
Preparation and application of hydroxyapatite(HA)nanoparticles/NR2B-siRNA complex
YANG Hui(杨 惠)1, HUANG Dong(黄 东)1, 2, ZHU Shai-hong(朱晒红)1, YAN Xue-bin(阎雪彬)1,
GU Yong-hong(谷永红)1, YAN Hui(颜 辉)1, WU Li-xiang(邬力祥)2
1. The Third Xiangya Hospital, Central South University, Changsha 410013, China;
2. School of Basic Medicine, Central South University, Changsha 410013, China
Received 28 March 2008; accepted 15 May 2008
Abstract: Hydroxyapatite(HA) nanoparticles were prepared by coprecipitation-hydrothermal synthesis and their exosyndrome was estimated via transmission electron microscopy. Agarose gel electrophoresis and ultraviolet spectrophotometry were used to evaluate the ability of HA to bind NR2B-siRNA at different pH values and at different HA?NR2B-siRNA ratios. And the stability of the complex in saline was also evaluated. The effect of HA/NR2B-siRNA complex on chronic inflammatory pain was evaluated in vivo in mice. Transmission electron microscopy(TEM) reveals that HA nanoparticles are thin strips or short rod in shape and the one-dimensional particle size of HA nanoparticles is 40-50 nm. Under the acid or neutral condition, the Zeta potential of HA is positive; nanoparticles can completely bind NR2B-siRNA when the HA:NR2B-siRNA ratio is at or larger than 35?1; while under the alkaline condition, the affinity of HA to NR2B-siRNA is rather weak. HA/NR2B-siRNA complex is not dissociated when being resuspended in saline. The nociception of the tonic phase induced by formalin is significantly reduced in the HA/NR2B-siRNA treated mice as compared with the controls. Therefore, HA may be a new siRNA nano-vector material.
Key words: hydroxyapatite; nanoparticles; NR2B; siRNA; formalin pain; mice
1 Introduction
In the past decades, biomaterials have been increasingly widely applied in medicine. For example, BROWN and CHOW[1] found that the structure of hydroxyapatite(HA) was similar to that of the bone and tooth of the human, and later was successfully used in the repair of bone coloboma.
MATSUMOTO et al[2] prepared HA into nanoparticles and successfully used it as the slow-release carriers. In 2004, ZHU et al[3] observed that the transfection efficiency of HA nanoparticles could be as high as 50%-80% of the lipid body without obvious acute toxicity, but with satisfactory biocompatibility. SUN et al[4] successfully used HA nanoparticle to carry NT-3 gene in vitro and in vivo in the Cochlear neurons of the guinea pig.
NR2B, an NMDA receptor subunit existing in the procerebrum and spinal cord, plays an important role in adjustment of chronic pain. Silencing this gene could relieve chronic inflammatory pain.
In the current study, HA was prepared by chemical precipitation-hydrothermal synthetic technique. Its exosyndrome, optimal pH value and optimal HA nanoparticle:NR2B-siRNA ratio were evaluated. The transfection efficiency of the HA/NR2B-siRNA complex was estimated in vivo in mice.
2 Experimental
2.1 Preparation of HA nanoparticle powder and suspension
The HA nanoparticles were prepared by chemical precipitation-hydrothermal synthetic technique, then dissolved in the deionized water by ultrasonic cell disruptor (BRANSON SONIFIER 150, Rongshun Technological Instrument Factory, Ningbo) (Fig.1).
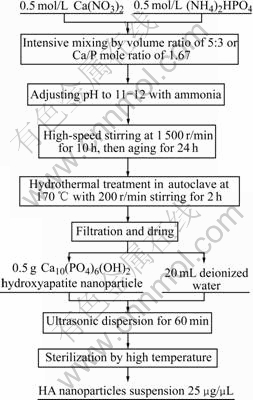
Fig.1 Experimental procedures of preparation of HA nanoparticle powder and suspension
2.2 Electron microscopy analysis and Zata potential measurement
0.1 mL of the above prepared HA nanoparticle suspension was diluted 10 times by double distilled water. One drop of the diluted suspension was dripped onto the copper network at room temperature. After drying, the nanoparticles were observed under a transmission electron microscope and pictures were taken. The HA nanoparticle suspension was further diluted to 0.5 μg/μL and adjusted to different pH values. Zeta potential of the HA nanoparticles at different pH values was measured via Zeta potential analyzer (Delsa 440SX Beckman Coulter Limited, USA).
2.3 Synthesis of HA nanoparticles and HA/NR2B- siRNA complex (HA/NR2B-siRNA)
The mice NR2B-siRNA designed by ourselves according to the Tuschl rules[5] was synthesized by Shanghai Genepharma Co Ltd, China.
2.3.1 Binding of HA nanoparticles to siRNA under different pH values
The HA nanoparticle suspension treated with 0.1% diethyl pyrocarbonate (DEPC, Sigma Corporation, USA) was diluted to 2.5 μg/μL, 20 μL of such suspension was taken into each eppendorf tube and the pH values were adjusted to 4, 7 and 10, respectively, followed by the addition of 1 μg NR2B-siRNA into each tube; while another tube containing 1 μg NR2B-siRNA without HA was used as control. After shaking and centrifugation, 3.75 μL supernatant from each tube was used for agarose gel electrophoresis.
2.3.2 Binding of HA nanoparticle to siRNA at different mass ratios
The HA nanoparticle suspension was diluted to 1.0 μg/μL, from which, 50, 45, 40, 35. 30, 25 and 10 μL was taken into 7 eppendorf tubes, respectively. The pH value of all the sample was adjusted to 6.5. Then 1 μg NR2B- siRNA was added into each tube; while another tube containing 1 μg NR2B-siRNA only was used as control. The following steps were as described above.
2.3.3 Measuring concentration of siRNA by ultraviolet spectrometer
2 μL supernatant from the above tubes containing different HA?siRNA ratios was taken from each tube to measure the concentration of unbound siRNA via the absorbance at 260 nm by ultraviolet spectrophotometer (Biophotometer ultraviolet spectrophotometer, Eppendorf, Germany).
2.3.4 Influence of saline on stability of HA/NR2B- siRNA complex
Tubes containing 1, 2, 3 and 4 μg NR2B-siRNA, respectively, were added with HA suspension to make the HA/NR2B-siRNA complex with the ratio of 35?1. After centrifugation, the supernatant was discarded. The complex was resuspended by 20 μL saline and centrifuged. 20 μL saline containing 1 μg NR2B-siRNA prepared at the same time served as control. 2 μL supernatant from each tube was taken to measure the unbound siRNA.
2.4 In vivo preliminary experiment
2.4.1 Preparation of HA/NR2B-siRNA complex and HA/ GFP-siRNA complex
4 μg NR2B-siRNA or 4 μg GFP-siRNA (the sequence of GFP-siRNA was from Ref.[6], and synthesized by Shanghai Genepharma Ltd., China) and 140 μg HA nanoparticles were mixed fully to prepare the HA/NR2B-siRNA complex or HA/GFP-siRNA complex, at the ratio of 35?1. The supernatant was discarded and replaced with 5 μL sterile saline before intrathecal injection.
2.4.2 Animal test
Twenty-four male Kunming mice, weighing 18-20 g, were randomly divided into 3 groups: 1) HN Group (8 mice), in which 5 μL HA/NR2B-siRNA complex was injected[7] into subarachnoid space of each mice; 2) HG Group (8 mice), 5 μL HA/GFP-siRNA complex was administered via the same way; 3) BC Group (8 mice), the same procedure was applied without administering any complex.
2.5 Formalin test
On the 7th day after injection, the planta surface of the right hindpaw of each mouse in all the groups was injected with 20 μL of 1% formalin solution for the formalin test observed in the following hour. The contralateral paw served as control. Each mouse was put into a resin cage for observation immediately. Pain scores were given[8] and the Time-Score Curves were plotted.
2.6 Statistics
The software SPSS 13.0 was used for statistical analysis. All data were reported as the mean±standard deviation ( ±s). The group comparison was analyzed using one-way analysis of variance. Statistical significance was established at P<0.05.
±s). The group comparison was analyzed using one-way analysis of variance. Statistical significance was established at P<0.05.
3 Results
3.1 Electron microscopy analysis of HA nanoparticle suspension
Static observation after ultrasonic dispersion for 60 min, compared with water in beaker, HA nanoparticle suspension has no stratification, but it looks like emulsion (Fig.2).

Fig.2 HA nanoparticle suspension ()
Transmission electron microscope(TEM)(H-600 TEM, Hitachi, Japan) analysis reveals that the one-dimensional particle size of HA nanoparticles is 40-50 nm, appearing as thin strips or short rods in shape (Fig.3).
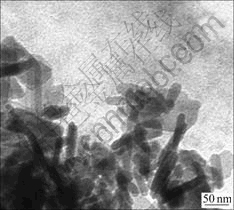
Fig.3 TEM image of HA nanoparticles
3.2 Zeta potential of HA nanoparticles
As shown in Fig.4, under acidic and neutral conditions, HA nanoparticles bear positive charges.
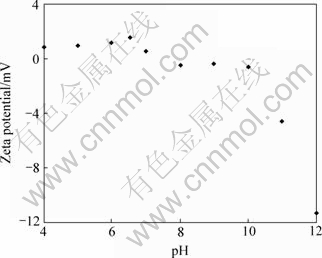
Fig.4 Zeta potential of HA nanoparticles at different pH values
3.3 Synthesis of HA nanoparticles and NR2B-siRNA complex
3.3.1 Stability of HA nanoparticle/siRNA at different pH values
The electrophoresis demonstrates that at pH values of 4 and 7 (corresponding to lines 1 and 2, Fig.5) there is no unbound NR2B-siRNA, while at pH 10 (line 3, Fig.5) there is some trace of free NR2B-siRNA as compared with the control that contains pure free NR2B-siRNA (line 4, Fig.5).

Fig.5 Stability of HA/NR2B-siRNA complex at different pH values
3.3.2 Binding of HA nanoparticles to siRNA at different mass ratios
The electrophoresis displays that at the HA: NR2B-siRNA ratios of 50?1 or 45?1 or 40?1 or 35?1 (corresponding to lines 1 to 4 in Fig.6) there is no unbound NR2B-siRNA, while at the ratio of 30?1 or 25?1 (corresponding lines 5 and 6 in Fig.6) there is some trace of unbound NR2B-siRNA, whereas at 10?1 ratio (line 7 in Fig.6) there is obvious free NR2B-siRNA as compared with the control that contains pure free NR2B-siRNA (line 8, in Fig.6).
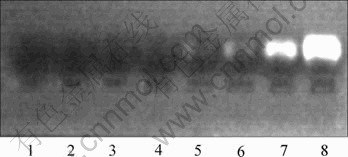
Fig.6 Binding condition of HA/NR2B-siRNA complex at different mass ratios
3.3.3 Assay of ultraviolet spectrometer
It is demonstrated in Table 1 that unbound siRNA is undetectable by Ultraviolet Spectrometer in the supernatants with HA?NR2B-siRNA ratio at or over 35?1.
Table 1 A260 values of supernatants and concentrations of unbound siRNA at different HA:NR2B-siRNA mass ratios
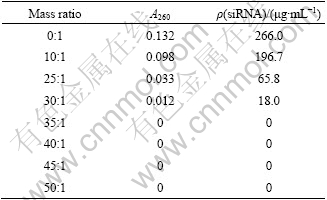
3.3.4 Influence of saline on stability of HA/NR2B- siRNA complex
As displayed in Table 2 that there is no unbound siRNA detectable in the supernatants of the HA/NR2B- siRNA complex resuspended by saline, while the large amount of unbound siRNA is detected in the control tube.
Table 2 A260 values of supernatants and concentrations of unbound siRNA in HA/NR2B-siRNA complex resuspended by saline

3.4 Formalin test
Following subcutaneous injections of 1% formalin in their hindpaws, the mice displays nociceptive behaviors such as restlessness, uplifting, licking, biting or shaking their injected hindpaw.
The nociceptive behavior in the mice demonstrated a typical response of double phases, I.E. the first phase is from 0 to 5 min, while the second phase is from 15 to 60 min. The nociceptive response of the mice in the first phase (acute phase) was indistinguishable among all groups (P>0.05). However, the nociceptive response of HN group in the second phase (tonic phase) was decreased significantly as compared with the other groups (P<0.05). There was no significant difference between HG group and BC group (P>0.05) (Fig.7). Furthermore, there were no body mass or daily behavior changes observed in the HN and HG groups as compared with that in the control group during the 7 d after intrathecal injection.
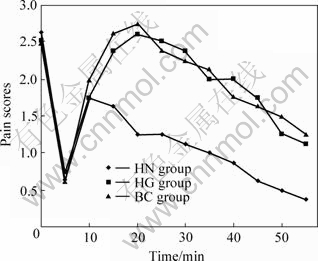
Fig.7 Time-score curves for results of formalin-induced nociception (In the second phase during 15-60min, there was significant difference between HN group and Hg group, or between HN group and BC group (P<0.05))
4 Discussion
RNA interference(RNAi) could be used in gene therapy by transferring small fragments of double-strand RNA into the target cell to silence the target gene at the transcriptional level. However, it is difficult for naked RNA to penetrate cellular lipid membranes[9], therefore, numerous vectors have been developed to facilitate the transfer of siRNA into the target cells[10]. Data accumulated have demonstrated that the traditional transportation system of virus carriers in gene therapy can exert well on delivery[11-12]. Despite its relatively high transfection efficiency, however, the use of virus carriers was stringent due to the security consideration and the high cost.
The nonvirus carriers have advantages in these aspects. Nanoparticles have become increasingly important as nonvirus carriers because of their unique properties, such as the small size effect and the surface effect. According to their chemical properties, nanocarriers can be categorized as organic or inorganic vectors. The main inorganic materials include the hydroxyapatite[4], silicon-oxidize[13], ferro-oxidize[14], calcium phosphate[15], calcium carbonate[16]. The main organic materials are polycations, including the improved PEI nanoparticles[17-18].
Compared with other nonvirus carriers, the major advantages of HA are its higher biocompatibility, stronger affinity to proteins and nucleotides, lower cost and higher transfection efficiency.
Presently, the main domestic preparation of the nano hydroxyapatite powder includes coprecipitation, hydrothermal synthesis and solution-galation, while we used the chemical coprecipitation-hydrothermal synthesis[3] in the current study. The HA nanoparticles were short rods when being observed under TEM, with a linear length of approximately 40-50 nm. Their Zeta potential showed that in acidic or neutral condition, HA nanoparticles bear positive charges.
Our results show that HA nanoparticles can bind to NR2B-siRNA effectively in the acidic or neutral condition, while their affinity to NR2B-siRNA is decreased in the basic condition. It is proposed that the HA/NR2B-siRNA complex is formed by the mutual effects of their charges. The varying affinity of HA to NR2B-siRNA is probably due to the change of their surface charges at different pH conditions for pH can affect the surface charge of the nanoparticles.
Our results also demonstrate that when the HA:NR2B-siRNA mass ratio is below 35?1, the affinity of HA to NR2B-siRNA is decreased. When the ratio at or above 35?1, HA can bind the NR2B-siRNA completely, leaving no free siRNA in the supernatant. In addition, when being resuspended with saline, the complex remains stable, implying that HA/NR2B-siRNA complex can always be transported to the target cell in the physiological condition.
However, it is also true that the pH value of HA nanoparticles suspension can not be adjusted downwards unboundedly, because when pH value is below 3, the emulsus suspension turns to be transparent immediately (data not shown), implying the two hydroxyl groups of HA are neutralized by H+ and the properties of HA have been changed.
Furthermore, Zeta potential shows that positive charges of HA nanoparticles under acidic and neutral conditions are far less than its negative charges under basic condition. In order to boost the ability of carrying siRNA, further research should be directed to improve their Zeta potential. In addition, further improvement in manufacturing HA nanoparticles should be conducted to reduce the HA:NR2B-siRNA mass ratio. All of these will be carried out in the future study.
In this study, HA/NR2B-siRNA complex can significantly reduce formalin-induced nociception in the tonic phase in mice. Moreover, in the two groups using HA nanoparticles as siRNA vector shows no impacts on the body mass and daily behavior as compared with the control group. Therefore, it can be concluded that HA may have great potential to become a novel siRNA nano-vector in gene therapy.
5 Conclusions
1) The one-dimensional size of the HA nanoparticles produced by chemical precipitation- hydrothermal synthesis in the current study is 40-50 nm; Zeta potential shows under acidic or neutral condition, HA nanoparticles are positively charged, and HA can completely bind to NR2B-siRNA at 35?1 or higher mass ratio.
2) HA/NR2B-siRNA complex is stable in saline.
3) Via intrathecal injection, HA/NR2B-siRNA complex can relieve formalin-induced nociception in the tonic phase in mice.
4) HA may be a potential novel siRNA nano-vector material.
References
[1] BROWN W E, CHOW L C. A new calcium phosphate, watersetting cement [M]// BRDWN P W. Cement Research Progress. Ohio: American Ceramic Society, 1987: 352-379.
[2] MATSUMOTO T, OKAZAKI M, INOUE M, YAMAGUCHI S, KUSUNOSE T, TOYONAGA T, HAMADA Y, TAKAHASHI J. Hydroxyapatite particles as a controlled release carrier of protein [J]. Biomaterials, 2004, 25(17): 3807-3812.
[3] ZHU Shai-hong, HUANG Bo-yun, ZHOU Ke-chao, HUANG Su-ping, LIU Fang, LI Yi-ming, XUE Zhi-gang, LONG Zhi-gao. Hydroxyapatite nanoparticles as a novel gene carrier [J]. Journal of Nanoparticle Research, 2004, 6(2): 307-311.
[4] SUN Hong, JIANG Ming, ZHU Shai-hong. In vitro and in vivo studies on hydroxyapatite nanoparticles as a novel vector for inner ear gene therapy [J]. Chin J Otorhinolaryngol Head Neck Surg, 2008, 43(1): 51-56. (in Chinese)
[5] ELBASHIR S M, HARBORTH J, WEBER K, TUSCHL T. Analysis of gene function in somatic mammalian cells using small interfering RNAs [J]. Methods, 2002, 26: 199-213.
[6] TAN P H, YANG L C, SHIH H C, LAN K C, CHANG J T. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat [J]. Gene Therapy, 2005, 12(1): 59-66.
[7] FAIRBANKS C A. Spinal delivery of analgesics in experimental models of pain and analgesia [J]. Advanced Drug Delivery Reviews, 2003, 55: 1007-1041.
[8] ISHIZAKI K, SASAKI M, KARASAWA S, OBATA H, NARA T, GOTO F. The effect of intrathecal magnesium sulphate on nociception in rat acute Pain models [J]. Anaesthesia, 1999, 54: 241-246.
[9] LI C X, PARKER A, MENOCAL E, XIANG S L, BORODYANSKY L, FRUEHAUF J H, BORODYANSKY, FRUEHAUF J H. Delivery of RNA Interference [J]. Cell Cycle, 2006, 5(18): 2103-2109.
[10] ZHANG Shu-biao, ZHAO Bu-diao, JIANG Hui-ming, WANG Bing, MA Bai-chao. Cationic lipids and polymers mediated vectors for delivery of siRNA [J]. Journal of Controlled Release, 2007, 123(1): 1-10.
[11] BRUMMELKAMP T R, BERNARDS R, AGAMI R. Stable suppression of Tumorigenicity by virus-mediated RNA interference [J]. Cancer Cell, 2002, 2(3): 243-247.
[12] DEVROE E, SILVER P A. Therapeutic potential of retroviral RNAi vectors [J]. Expert Opinion On Biological Therapy, 2004, 4(3): 319-327.
[13] HE X X, WANG K M, TAN W H, LIU B, LIN X, HE C M, LI D, HUANG S S, LI J. Bioconjugated nanoparticles for DNA protection from cleavage [J]. J Am Chem Soc, 2003, 125(24): 7168-7169.
[14] DOBSON J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery [J]. Gene Therapy, 2006, 13(4): 283-287.
[15] BISHT S, BHAKTA G, MITRA S, MAITRA A. pDNA loaded calcium phosphate nanoparticles: Highly efficient non-viral vector for gene delivery [J]. Int J Pharm, 2005, 288(1): 157-168.
[16] HE X W, LIU T, CHEN Y X, CHENG D J, LI X R, XIAO Y, FENG Y L. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo [J]. Cancer Gene Therapy, 2008, 15(3): 193-202.
[17] MYKHAYLYK O, VLASKOU D, TRESILWISED N, PITHAYANUKUL P, MOLLER W, PLANK C. Magnetic nanoparticle formulations for DNA and siRNA delivery [J]. Journal of Magnetism and Magnetic Materials, 2007, 311(1): 275-281.
[18] SWAMI A, KURUPATI R K, PATHAK A, SINGH Y, KUMAR P, GUPTA K C. A unique and highly efficient non-viral DNA/siRNA delivery system based on PEI-bisepoxide nanoparticles [J]. Biochemical and Biophysical Research Communications, 2007, 362(4): 835-841.
Foundation item: Project(07JJ5035) supported by the Natural Science Foundation of Hunan Province, China; Project(2007WK3031) supported by Science and Technology Foundation of Hunan Province, China
Corresponding author: HUANG Dong; Tel: +86-731-8618326; E-mail: huangdong6619@yahoo.com.cn
(Edited by LI Xiang-qun)