J. Cent. South Univ. (2017) 24: 2565-2571
DOI: https://doi.org/10.1007/s11771-017-3670-y

Mechanism studies of 5-HMF pyrolysis by quantum chemistry
CHEN Bo(陈波)1, SHI Zhang-ming(时章明)1, JIANG Shao-jian(蒋绍坚)1, TIAN Hong(田红)2
1. School of Energy Science and Engineering, Central South University, Changsha 410083, China;
2. Institute of Energy & Power Engineering, Changsha University of Science and Technology, Changsha 410076, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2017
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2017
Abstract: The pyrolysis of 5-HMF was investigated using density functional theory methods at B3LYP/6-31G++(d, p) level. Two possible pyrolytic pathways were proposed and full optimization of the energy gradient for the structures of reactants, products, intermediates and transition states of various reactions was implemented. The standard kinetic parameters in each reaction pathway were calculated and the formation and evolution mechanism of main pyrolysis products were analyzed. Bond dissociation energies calculation results show that the bond dissociation energy of CH3—OH of 5-HMF is the lowest and the order of all kinds of bond dissociation energy is CH3—OH<C—H<CH3OH—Caromatic<CHO—Caromatic<Caromatic—H. In pathway (1), the energy barrier of furfural is 322.8 kJ/mol, the energy barrier of 2-furfuryl alcohol is 375.4 kJ/mol; the energy barrier of furan-2,5-dicarbaldehyde is 496.1 kJ/mol; the energy barrier of 5-methyl furfural is 375.8 kJ/mol, and the energy barrier of 2-methyl furan is 375.8 kJ/mol. In pathway (2), the activation energy required for open-loop with H2O is higher.
Key words: 5-HMF; pyrolysis mechanisms; quantum chemistry
1 Introduction
With the shortage of fossil energy, biomass energy has been paid increasing attention as the world’s fourth largest energy. Biomass gasification technology has become the main development direction of biomass energy utilization, but large amounts of tar will be produced in the gasification process [1-5]. The composition of the tar always changes as the pyrolysis temperature increases. The tar is divided into primary tars, secondary tars and tertiary tars based on the temperature and tar composition [6, 7]. As one of the representative components of primary tars and the main compound of furans produced by cellulose and semi-fiber pyrolysis, 5-HMF is generally regarded as the final product of cellulose and semi-fiber pyrolysis because of its steady chemical property [8-10].
At present, studies about 5-HMF have been focused on the formation of 5-HMF from glucose and fructose, and most of them are based on macro-experiments. Experimental study was carried out on degradation of glucose to 5-HMF using ZrO2 as catalyst [11]. OKANO et al [12] drew a conclusion that the yield of 5-HMF came to 88.7% by degrading fructose experiments in the ionic solution using [MBCIm]SO3Cl as a catalyst. JADHAV et al [13] obtained that the yield of 5-HMF reached up to 92.3% by degrading fructose experiments in the double anionic ionic liquids. BINDER [14] et al developed the way of producing 5-HMF from biomass stalk cornstalks with a yield of 48%. LU et al [15] offered a promising way of low-temperature fast pyrolysis of fructose to produce 5-hydroxymethyl furfural (HMF) with furfural (FF) as an important by-product. HUANG et al [16] studied the pyrolysis mechanism of glucopyranose by using density functional theory (DFT) methods at B3LYP/6-31G++(d, p) level, the results showed that 5-HMF was the main pyrolysis product, and the activation energies of the two pathway were 284.49 kJ/mol and 297.02 kJ/mol. WANG et al [17] conjectured that glucopyranose produced a ring-opening chain firstly by cleavage of C(1)—O bond and then cyclized to form an intermediate by dehydration of hydroxyl groups on C(2) and C(5), afterwards 5-HMF was generated through two hydroxyl dehydration reaction. While some other scholars [18] believed that 5-HMF is formed through the way that the open-loop glucose chain forms two double after twice dehydration, followed by cyclization reaction.
Further studies on the cleavage of 5-HMF are rare, and most of them are mainly focused on pyrolysis tests. HUBER et al [19] reported that the 5-HMF underwent an aldol condensation reaction under the action of a solid base catalyst to form a C(9)-C(15) organic compound. DENG et al [20] suggested that levulinic acid and equivalent formic acid could be obtained by further hydrolysis of 5-HMF. SHIN et al [21] studied the pyrolysis of 5-HMF; the results showed that single molecule degradation was the main pyrolysis mechanism, but benzene ring might be produced through the addition reaction. ZHAO et al [22] carried out catalytic pyrolysis of 5-HMF, and the yield of aromatics was 48.99% under the optimum conditions. LIAO et al [23] conducted pyrolysis experiment of 5-HMF by PY-GC-MS, and the mechanism of pyrolysis was deduced from the pyrolysis products. Mechanism study of 5-HMF pyrolysis was analyzed by density functional theory and the energy barrier of reaction pathways can be concluded, which can explain the related experiment results. The study is also helpful to understand the secondary reaction of biomass pyrolysis and the evolution mechanism of the biomass tar.
2 Analytical method
The structure optimization and frequency calculation of intermediates, transition states and products of 5-HMF and pyrolysis process were carried out by DFT. The standard thermodynamic parameters under pressure 1.13×105 Pa and temperature 298 K were obtained. The optimized structures of all the molecules were verified by the virtual frequency, for being sure to be a minimum point on the potential energy surface. All the thermodynamic parameters were corrected by the zero point energy (ZPE). The activation energy was defined as the energy difference between the transition state and the reactant. The feasibility of the reaction pathway was judged by calculating the activation energy of the reaction, so that the most likely route was found in the presumed reaction pathway. The Opt (TS) method was used to find the transition state, and the unique frequency was determined by the vibration frequency calculation. The standard thermodynamic variables were calculated as the thermodynamic quantities of the product minus the thermodynamic quantities of the reactants and corrected by the zero energy correction. The bond dissociation energy for the main bond dissociation reactions was calculated as [24]:
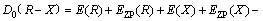
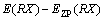 (1)
(1)
where D0 is the bond dissociation energy considering the zero point correction; E is the molecular energy; and EZP is the correction value of zero point energy.
3 Simulation results and discussion
3.1 Bond dissociation energy
The bond dissociation energy is an important parameter indicating the bond strength. It reflects combinative firm degree between the two bonding atoms in the molecule. It is generally believed that the greater the bond dissociation energy shows, the better the thermal stability of the bond is. Figure 1 shows the chemical structure of 5-hydroxymethyl furfural and 8 kinds of molecular bond split modes. Table 1 shows the calculation results of dissociation energies of various primary bonds.
As shown in Table 1, the bond dissociation energy of hydroxymethyl group is higher than that of aldehyde group (the bond dissociation energy of methylene group is 415.8 kJ/mol and the dissociation energy of aldehyde group is 433.6 kJ/mol). C(8)—O(9), where the lowest dissociation energy is 278.7 kJ/mol, followed by the dissociation energy of C—H on the hydroxymethyl group, which is 310.3 kJ/mol. The maximum of the bond dissociation is Caromatic—H on the furan ring (C(3)—H(10) is 493.5 kJ/mol, and C(5)—H(11) is 492.2 kJ/mol). The order of the dissociation energies of the various bonds is: CH3—OH3OH—Caromaticaromatic< Caromatic—H. During the pyrolysis process, the hydroxymethyl groups are easier to break than the aldehydes. This is consistent with the results of pyrolysis experiment of 5-HMF, where 2-furfuryl alcohol comes into being after furfural.

Fig. 1 Chemical structural formula of 5-HMF and its eight homolytic cleavage ways
Table 1 Bond dissociation energies (D) of major bonds in 5-HMF (unit: kJ/mol)

3.2 Analysis of product formation mechanism
The results of pyrolysis experiment of 5-HMF [23] indicated that the fission products of furan ring were furan-2, 5-dicarbaldehyde, 5-methyl furfural, furfural, 2-furfuryl alcohol and 2-methylfuran in the case of the fact that furan ring was not open. Furfural and 5-methyl furfural were mainly generated at 130-230 °C, furfuryl alcohol was mainly formed after 250 °C, 2-methyl-furan was produced after 336 °C and 5-furfuraldehyde forms in the whole process. SHIN et al [21] found that the two weakest bonds in the 5-HMF were located on the hydroxymethyl group from the point of view of chemical bond energy, so the possibility of hydroxymethyl cleavage was greater than that of the aldehyde, leading to furfural dominated in the further decomposition product of 5-HMF. The main pyrolysis products were the chain ketone structure materials in the case of the fact that furan ring was open [25]. Two possible pyrolysis pathways for 5-HMF are proposed based on the calculations of bond dissociation energies and the experimental results. The first reaction pathway is that the furan ring does not undergo furan ring opening, and the second pathway undergo furan ring opening. Two possible reaction pathways are shown in Fig. 2 and Fig. 3. Two potential energy profiles are shown in Fig. 4 and Fig. 5. Optimized structures of reactants, products and transition states in reaction pathway of 5-HMF pyrolysis are shown in Table 2 and Table 3.
In the pathway (1), the CH3—OH bond of 5-HMF breaks firstly, forming intermediate IM3 and hydroxyl, and the heat of absorption is 278.7 kJ/mol. The intermediate IM3 is further hydrogenated to form 5-methylfurfural. The hydrogenation reaction is exothermic, with the heat of 340.7 kJ/mol. 5-methylfurfural could decarbonylate further to form 5-methylfuran. The reaction releases heat 32.1 kJ/mol, and the reaction energy barrier is 313.8 kJ/mol. The hydrogen atom on the methylol of 5-HMF is removed to form intermediates IM1, with the absorption of heat 310.3 kJ/mol. IM1 generates furan-2,5-dicarbaldehyde through dehydrogenation reaction (the transition state TS1), absorbing energy 271.4 kJ/mol, and the reaction energy barrier is 185.8 kJ/mol. 5-HMF can completely remove the methylol to form formaldehyde and furfural.
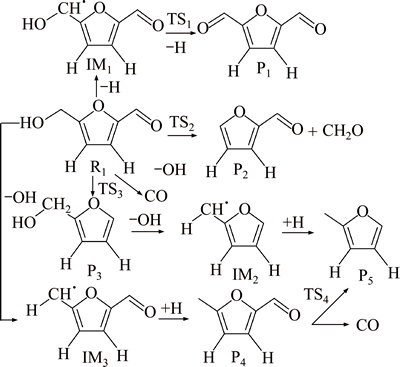
Fig. 2 Proposed pyrolysis processes of 5-HMF in reaction pathway (1)

Fig. 3 Proposed pyrolysis processes of 5-HMF in reaction pathway (2)
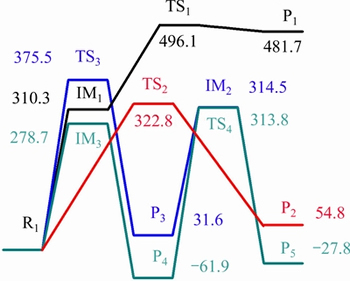
Fig. 4 Potential energy profiles along reaction pathway (1) of 5-HMF(unit: kJ/mol)

Fig. 5 Potential energy profiles along reaction pathway (2) of 5-HMF (unit: kJ/mol)
Table 2 Optimized structure of reactants, products, intermediates and transition states in reaction pathway (1) of 5-HMF pyrolysis (unit: 0.1 nm)

Table 3 Optimized structure of reactants, products, intermediates and transition states in reaction pathway (2) of 5-HMF pyrolysis (unit: 0.1 nm)

The reaction energy is 54.8 kJ/mol and the reaction energy barrier is 322.8 kJ/mol. 5-HMF can also remove the aldehydes, and pass the transition state of TS3 to produce furfuryl alcohol and CO, absorbing heat 31.6 kJ/mol. The reaction energy barrier is 375.4 kJ/mol. Furfuryl alcohol takes off hydroxyl to produce intermediates IM2, absorbing heat 314.5 kJ/mol. The intermediate IM2 is further hydrogenated to form 2-methylfuran, giving a heat of 344.3 kJ/mol. 2-methyl furan can be generated through the process of hydroxyl group removal, then hydrogenation, and last aldehyde group removal, or firstly aldehyde removal, then hydroxyl group taking off, and lastly hydrogenation. The energy barrier of the way that hydroxyl group removal reaction happened firstly is low, and the reaction is more likely to occur from the potential energy profile.
Those verify the same results of in-situ FTIR experiment [23]: if the formation of 2-methyl furfuryl alcohol is as an intermediate product, the signal of the formation of CO should be relatively early and strong. As the emergence of CO is late and the signal is weak, it is more likely that 5-methyl furfural is the intermediate product.
In the pathway (2), the pyrolysis of 5-HMF is mainly the ring-opening of the furan ring, instead of the removal of the functional group. Through integrating the ring-opening mechanism of the furan ring and the product composition of pyrolysis reaction, the ring opening of 5-HMF is divided into open-loop behavior with H2O and open-loop behavior without H2O.
5-HMF undergoes the transition state TS5 and the enol type product of P6 forms in the presence of H2O. This process can be described as follows: C—O bond on furan ring is gradually weakened, fracture and open-loop occur in the presence of H2O. The H atom in the H2O atom bonds to the O atom in the ring and a hydroxyl group forms. The hydroxyl group formed in the dehydrogenation of H2O bonds to the C formed in the cleavage of C—O on the furan ring. The heat absorption is 73.9 kJ/mol and the reaction energy barrier is 287.6 kJ/mol. 5-HMF undergoes the transition state TS6 to form the enolate product P6 in the absence of H2O. This process can be described as follows: The aldehyde group forms a hydrogen radical and migrates to the carbon atom on the furan ring, resulting in the ring opening of the furan ring. The reaction endotherm is 35.6 kJ/mol and the reaction energy barrier is 279.1 kJ/mol. The activation energy required for open-loop with H2O is higher.
3.3 Thermodynamic analysis
The standard thermodynamic parameters ΔH and ΔG of each reaction are calculated at different temperatures (298, 400, 550, 680, 800 and 950 K, chosen according to the experiments [21]). The results are shown in Table 4 and Fig. 6. ΔH indicates the standard enthalpy change of the reaction. When ΔH<0, the reaction emits heat. Otherwise, it absorbs heat. ΔG represents the standard free energy variable value, which is the thermodynamic parameter to determine whether the reaction can proceed spontaneously and the reactant conversion rate when the reaction reaches equilibrium. When ΔG<0, the reaction can be carried out spontaneously. The smaller the ΔG is, the more easily the reaction happens and the more the products would be got when reaction reaches equilibrium.
As shown in Table 4 and Fig. 6, the enthalpy change of reaction 6 and reaction 8 are less than zero and the reactions are exothermic. The other reactions are endothermic, where the enthalpy change of reaction 1 is the biggest. It can be concluded that the breaking of aldehyde group needs more heat than the breaking of methylol group and the generation of 2-furfuryl alcohol is more easily than furfural thermodynamically. The enthalpy changes of reactions 4 and 9 are smaller than those of the other reactions at each temperature, indicating that the cleavage of aldehyde group needs less heat and 5-HMF is easy to generate furfural in the state of heating. The Gibbs free energies of reactions 4, 6, and 8 are all less than zero and the reaction can proceed spontaneously. The Gibbs free energies of reactions 6, 8 and 10 increase with the rise in temperature, indicating that the increase of temperature is not conducive to the formation of P4, P5 and P6.
Table 4 Change of standard thermodynamic parameters at different temperatures
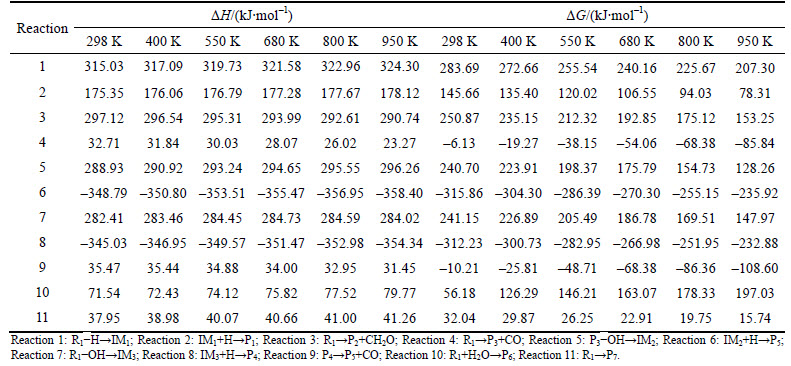
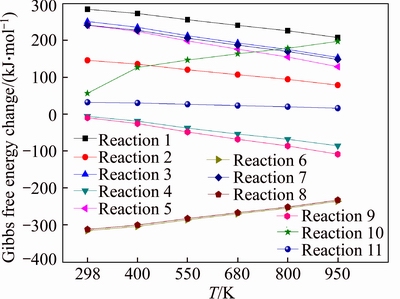
Fig. 6 Gibbs free energy changes at different temperatures
4 Conclusions
1) The results show that the dissociation energy of CH3-OH bond of 5-HMF is the smallest, and the dissociation energy of each bond is in the order of
CH3—OH3OH—Caromaticaromatic<Caromatic—H.
2) Two possible pyrolysis routes were proposed for pyrolysis of 5-HMF. In the pathway (1), the reaction energy barrier of furfural is 322.8 kJ/mol; the reaction energy barrier of 2-furfuryl alcohol is 375.4 kJ/mol; the reaction energy barrier of furan-2,5-dicarbaldehyde is 496.1 kJ/mol. The reaction energy barrier of 5-methyl furfural is 278.7 kJ/mol and the reaction energy barrier of 2-methyl furan is 375.8 kJ/mol. The open-loop energy barrier with H2O in the pathway (2) is 287.6 kJ/mol, and the open-loop energy barrier without H2O is 279.1 kJ/mol.
3) The cleavage of aldehyde group of 5-HMF needs less heat than that of hydroxymethyl. The reaction energy barrier of aldehyde group is higher than that of hydroxymethyl. The Gibbs free energy of cleavage of aldehyde group is less than zero and the reaction can occur spontaneously.
References
[1] GAO Zheng-wei, WU Zhen, CHEN Wang-qi, KANG Tian-shan. The features and elimination of tar in biomass gasification processes [J]. Guangzhou Chemical Industry, 2015, 43(23): 50-53. (in Chinese)
[2] AHMED I I, GUPTA A K. Kinetics of woodchips char gasification with steam and carbon dioxide [J]. Applied Energy, 2011, 88(5): 1613-1619.
[3] UMEKI K, NAMIOKA T, YOSHIKAWA K. Analysis of an updraft biomass gasifier with high temperature steam using a numerical model [J]. Applied Energy, 2012, 90(1): 38-45.
[4] LIU Hai-li, E Jia-qiang, DENG Yuan-wang, XIE Chang-qing, ZHU Hao. Experimental study on pyrolysis characteristics of the tobacco stem based on the microwave heating method [J]. Applied Thermal Engineering, 2016, 106: 473-479.
[5] LIU Hai-li, E Jia-qiang, MA Xiao-qian, XIE Chang-qing. Influence of microwave drying on the combustion characteristics of food waste [J]. Drying Technology, 2016, 34(12): 1397–1405.
[6] ROBERT J E, THOMAS A M. Molecular characterization of the pyrolysis of biomass 1. Foudamental [J]. Energy & Fuels, 1987, 1(2): 123-137.
[7] ROBERT J E, THOMAS A M. Molecular characterization of the pyrolysis of biomass 2. Applications [J]. Energy & Fuels, 1987, 1(4): 311-319.
[8] LU Q, YANG X, DONG C, ZHANG Z, ZHANG X, ZHU X. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study [J]. Journal of Analytical and Applied Pyrolysis, 2011, 92(2): 430-438.
[9] KATO K. Pyrolysis of cellulose, Part III. Comparative studies of the volatile compounds from pyrolysates of cellulose and its related compounds [J]. Agricultural and Biological Chemistry, 1967, 31: 657-663.
[10] WAN P R, WAN J C, DE J E, RASRENDRA C B, HEERES H J, DE J G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources [J]. Chemical Reviews, 2013, 113(3): 1499-1597.
[11] OSATIASHTIANI A, LEE A F, BROWN D R, MELERO J A, MORALES G, WILSON K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose [J]. Catalysis Science & Technology, 2014, 4(2): 333-342.
[12] OKANO T, QIAO K, BAO Q, YOKOYAMA C. Dehydration of fructose to 5-hydroxymethylfurfural (HMF) in an aqueous acetonitrile biphasic system in the presence of acidic ionic liquids [J]. Applied Catalysis A: General, 2013, 451: 1-5.
[13] JADHAV A H, CHINNAPPAN A, PATIL R H, KOSTJUK S V, KIM H. Green chemical conversion of fructose into 5-hydroxymethylfurfural (HMF) using unsymmetrical dicationic ionic liquids under mild reaction condition [J]. Chemical Engineering Journal, 2014, 243: 92-98.
[14] BINDER J B, RAINES R T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals [J]. Journal of the American Chemical Society, 2009, 131(5): 1979-1985.
[15] LU Qiang, LIAO Hang-tao, ZHANG Yang, ZHANG Jun-jiao,DONG Chang-qing. Reaction mechanism of low-temperature fast pyrolysis of fructose to produce 5-hydroxymethyl furfural [J]. Journal of Fuel Chemistry and Technology, 2013, 41(9): 1070-1075. (in Chinese)
[16] HUANG Jin-bao, TONG Hong, LI Wei-min, WU Dan. Quantum chemistry theoretical studies on pyrolysis mechanism of glucopyranose [J]. Chemical Research and Application, 2013, 25(4): 479-483. (in Chinese)
[17] WANG Shu-rong, LUO Zhong-yang. Pyrolysis of biomass components [M]. Beijing: Science Press, 2013. (in Chinese)
[18] SHEN D K, GU S. The mechanism for thermal decomposition of cellulose and its main products [J]. Bioresource Technology, 2009, 100(24): 6496-6504.
[19] HUBER G W, CHHEDA J, BARRETT C B, DUMESIC J A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates [J]. Science, 2005, 308(27): 1446-1450.
[20] DENG L, LI J, LAI D M, GUO Q X. Catalytic conversion of biomass-derived carbohydrates into-valerolactone without using an external H2 supply [J]. Angewandte Chemie International Edition, 2009, 48(35): 6529-6532.
[21] SHIN E J, NIMLOS M R, EVANS R J. Kinetic analysis of the gas-phase pyrolysis of carbohydrates [J]. Fuel, 2001, 80(12): 1697-1709.
[22] ZHAO Yan, PAN Tao, ZUO Yong, GUO Qing-xiang, FU Yao. Production of aromatic hydrocarbons through catalytic pyrolysis of 5-hydroxymethylfurfural from biomass [J]. Bioresource Technology, 2013, 147: 37-42.
[23] LIAO Yan-fen, GUO Zhen-ge, CAO Ya-wen, MA Xiao-qian, LIN Yan. Analysis of pyrolysis mechanism of 5-hydroxymethyl furfural by using PY-GC-MS and in-situ FT-IR [J]. Journal of South China University of Technology: Natural Science Edition, 2015, 43(6): 15-18. (in Chinese)
[24] ZHANG Fang-pei, CHENG Xin-lu, LIU Zi-jiang, HU Dong, LIU Yong-gang. Density functional studies on the bond dissociation energy and pyrolysis mechanism of propyl nitrate [J]. Chinese Journal of High Pressure Physics, 2005, 19(2): 189-192. (in Chinese)
[25] JI Hui-ling, JIANG Nan, WANG Ji-gang. Theoretical study of the ring-opening mechanisms of constituent units with different numbers of furan rings during the degradation of furfuryl-alcohol resin [J]. Journal of Beijing University of Chemical Technology: Natural Science, 2011, 38(4): 43-46. (in Chinese)
(Edited by YANG Hua)
Cite this article as: CHEN Bo, SHI Zhang-ming, JIANG Shao-jian, TIAN Hong. Mechanism studies of 5-HMF pyrolysis by quantum chemistry [J]. Journal of Central South University, 2017, 24(11): 2565–2571. DOI:https://doi.org/10.1007/s11771-017-3670-y.
Foundation item: Project(51276023) supported by the National Natural Science Foundation of China
Received date: 2016-12-06; Accepted date: 2017-02-25
Corresponding author: JIANG Shao-jian, Professor; Tel: +86-731-88830269; E-mail: sjjiang@csu.edu.cn