(Sc,Zr) in Al-0.4Sc-0.2Zr (wt.%) alloy after aging at 380 °C for 48 h are 28 nm and 7.1×10-3, respectively.
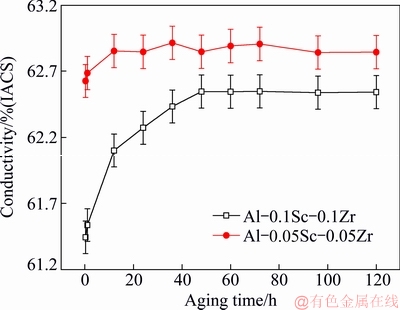
Fig. 9 Effect of aging time on conductivity of Al-Sc-Zr alloys aged at 380 °C
Table 1 |Δkp| values of Al-Sc-Zr alloys aged at 380 °C for 48 h
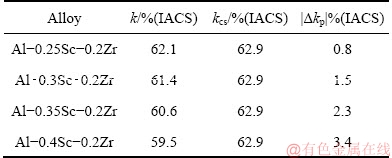
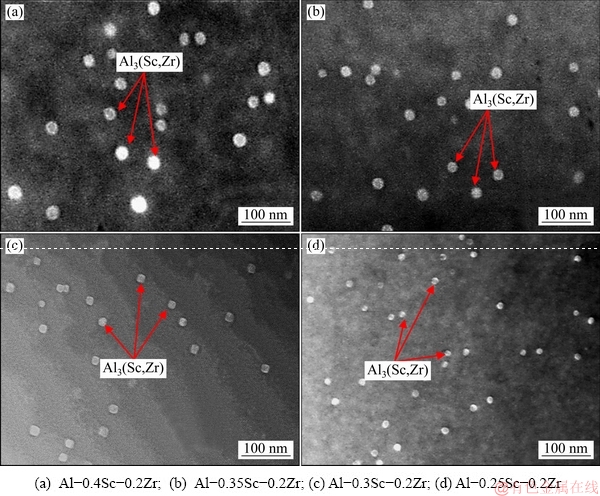
Fig. 10 STEM photographs of Al-Sc-Zr alloys after aging at 380 °C for 48 h
Table 2 Volume fraction and average diameter of Al3(Sc,Zr) precipitates after aging at 380 °C for 48 h
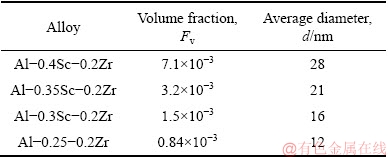
Figure 11 shows the relationship between Fv/d of precipitates and |Δkp| of Al-Sc-Zr alloys aged at 380 °C for 48 h. As can be seen from Fig. 11, the |Δkp| gradually increases with the increase of the Fv/d. The Δkp increases from 0.8% (IACS) to 3.4% (IACS), as Fv/d of the Al3(Sc,Zr) precipitates increases from 0.7×10-4 to 2.4×10-4 nm-1.
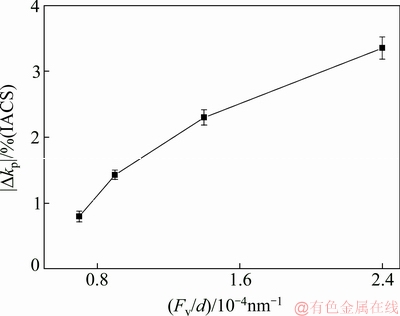
Fig. 11 Relationship between Fv/d of precipitates and |Δkp| of Al-Sc-Zr alloys aged at 380 °C for 48 h
3.2 Effect of cold deformation on microstructures and properties of Al-Sc-Zr alloys
Figure 12 shows the microstructures of Al-0.35Sc- 0.2Zr (wt.%) alloy after thermal treatment and cold deformation. When the area reduction ratio induced by cold deformation is 33%, the length and width of grains are 1170 μm and 630 μm, respectively. When the area reduction ratio is 60%, the length and width of grain are 2650 μm and 510 μm, respectively. When the area reduction is ratio 80%, the length and width of grain are 7500 and 550 μm, respectively.
Figure 13 shows the TEM photographs of Al-0.35Sc-0.2Zr (wt.%) alloy at longitudinal section with different area reduction ratios after cold deformation. When the area reduction ratio reached 33%, the opposite sign edge dislocations interacted in the parallel slip plane of the alloy, and the deformation band along the deformation direction was irregular due to uneven deformation, as shown in Fig. 13(a). With the increase of area reduction ratio, the sub-grains were elongated along the direction of deformation, the dislocations and the precipitates tangled, and thus the deformation resistance increased, as shown in Figs. 13(b) and (c).
Figure 14 shows TEM photographs of Al-0.35Sc- 0.2Zr (wt.%) alloy with different area reduction ratios after cold deformation. It can be observed that many fine second phases distributed homogeneously in Al matrix. These second phases effectively pined dislocations, and the dislocation density gradually increased.
Figure 15(a) shows the relationship between tensile strength and area reduction ratio of Al-0.35Sc-0.2Zr (wt.%) alloy after cold deformation. With the increase of area reduction ratio, the tensile strength of Al-0.35Sc- 0.2Zr (wt.%) alloy increases. When the area reduction ratio of the alloy is greater than 40%, the tensile strengths of Al-0.35Sc-0.2Zr (wt.%) alloy aged for 48 and 60 h are obviously higher than those of the alloy aged for 24 and 36 h. When the area reduction ratio of the alloy reaches 90%, the tensile strength of Al-0.35Sc-0.2Zr (wt.%) alloy reaches the peak value of 210.6 MPa. Figure 15(b) shows the relationship between elongation and area reduction ratio of Al-0.35Sc-0.2Zr (wt.%) alloy after cold deformation. With the increase of area reduction ratio, the elongation gradually decreases. Figure 15(c) shows the relationship between area reduction ratio and the conductivity of Al-0.35Sc-0.2Zr (wt.%) alloy aged for different time. With the increase of area reduction ratio, the conductivity of the alloy shows a gradual downward trend. When the area reduction ratio reaches 90%, the conductivity k3 of Al-0.35Sc-0.2Zr (wt.%) alloy aged at 380 °C for 48 h is 60.1% (IACS). The quantitative contribution of cold deformation to the conductivity of the alloy is |Δkε|=0.5% (IACS). The conductivity reduction of the alloys induced by cold deformation was small. This is possibly caused by microvoids closure during cold deformation [16], which offsets the partial increase in resistivity induced by dislocations. At the same time, the Al3(Sc,Zr) particles with a complex core–shell structure is almost unaffected by dislocations, and it is very stable [28]. So, the Al3(Sc,Zr) particles can intensively pin the dislocation. It is concluded that cold deformation can significantly improve the strength of Al-Sc-Zr alloys with a small conductivity reduction.
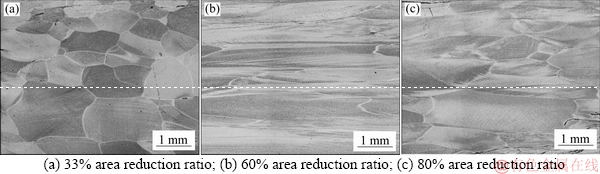
Fig. 12 Microstructures of Al-0.35Sc-0.2Zr (wt.%) alloy at longitudinal section after thermal treatment and cold deformation
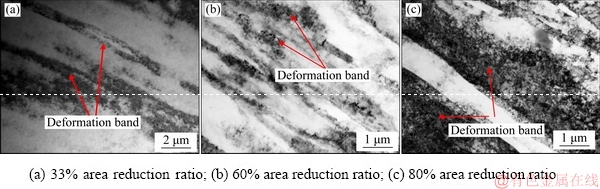
Fig. 13 TEM photographs of Al-0.35Sc-0.2Zr (wt.%) alloy at longitudinal section after cold deformation

Fig. 14 TEM photographs of Al-0.35Sc-0.2Zr (wt.%) alloy with different area reductions after cold deformation
Al-0.35Sc-0.2Zr (wt.%) alloy after solid solution at 630 °C for 72 h, aging at 380 °C for 48 h and cold deformation with area reduction ratio of 90% exhibits optimal comprehensive properties. The corresponding conductivity is 60.1% (IACS), the tensile strength is 210.6 MPa and the elongation is 7.6%. The properties of Al-0.35Sc-0.2Zr (wt.%) alloy are better than the standard of AT1, AT3 and AT4 heat-resisting aluminum alloy wires in IEC 62004-2007 (Standard of Aluminum Alloy Wires for Overhead Conductors).
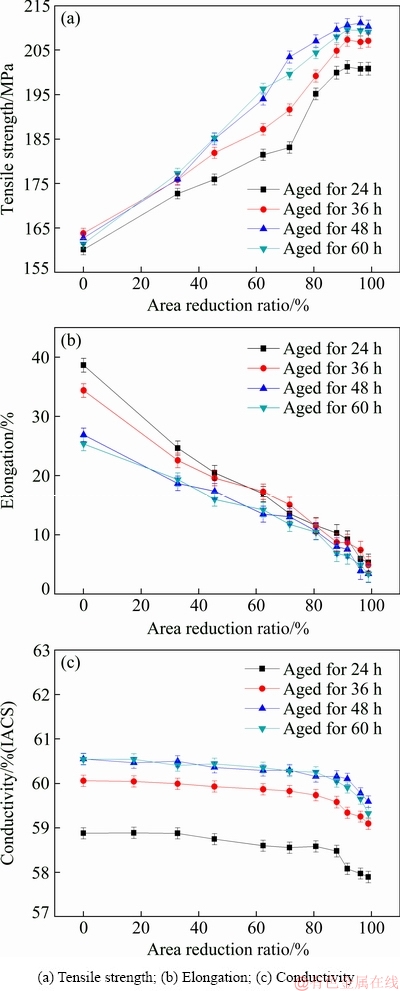
Fig. 15 Relationships between properties and area reduction ratio of Al-0.35Sc-0.2Zr (wt.%) alloy after cold deformation
3.3 Comparison of effects of solid solution atoms, precipitates and deformation on conductivity
According to the results, the solution atom contribution to conductivity reduction is |Δks|=10.5% (IACS). The precipitate contribution to conductivity reduction is |Δkp|=2.3% (IACS). The cold deformation contribution to conductivity reduction is |Δkε|=0.5% (IACS). The comparison of the effects of solid solution atoms, precipitates and cold deformation on conductivity reduction is shown in Fig. 16. This implies that the conductivity reduction induced by area reduction is slight but the conductivity reduction induced by solid solution atoms and precipitates is remarkable. So, cold working is an effective strengthening method with little effect on the conductivity.
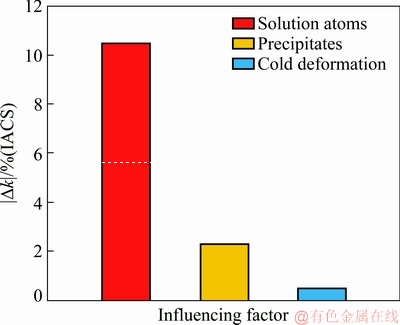
Fig. 16 Conductivity reduction |Δk| induced by solid solution atoms, precipitates and cold deformation of Al-Sc-Zr alloys
4 Conclusions
(1) The dynamic equilibrium time of the Al-Sc-Zr alloys aged at 380 °C was 48 h, and the critical solid solubility of the Al-Sc-Zr alloys aged at 380 °C was close to 0.05 wt.%.
(2) Solid solution for Al-0.35Sc-0.2Zr (wt.%) alloy could cause grain growth from 80 to 890 μm, tensile strength reduction from 107.4 to 76.3 MPa and conductivity increasing from 52.6% (IACS) to 53.5% (IACS). Aging could cause tensile strength and conductivity increasing, and the conductivity reduction changed as a function of the ratio of the volume fraction (Fv) to the diameter (d) of the Al3(Sc,Zr) precipitates (Fv/d). Cold deformation caused significant increasing of the tensile strength due to hard working, but its influence on conductivity was very little.
(3) The quantitative contribution of solution atoms to conductivity is 10.5% (IACS). The quantitative contribution of precipitates formed in aging to conductivity is 2.3% (IACS). The quantitative contribution of cold deformation to the conductivity of the alloys is 0.5% (IACS). So, cold working is an effective strengthening method with little influence on the conductivity, while the existing state of Sc and Zr atoms has a significant effect on the conductivity of the alloy.
References
[1] RALPH R S, CRAIG L J. Mechanical properties and microstructures of Al-Mg-Sc alloys [J]. Metallurgical Transactions A, 1990, 21(1): 421-430.
[2] JO H H, FUJIKAWA S I. Kinetics of precipitation in Al3Sc alloys and low temperature solid solubility of scandium in aluminium studied by electrical resistivity measurements [J]. Materials Science and Engineering A, 1993, 171(1-2): 151-161.
[3] LI Bo, PAN Qing-lin, CHEN Cong-ping, YIN Zhi-min. Effect of aging time on precipitation behavior, mechanical and corrosion properties of a novel Al-Zn-Mg-Sc-Zr alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2263-2275.
[4] LI Hong-ying, GAO Zhao-he, YIN Hao, JIANG Hao-fan, SU Xiong-jie, BIN Jie. Effects of Er and Zr additions on precipitation and recrystallization of pure aluminum [J]. Scripta Materialia, 2013, 68(1): 59-62.
[5] ELAGIN V I, ZAKHAROV V V, ROSTOVA T D. Some features of decomposition for the solid solution of scandium in aluminum [J]. Metal Science and Heat Treatment, 1983, 25(7): 546-549.
[6] ZHANG Wei, XING Yuan, JIA Zhi-hong, YANG Xiao-fang, LIU Qing, ZHU Chang-luo. Effect of minor Sc and Zr addition on microstructure and properties of ultra-high strength aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3866-3871.
[7] XU Cong, DU Rou, WANG Xue-jiao, HANADA S, YAMAGATA H, WANG Wen-hong, MA Chao-li. Effect of cooling rate on morphology of primary particles in Al-Sc-Zr master alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(7): 2420-2426.
[8] GUAN Ren-Guo, TIE Die. A review on grain refinement of aluminum alloys: Progresses, challenges and prospects [J]. Acta Metallurgica Sinica (English Letters), 2017, 30(5): 409-432.
[9] GUAN Ren-guo, SHEN Yong-feng, ZHAO Zhan-yong, WANG xiang. A high-strength, ductile Al-0.35Sc-0.2Zr alloy with good electrical conductivity strengthened by coherent nanosized- precipitates [J]. Journal of Materials Science & Technology, 2017, 33(3): 215-223.
[10] CHANG W S, RAJESH S R, CHUN C K, KIM H J. Microstructure and mechanical properties of hybrid laser-friction stir welding between AA6061-T6 Al alloy and AZ31 Mg alloy [J]. Journal of Materials Science & Technology, 2011, 27(3): 199-204.
[11] BAHU S, JANAKI R G D, VENKETAKRISNAN P V, MADHUSUDHAN R G, PRASAD R K. Microstructure and mechanical properties of friction stir lap welded aluminum alloy AA2014 [J]. Journal of Materials Science & Technology, 2012, 28(5): 414-426.
[12] COSTA S, PUGA H, BARBOSA J, PINTO A M P. The effect of Sc additions on the microstructure and age hardening behaviour of as cast Al-Sc alloys [J]. Materials & Design, 2012, 42: 347-352.
[13] DU Xiao-dong. Study on ageing and creep of Al-0.1Zr alloy [J]. Materials Science and Engineering A, 2006, 432(1-2): 84-89.
[14] KEITH E K, RICHARD A K, CONSTANCE P L, DAVID C D, DAVID N S. Precipitation evolution in Al-0.1Sc, Al-0.1Zr and Al-0.1Sc-0.1Zr (at.%) alloys during isochronal aging [J]. Acta Materialia, 2010, 58(15): 5184-5195.
[15] VLACH M, STULIKOVAI, SMOLA B, ZALUDOVA N, CERAN J. Phase transformations in isochronally annealed mould-cast and cold-rolled Al-Sc-Zr-based alloy [J]. Journal of Alloys and Compounds, 2010, 492(1-2): 143-148.
[16] ZHOU Wei-wei, BIN Cai, LI Wei-Jie, LIU Zhong-Xia, YANG Sheng. Heat-resistant Al-0.2Sc-0.04Zr electrical conductor [J]. Materials Science and Engineering A, 2012, 552: 353-358.
[17] CLOUET E, BARBU A. Using cluster dynamics to model electrical resistivity measurements in precipitating AlSc alloys [J]. Acta Materialia, 2007, 55(1): 391-400.
[18] MARSHA E V D, THOMAS G, DAVID C D, DAVID N S. Effects of Yb and Zr microalloying additions on the microstructure and mechanical properties of dilute Al-Sc alloys [J]. Acta Materialia, 2011, 59(20): 7615-7626.
[19] CHRISTIAN B F, DAVID N S. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part II-Coarsening of Al3(Sc1-xZrx) precipitates [J]. Acta Materialia, 2005, 53(20): 5415-5428.
[20] FORBORD, LEFEBVRE B W, DANOIX F, HALLEM H, MARTHINSEN K. Three dimensional atom probe investigation on the formation of Al3(Sc,Zr)-dispersoids in aluminium alloys [J]. Scripta Materialia, 2004, 51(4): 333-337.
[21] LEFEBVRE W, DANOIX F, HALLEM H, FORORD B, BOSTEL A, MARTHINSEN K. Precipitation kinetic of Al3(Sc,Zr) dispersoids in aluminium [J]. Journal of Alloys and Compounds, 2009, 470(1-2): 107-110.
[22] TOLLEY A, RADMILOVIC V, DAHMEN U. Segregation in Al3(Sc,Zr) precipitates in Al-Sc-Zr alloys [J]. Scripta Materialia, 2005, 52(7): 621-625.
[23] CHRISTOPHER B M, DAVID C D, DAVID N S. Coarsening resistance at 400 °C of precipitation-strengthened Al-Zr-Sc-Er alloys [J]. Acta Materialia, 2011, 59(18): 7029-7042.
[24] RADMILOVIC V, TOLLEY A, MARQUIS E A, ROSSELLl M D, LEE Z, DAHMEN U. Monodisperse Al3(LiScZr) core/shell precipitates in Al alloys [J]. Scripta Materialia, 2008, 58(7): 529-532.
[25] DESCHAMPS A, LAE L, GUYOT P. In situ small-angle scattering study of the precipitation kinetics in an Al-Zr-Sc alloy [J]. Acta Materialia, 2007, 55(8): 2775-2783.
[26] MARTIN V, JAKUB C, OKSANA M, IVANA S, BOHUMIL S, TOMAS K, HANA K, RYOTA G, VOLKMAR N. Early stages of precipitation process in Al-(Mn-)Sc-Zr alloy characterized by positron annihilation [J]. Metallurgical & Materials Transactions A, 2015, 46(4): 1556-1564.
[27] KEITH E K, DAVID N S, DAVID C D. Ambient- and high-temperature mechanical properties of isochronally aged Al-0.06Sc, Al-0.06Zr and Al-0.06Sc-0.06Zr (at.%) alloys [J]. Acta Materialia, 2011, 59(3): 943-954.
[28] VLACH M, CIZEK J, SMOLA B, STULIKOVA I, HRUSKAA P, KODETOVAA V, DANISA S, TANPRAYOONB D, NEUBERTC V. Influence of dislocations on precipitation processes in hot-extruded Al-Mn-Sc-Zr alloy [J]. International Journal of Materials Research, 2018, 109(7): 583-592.
管仁国1,2,金红梅2,Wensen JIANG3,王 祥2,王宇翔2,李 征1,张 俭2,Huinan LIU3,4
1. 西北工业大学 凝固技术国家重点实验室 先进润滑与密封材料研究中心,西安 710072;
2. 东北大学 材料科学与工程学院,沈阳 110819;
3. Materials Science and Engineering Program, University of California at Riverside, 900 University Avenue, Riverside, California 92521, USA;
4. Department of Bioengineering, University of California at Riverside, 900 University Avenue, Riverside, California 92521, USA
摘 要:为了研究固溶原子、析出相粒子和冷变形对Al-Sc-Zr合金组织和性能的影响,对连续流变挤压制备的Al-Sc-Zr合金进行形变热处理,采用电导率、强度、显微硬度测试以及光学显微镜、TEM、STEM对合金在不同处理状态下的显微组织和性能进行研究。建立固溶原子、析出相粒子和冷变形对合金导电率定量贡献的数学模型。结果表明:固溶、时效和冷变形都能使合金的强度得到显著的提升,而固溶原子、沉淀粒子和冷变形对Al合金导电率影响值分别是10.5% (IACS)、2.3% (IACS)和0.5% (IACS)。时效和冷变形处理是获得高强高导铝合金导线的关键。
关键词:Al-Sc-Zr 合金;热处理;冷变形;力学性能;导电率
(Edited by Bing YANG)
Foundation item: Project (51674077) supported by the National Natural Science Foundation of China; Project (2018YFB2001800) supported by the National Research and Development Program of China
Corresponding author: Hong-mei JIN; Tel: +86-13840321037; E-mail: jinhong.ru@163.com
DOI: 10.1016/S1003-6326(19)65000-4

