Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides(L.) Roberty)
来源期刊:中南大学学报(英文版)2019年第2期
论文作者:吴川 Manikandan RAJENDRAN 安文慧 李伟展 Venkatachalam PERUMAL Shivendra Vikram SAHI Santosh Kumar SARKAR
文章页码:489 - 500
Key words:chromium; Chrysopogon zizanioides; detoxification mechanism; antioxidant enzymes; phytoremediation
Abstract: This study investigated the chromium (Cr) detoxification mechanism-induced changes in growth and antioxidant defence enzyme activities in Chrysopogon zizanioides. Plant growth decreased by 36.8% and 45.0% in the shoots and roots, respectively, in the 50 mg/L Cr treatment. Cr accumulation was higher in root (9807 μg/g DW) than in shoots (8730 μg/g DW). Photosynthetic pigments and malondialdehyde content increased up to the 30 mg/L Cr treatment, whereas they declined at higher doses. The activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) were increased significantly with increasing of Cr dose but slightly declined at higher doses. Isozyme banding patterns revealed the expression of multiple bands for SOD, CAT and POX enzymes, and the band intensity decreased at high doses of Cr exposure. These results indicate that higher Cr doses increased the oxidative stress by over production of reactive oxygen species (ROS) in vetiver that had potential tolerance mechanism to Cr as evidenced by enhanced level of antioxidative enzymes, photosynthetic pigments, MDA contents. Therefore, vetiver has evolved a mechanism for detoxification and accumulates higher concentration of toxic Cr. This study provides a better understanding of how vetiver detoxifies Cr.
Cite this article as: Manikandan RAJENDRAN, AN Wen-hui, LI Wai-chin, Venkatachalam PERUMAL, WU Chuan, Shivendra Vikram SAHI, Santosh Kumar SARKAR. Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides(L.) Roberty) [J]. Journal of Central South University, 2019, 26(2): 489–500. DOI: https://doi.org/10.1007/s11771-019-4021-y.

ARTICLE
J. Cent. South Univ. (2019) 26: 489-500
DOI: https://doi.org/10.1007/s11771-019-4021-y
Manikandan RAJENDRAN1, 2, AN Wen-hui(安文慧)1, LI Wai-chin(李伟展)3,Venkatachalam PERUMAL2, WU Chuan(吴川)1, Shivendra Vikram SAHI4, Santosh Kumar SARKAR5
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Plant Genetic Engineering and Molecular Biology Lab, Department of Biotechnology, Periyar University, Salem 636 011, Tamil Nadu, India;
3. Department of Science and Environmental Studies, The Education University of Hong Kong, Tai Po, Hong Kong, China;
4. Department of Biology, Western Kentucky University, Bowling Green, KY 42101-1080, USA;
5. Department of Marine Science, University of Calcutta, Calcutta 700019, West Bengal, India
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: This study investigated the chromium (Cr) detoxification mechanism-induced changes in growth and antioxidant defence enzyme activities in Chrysopogon zizanioides. Plant growth decreased by 36.8% and 45.0% in the shoots and roots, respectively, in the 50 mg/L Cr treatment. Cr accumulation was higher in root (9807 μg/g DW) than in shoots (8730 μg/g DW). Photosynthetic pigments and malondialdehyde content increased up to the 30 mg/L Cr treatment, whereas they declined at higher doses. The activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) were increased significantly with increasing of Cr dose but slightly declined at higher doses. Isozyme banding patterns revealed the expression of multiple bands for SOD, CAT and POX enzymes, and the band intensity decreased at high doses of Cr exposure. These results indicate that higher Cr doses increased the oxidative stress by over production of reactive oxygen species (ROS) in vetiver that had potential tolerance mechanism to Cr as evidenced by enhanced level of antioxidative enzymes, photosynthetic pigments, MDA contents. Therefore, vetiver has evolved a mechanism for detoxification and accumulates higher concentration of toxic Cr. This study provides a better understanding of how vetiver detoxifies Cr.
Key words: chromium; Chrysopogon zizanioides; detoxification mechanism; antioxidant enzymes; phytoremediation
Cite this article as: Manikandan RAJENDRAN, AN Wen-hui, LI Wai-chin, Venkatachalam PERUMAL, WU Chuan, Shivendra Vikram SAHI, Santosh Kumar SARKAR. Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides(L.) Roberty) [J]. Journal of Central South University, 2019, 26(2): 489–500. DOI: https://doi.org/10.1007/s11771-019-4021-y.
1 Introduction
Chromium contamination is one of the primary environmental concerns worldwide, and the contamination occurs in various oxidation states such as trivalent chromic (Cr3+) and hexavalent chromate (Cr6+) ions. Chromium is released into soil and water bodies from various industries including electroplating, metallurgical, tanneries, textile dyeing and chemical industries [1].
Among these sources, the leather industry is a primary source for Cr release into the biosphere, accounting for approximately 40% of industrial usage [2]. Heavy metals are easily transferred into food chains via adsorption, uptake and accumulation by crop plants [3–5]. Humans are also consuming high concentrations of heavy metals via food and contaminated waters that can damage organs and cause cancers [6, 7].
Heavy metals may induce toxicity to plants by generating increased levels of ROS. ROS like superoxide radical (O2·), hydroxyl radical (OH·) and hydrogen peroxide (H2O2) may produce various by-products associated with membrane transport and other metabolic pathways. Plants have effective antioxidant defence mechanisms to protect them from oxidative damage induced by ROS, and the detoxification activities are highly complex mechanisms and compartmentalized among plant cells. In plants, the ROS level is regulated by an antioxidative defence system that includes various enzymes such as SOD, CAT, APX, and POX. SOD is a primary ROS scavenger under stress conditions, with the superoxide anion converted into H2O2 and O2. Subsequently, catalases in the peroxisomes scavenge H2O2 by converting it to water and oxygen molecules. Additionally, POX may reduce H2O2 using various reductants of phenolic compounds [8].
Chromium does not degrade in the environment; hence, effective methods are required to remove heavy metals from polluted sites. Remediation of heavy metals contaminated soils with traditional methods such as physical and chemical techniques is expensive and not applicable to large area. XUE et al [9] described that the various industrial wastes are used for soil remediation. For example, ZOU et al [10] reported that, the red mud modified biochar reduced the arsenic in soil. XUE et al [11] mentioned that, the phosphogypsum addition reduced the soil pH and increased the heavy metals adsorption. Green phytoremediation technology has emerged as one of the potential strategies for the extraction of heavy metals contamination from soil and water sources by growing various hyperaccumulators.
Previous studies have reported potential utilization of vetiver to decontaminated heavy metals from soil, wastewater treatment, mine tailings and recently used for heavy metal removal from red mud. However, few reports available regarding Cr impact on vetiver growth and accumulation [12] have been published, but to the best of our knowledge, no data available of Cr effect on photosynthetic pigments, antioxidative enzymes, isoenzyme patterns and protein profile changes in vetiver under the Cr treatments. Therefore, the present study was to investigate the effect of Cr-induced toxicity on growth, Cr accumulation, chlorophyll content, antioxidative enzyme activity, protein, isoenzyme pattern change and MDA level in vetiver.
2 Materials and methods
Uniform sized vetiver tillers were collected and washed thoroughly with running tap water, and then grown hydroponically [13]. After adaptation, plants were treated with different concentrations of Cr (0, 10, 20, 30, 40 and 50 mg/L). Chromium was supplied as potassium dichromate (K2Cr2O7), whereas medium without Cr served as the control. Plants were removed from the hydroponic solution after 16 d of treatment and rinsed with distilled water for removal of surface particles.
2.1 Determination of plant growth and biomass
Treated plants were harvested and separated into the root and shoot tissues and their fresh weight (FW) was measured immediately. That same root and shoot tissues were dried in a hot air oven for 48 h at 65 °C to quantify dry weight (DW) biomass. The index of tolerance (IT) was calculated according to follows [14]:
IT=MLCr/MLcontrol×100% (1)
where MLCr is the maximum length of Cr treated plant root (or) shoot and MLcontrol is the maximum length of control plant root (or) shoot.
2.2 Anatomical study
Treated and control plants roots and leaves were cut into 5–10 cm pieces and fixed at 48 h in formalin acetic acid alcohol (FAA) then preserved in 70% alcohol [15]. Cross section of leaves and roots were taken by hand. Sections were dehydrated in ethyl alcohol and stained by safranin. Then well-stained sections were photographed with an Olympus MLXI.
2.3 Analysis of photosynthetic pigment content
The chlorophyll a and b and carotenoids contents were determined according to ARNON [16] method. The photosynthetic pigments contents were calculated according to MANIKANDAN et al [13].
2.4 Analysis of Cr content and translocation factor
For Cr content estimation, dried roots and shoots (0.5 g) were digested with a mixture of HCl and HNO3 (7:3 volume ratio) by heating on a hot plate at 70 °C till the mixture turned brown to light yellowish. The digested samples were filtered and determined Cr concentration by using AAFES (Atomic absorption flame emission spectrophoto- meter–6200; Shimadzu). The translocation factor (TF) was calculated according to DAS et al [17].
IT=Cr content in shoots/Cr content in roots×100% (2)
2.5 Determination of antioxidative enzyme activities and native PAGE analysis
Chromium-caused oxidative stress in plants is associated with the production of ROS, which can rapidly affect various biomolecules like proteins, nucleic acids, and pigments. Plants have evolved a unique set of antioxidant defence enzymes that act against such oxidative damages [18]. Therefore, the role of SOD, CAT and POX activities towards the reduction of ROS production in Cr-stressed vetiver plants requires study.
About 200 mg of fresh root or shoot samples were ground in mortar and pestle with 2 mL of phosphate buffer (pH 7.5). The homogenate samples were centrifuged at 10000 r/min for 10 min and the supernatant was collected for analyses of antioxidative enzymes activities and PAGE.
The activity of SOD was measured by photochemical reduction of nitrobluetetrazolium (NBT) by DHINDSA et al [19]. CAT activity was assayed according to AEBI [20] method. The activity of POX was determined according to the method of CASTILLO et al [21]. The analyses of SOD, CAT and POX activities and units were expressed according to our previous study [13].
SOD, CAT and POX isoenzyme banding patterns were determined on native polyacrlymidegel electrophoresis according to our previous study [13].
The SOD isoenzyme banding pattern was visualized according to BEAUCHAMP et al [22]. CAT isoenzyme patterns were analyzed according to the method of VERMA et al [23]. POX isoenzyme pattern analysis was conducted by the protocol of ANDERSON et al [24]. The images of isoenzyme polyacrlymide gels were documented by using Alpha Innotech gel image system (San Jose, California, USA).
2.6 Estimation of protein, lipid peroxidation and H2O2 contents
The content of total soluble protein in the supernatant was determined by the method of BRADFORD [25]. Bovine Serum Albumin (BSA) was used as standard and total soluble protein content was expressed in mg/g FW.
The lipid peroxidation level was measured as content using the procedure of DAVENPORT et al [26]. The MDA content was calculated according to MICHEAL et al [27]. The content of hydrogen peroxide was measured according to the method of SERGIEV et al [28] and content was expressed in nmol/g FW.
2.7 Statistical analyses
For statistical analysis, each experiment was performed with 3 replicates for measurements of plant growth, photosynthetic pigments, antioxidative enzyme activities, MDA and H2O2 content, with their mean values considered. Analysis of variance (ANOVA) and Duncan’s multiple range tests (DMRT) were conducted using the SPSS 20 statistical software package (IBM SPSS 20 Statistics). Student-Newman-Keuls tests were used to identify the significant differences among means at the P<0.05 significance level.
3 Results and discussion
3.1 Effect of chromium on growth and biomass changes in vetiver
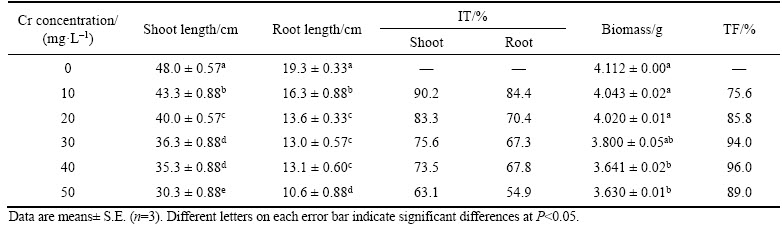
The primary focus of present study was to evaluate the effect of Cr exposure on growth, biomass, photosynthetic pigments, antioxidative enzyme activity and alteration in isoenzyme banding profiles in vetiver. Shoot and root growth varied significantly depending on the concentration of Cr treatment. After 16 d of exposure, plant growth decreased by 36.8% and 45.0% in shoots and roots, respectively, in the 50 mg/L Cr treatment (Table 1). Similarly, the root and shoot index of tolerance (IT) values also decreased under Cr-induced stress conditions (Table 1). However, the decline in root length was more pronounced than that of shoot length under Cr stress. Similar, results were also reported in Zea mays [29]. SHAHID et al [30] claimed that decrease in shoot length can be due to the fact that the Cr decreased the root growth and inhibited the nutrients translocation from root to shoot parts, which affect the shoots cellular metabolism, thereby reduce the shoot growth. Altered plant biomass by heavy metal induced toxicity is considered a good biological indicator in plants. Vetiver plant biomass declined with increasing Cr concentration in the growth medium (Table 1). The maximum biomass reduction was 12.1% in the 50 mg/L Cr treatment. RASHAD et al [31] observed that, Cr inhibited plant growth and decreased biomass level in Citrus reshni, and Citrus limonia under Cr treatments.
Table 1 Effect of Cr stress on growth, tolerance indices (TI), biomass and translocation factor (TF) of vetiver
3.2 Chromium accumulation
Root and shoot Cr accumulation was increased gradually with the increase of Cr concentration (Figure 1). The higher level of Cr accumulation was 9808 μg/g DW and 8731 μg/g DW for roots and shoots, respectively, at the 50 mg/L Cr concentration, with Cr accumulation higher in root than in shoot. This result indicated that roots served as a partial barrier against the transport of Cr to the shoot and Cr concentration was largely acquired then stored in vetiver roots. SEN et al [32] mentioned that, the Cr accumulation was lower in shoots than in roots due to the reduction of Cr6+ to Cr3+, which decreased the Cr mobility from root to shoot. SIEGEL [33] also claimed that, root accumulated Cr complexed with —COOH group and inhibited the Cr translocation from root to shoot.
The translocation factor (TF) values increased up to the 40 mg/L Cr treatment but slightly declined at the 50 mg/L Cr concentration (Table 1). Similar results were also reported in Tradescantia pallid [34]. Results indicated that the effective acquisition of Cr by vetiver plants could be due to the occurrence of an efficient physiological mechanism within the root system, and results also showed high hyperaccumulation potential in shoots via Cr heavy metal detoxification by regulating an antioxidant defence mechanism.
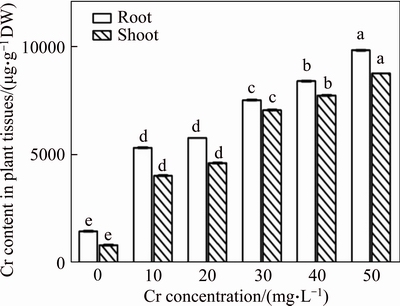
Figure 1 Chromium accumulation in root and shoot tissues of vetiver (Data are represented as means± S.E. (n=3). Means with different letters indicate significant differences at P<0.05)
3.3 Histochemical detection of Cr localization in root and shoot tissues of vetiver
Vetiver root xylem, phloem, cortex, pith and epidermis were affected by Cr toxicity. The epidermis consisted of small and normal cells in control plants (Figure 2(a)), but in Cr treatments, the root epidermis was damaged and consisted of disordered cells (Figure 2(b)).
The localization of Cr in root and leaf anatomical structures was confirmed by the appearance of a strong staining that ranged from red-brown to black-brown (Figures 2(b) and (d)). As shown in Figure 2(b), chromium accumulation was mostly observed in root pith tissues, with deposits also observed in vacuole bundles and parenchyma cells of leaf tissues at the 50 mg/L Cr exposure (Figure 2(d)). Similarly, SAMARDAKIEWICZ et al [35] showed the maximum level of heavy metal deposition in small vacuoles of Lemna minor. The possible reason for the localization of Cr between vacuoles and the cell wall was the redistribution of Cr metal ions, which reflected increased apoplastic transport.
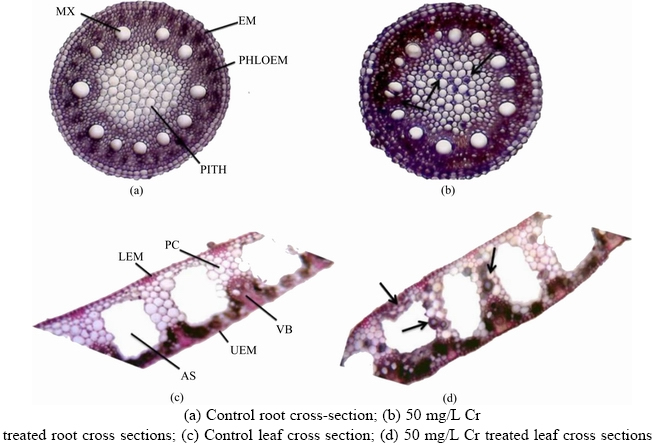
Figure 2 Chromium localization in root and leaf tissues of vetiver plant:(MX–Metaxylem; EM–Endodermis; LEM–Lower epidermis; PC–Parenchyma; AS–Airspace; UEM–Lower epidermis; VB–Vascular Bundle; Arrow marks–Cr deposits)
3.4 Effect of chromium on pigment composition
Effect of Cr on chlorophyll and carotenoide contents of vetiver is depicted in Table 2. Notably, the levels of chlorophyll a and b, total chlorophyll and carotenoids increased up to the 30 mg/L Cr treatment, whereas the levels slightly reduced at the higher doses. The maximum percent of pigment content recorded was 72.2%, 155.3%, 98.4% and 37.6% for Chl a, Chl b, total Chl and Car, respectively, in the 30 mg/L Cr treatment, whereas content decreased to 36.9%, 66.7%, 45.0% and 26.1%, respectively, in the 50 mg/L Cr exposure. This indicated that enhanced level of MDA reduced the chlorophyll biosynthesis in vetiver. Moreover, this increased MDA content was correlated with the maximum level of POX activity, which is one of the protective mechanisms of the cell membrane [13]. Present results were corroborated with previous finding in Cr treated Pistia stratiotes [36] and Citrus reshni, and Citrus limonia [31].
3.5 Effect of Cr on antioxidative enzyme activities
The SOD activity was gradually increased with increasing Cr concentrations in both shoots and root tissues (Figure 3(a)). The maximum percentage of SOD activity observed was 513.7% and 273.8% for shoots at the 40 mg/L Cr exposure and roots at the 20 mg/L Cr treatment, respectively. It may reduce the superoxide generation and protect vetiver plants from oxidative damage.
However, SOD activity slightly decreased in both shoot and root tissues at the higher doses of Cr treatment, which might be due to the Cr inhibitory effect on antioxidative defence enzyme systems [2].
Table 2 Effects of chromium on photosynthetic pigments in leaf tissues of vetiver
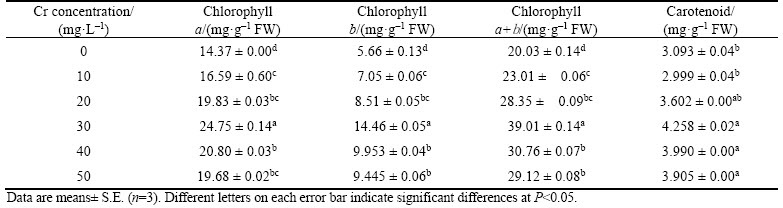
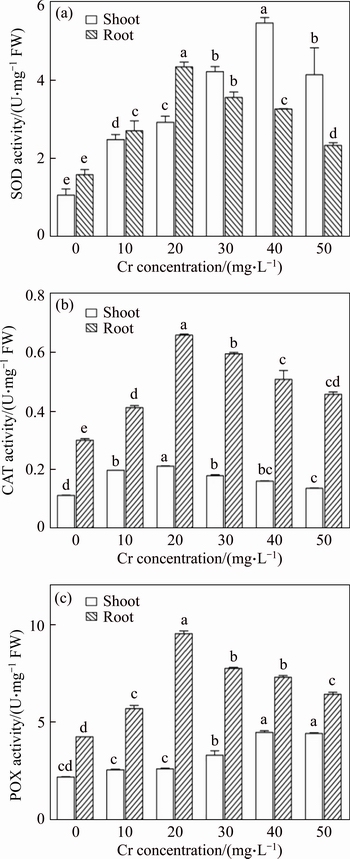
Figure 3 Effects of chromium on SOD (a), CAT (b) and POX (c) activities in shoot and root tissues of vetiver (Data are represented as means ± S.E. (n=3). Means with different letters indicate significant differences at P<0.05)
These results indicated that, Cr induced O2·– generation was higher at 50 mg/L Cr treatment, which may be attributed to decreased level of SOD activity at 50 mg/L Cr concentration. Moreover, the decreased SOD activity significantly increase of H2O2 was observed at 50 mg/L Cr treatment, which indicated enhanced accumulation of H2O2 in vetiver. Similar results were also reported in Pisum sativum [37] under the Cr stress.
CAT activity increased significantly up to the 20 mg/L Cr exposure and then slightly reduced at higher concentrations in both shoot and root (Figure 3(b)). The maximum CAT activity was 189.2% and 218.9% for shoots and roots, respectively, at 20 mg/L Cr exposure. This result indicated CAT actively involved in rapid removal of H2O2 produced as a result of SOD activity. However, CAT activity decreased at higher Cr concentrations, which might be due to the over production and substantial accumulation of H2O2 in vetiver. PANDEY et al [37] have observed the increased CAT activity in Brassica juncea and Pisum sativum under the Cr exposure.
In shoot tissues, POX activity was significantly increased with increasing Cr concentration. The maximum percent of POX activity observed was 203.9% in shoot tissues at 40 mg/L Cr treatment. In root, POX activity increased up to the 20 mg/L Cr concentration but decreased with a further increase in the Cr dose (Figure 3(c)). These results clearly indicated that vetiver plants have an effective detoxification mechanism to overcome the Cr stress-induced cell membrane damage. Increased POX activity has been reported in Salvinia natans [38]. The increased SOD, CAT, and POX activities in vetiver may be concluded as indirect evident for this developed tolerance mechanism against Cr induced toxicity. ELSTNER [39] claimed that, the changes of SOD, CAT and POX activities were due to Cr toxicity induced accumulation of superoxide and H2O2.
To confirm the above antioxidant enzyme activities, isoenzyme patterns of SOD, CAT and POX from shoots were determined by native PAGE analysis. Isoenzyme banding patterns can reflect the molecular level expression of respective antioxidative defence genes [40]. In the present study, expression of two isoforms (SOD I & II) was observed from both control and Cr treatments (Figures 4(a) and (d)). The intensity of SOD isoform II gradually increased up to the 40 mg/L Cr concentration and then reduced at the 50 mg/L of Cr dose. As shown in Figures 4(b) and (e), one CAT isoform was observed on the gel, and the CAT band intensity reduced with increasing Cr doses in the growth medium. For POX, four isoforms (POX I, II, III and IV) were observed, and the POX II isoform was not evident at higher concentrations of Cr exposure. The band intensity of other POX isoforms increased with increasing Cr concentration but then reduced at higher concentrations of Cr treatment (Figures 4(c 1) and (c2)). These results indicated that the increase of POX isoenzymes band intensity could be associated with increased POX activity under Cr exposure at lower doses. SUPARNA PAL et al [41] reported that, the appearance of additional APX and SOD isoenzyme bands are evidences of the plant developed detoxification mechanism to Cr toxicity.
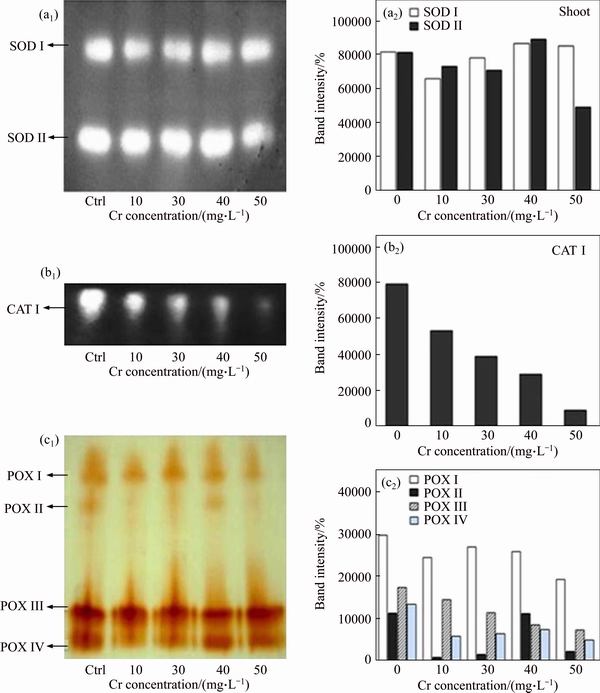
Figure 4 Effects of Cr on isoenzyme patterns of SOD (a1), CAT (b1) and POX (c1) in shoot tissues of vetiver and corresponding band intensities of SOD (a2), CAT (b2) and POX (c2) isozymes expressed as a percentage of control values of isozyme corresponding to various isozymes
3.6 Effect of chromium on protein content and protein profiles
In cell, protein molecules are more important constituents and they were easily affected by the various environmental stress conditions. Therefore, any changes occurring in these molecules may be considered a reliable indicator of oxidative heavy metals stress in plants [42]. In this study, the total soluble protein content was increased with increasing the Cr dose in both shoots and root tissues (Figure 5(a)). The maximum level of protein content was 128.9% and 11.5% for shoot and root tissues at 40 and 20 mg/L Cr concentrations, respectively. However, the protein content slightly declined at higher Cr treatment, indicating that the higher Cr dose can affect the amino acid side chains. MALAR et al [43] reported similar results. Based on these results, the increase of protein content might be a good indicator of the immense potential of vetiver to withstand Cr induced stress conditions. A possible reason for the increase in protein content might be an increase in the number of nuclei, which could lead to an increase in mRNA synthesis [18]. Considerable change in the total soluble protein pattern was observed in shoot tissues of vetiver plants after 16 d of Cr treatment (Figure 5(b)). The SDS–PAGE analysis showed polypeptides that ranged from 29.0 to 97.4 kDa, and the intensity of some polypeptides decreased and disappeared. A total of six polypeptides were observed in control. One of these polypeptides (29.0 kDa) exhibited higher expression than the others in control and Cr-treated. The 80.0 and 43.0 kDa polypeptides had intense staining in the control compared with that in Cr-treated shoot tissues. The polypeptides at 97.4, 66.0 and 43.0 kDa disappeared in the 50 mg/L Cr treatment. These results indicated that some of the genes responsible for those proteins had been completely suppressed and lost the ability to synthesize this protein under Cr stress conditions. By contrast, a newly synthesized polypeptide (50.0 kDa) was observed in the 40 mg/L Cr treatment. The appearance of a new polypeptide might be associated with a Cr stress-induced gene, which could develop the heavy metal tolerance mechanisms in vetiver plant. SINHA et al [36] reported similar observations in Pisita stratiotes.
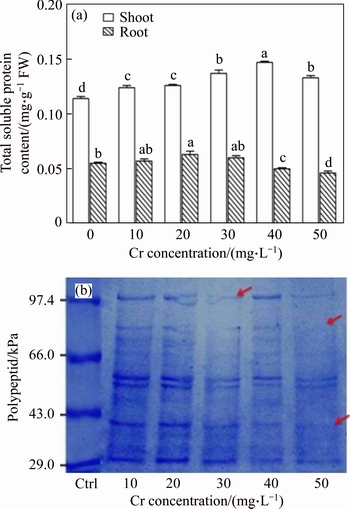
Figure 5 Effects of Cr on total soluble protein content in shoot and root tissues of vetiver (a) and (b) electrophoretic pattern (SDS–PAGE) of protein in leaves of vetiver (Data are represented as means± S.E. (n=3). Means with different letters indicate significant differences at P<0.05. Lane M–molecular marker; Lane Ctrl–Control; Lane 10 to 50 mg/L Cr treatment; Areo marks indicted disappearance of normal bands)
3.7 Effect of chromium on MDA and H2O2 content
In the present study, the level of MDA content in shoots and roots increased gradually with increasing of Cr doses. The maximum MDA content observed was 137% and 205% in shoots and roots, respectively, in the 30 mg/L Cr treatment. This result suggested that membrane damage to the plant cells occurred due to formation and accumulation of excess ROS under Cr stress. Moreover, the MDA content decreased in both shoots and roots at the 50 mg/L Cr exposure (Figure 6(a)). The increased POX activity under Cr-induced stress might be one of the possible defence mechanisms to protect cell membrane lipids by scavenging excess ROS. XIAO et al [44] described that, the Cr (VI) bound with proline and they deposited in root cell wallm, which reduced the MDA content in rice seedlings under the Cr stressed conditions.
The H2O2 level was increased gradually with increasing of Cr dose in the growth medium. The maximum percent of H2O2 was 143.8% and 139.2% in shoots and roots, respectively (Figure 6(b)). The increased SOD, CAT and POX activities in roots and shoots of vetiver could be an indirect and/or direct response to the enhanced level of H2O2 under Cr-induced oxidative stress. Similarly, an increase in H2O2 level under heavy metal stress is reported in rice seedlings [45]. According to DAT et al [46], a low level of H2O2 is considered messenger molecules and it was involved in various processes like acclamatory signalling and improved the tolerance against environmental stresses, whereas a high level may destroy cell membranes and induce programmed cell death in plants. Hence, it is hypothesized that elevation of H2O2 in Cr-treated root and shoot tissues might be due to the induction of increased levels of antioxidative defence enzyme activities in response to metal stress.
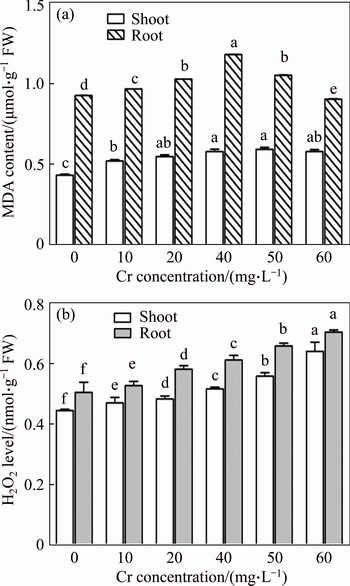
Figure 6 Effect of Cr on MDA (a) and hydrogen peroxide (b) content in shoot and root tissues of vetiver (Data are represented as means± S.E. (n=3). Means with different letters indicate significant differences at P<0.05)
4 Conclusions
Overall, the growth and antioxidative responses in C. zizanioides exposed to Cr toxicity showed the occurrence of an effective detoxification mechanism in this plant. Further, the activities of ROS-scavenging enzymes, including SOD, CAT and POX are considered important protective mechanisms to minimize oxidative damage caused by Cr toxicity. Therefore, increased enzyme activities were considered typical defence mechanisms triggered against Cr-induced toxicity. Increased levels of total soluble protein content suggested that this plant species possess an effective tolerance mechanism to overcome the Cr-induced toxicity by regulating antioxidative defence systems. Additionally, the production of increased levels of hydrogen peroxide and MDA contents was strongly correlated with the occurrence of an effective defence mechanism against Cr-induced toxicity in vetiver plants.
References
[1] CHEN T L, WISE S S, KRAUS S, SHAFFIEY F, LEVINE K, THOMPSON D W, ROMANO T, HARA T, WISE J P. Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts [J]. Environmental and Molecular Mutagenesis, 2009, 50: 387–393. DOI: 10.1002/em.2047.
[2] SHANKER A K, CERVANTES C, LOZATAVERAC H, AVUDAINAYAGAM S. Chromium toxicity in plants [J]. Environment International, 2005, 31: 739–753.
[3] DUBE B K, TEWARI K, CHATTERJEE J, CHATTERJEE C. Excess chromium alters uptake and translocation of certain nutrients in Citrullus [J]. Chemosphere, 2003, 53: 1147–1153. DOI: 10.1016/S0045-6535(03)00570-8.
[4] WANG Jun, CHENG Qing-yu, XUE Sheng-guo, RAJENDRAN M, WU Chuan, LIAO Jia-xin. Pollution characteristics of surface runoff under different restoration types in manganese tailing wasteland. [J] Environmental Science and Pollution Research, 2018, 10: 9998–10005. DOI: 10.1007/s11356-018-1338-2.
[5] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANG Qiong-li, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286. DOI: 10.1016/j.jes.2018.10.010.
[6] ZHANG A, FENG H, YANG G. Unventilated indoor coal-fired stoves in Guizhou province, China: Cellular and genetic damage in villagers exposed to arsenic in food and air [J]. Environmental Health Perspectives, 2007, 115: 653–658. DOI: 10.1289/ehp.9272.
[7] WU Chuan, SHI Li-zheng, XUE Sheng-guo, LI Wai-chin, JIANG Xing-xing, RAJENDRAN M, QIAN Zi-yan. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils [J]. Science of the Total Environment, 2019, 647: 1158–1168. DOI: 10.1016/j.scitotenv.2018.08. 087.
[8] GASPAR T, PENEL C, HAGEGE D, GREPPIN H. Peroxidases in plant growth: Differentiation and development processes [C]// LOBARZEWSKI J, GREPPIN H, PENEL C, GASPAR T. Biochemical, Molecular and Physiological Aspects of Plant Peroxidases. Lublin, 1991: 249–280 Lublin.
[9] XUE Sheng-guo, WU Yu-jun, LI Yi-wei, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, YE Yu-zhen. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288.
[10] ZOU Qi, AN Wen-hui, WU Chuan, LI Wai-chin, FU An-qin, XIAO Rui-yang, CHEN Hui-kang, XUE Sheng-guo. Red mud modified biochar reduces soil arsenic availability and changes bacterial composition [J]. Environmental Chemistry Letters, 2018, 16(3): 615–622. DOI: 10.1007/s10311-017- 0688-1.
[11] XUE Sheng-guo, LI Meng, JIANG Jun, MILLAR G J, LI Chu-xuan, KONG Xiang-feng. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1–10. DOI: 10.1016/j.jes.2018.05.016.
[12] SINGH V, THAKUR L, MONDAL P. Removal of lead and chromium from synthetic wastewater using Vetiveria zizanioides [J]. Clean Soil Air Water, 2015, 43–44: 538–543. DOI: 10.1002/clen.201300578.
[13] MANIKANDAN R, EZHILI N, VENKATACHALAM P. Phosphorus supplementation alleviates the cadmium induced toxicity by modulating oxidative stress mechanisms in vetiver grass (Chrysopogon zizanioides) [J]. Journal of Environmental Engineering, 2016. DOI: 10.1061/(ASCE)EE. 1943-7870.0001112.
[14] LIU W, YANG Y S, LI P J, ZHOU Q X, XIL L J, HAN X P. Risk assessment of cadmium contaminated soil on plant DNA damage using RAPD and physiological indices [J]. Journal of Hazards Materials, 2009, 161: 878–823. DOI: 10.1016/j.jhazmat.2008.04.038.
[15] AL-SAADI S A A M, AL-SAADI W M, AL-WAHEEB A N H, The effect of some heavy metals accumulation on physiological and anatomical characteristic of some Potamogeton L. plant [J]. Journal of Ecology and Environmental Science, 2013, 4: 100–108. DOI: 10.1134/S1995082917020031.
[16] ARNON D I. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta valgaris [J]. Plant Physiology, 1949, 24: 1–15. DOI: 10.1104/pp.24.1.1.
[17] DAS M, MAITI S K. Metal accumulation in five native plants growing on abandoned cu-tailings ponds [J]. Applies Ecology Environmental Research, 2007, 5: 27–35. DOI: 10.15666/aeer/0501_027035.
[18] DAUD K, MEI L, VARIATH M T, ALI S, LI C, RAFIQ M T, ZHU S J. Chromium (VI) uptake and tolerance potential in cotton cultivars: Effect on their root physiology, ultramorphology, and oxidative metabolism [J]. Biomedical Research International, 2014: Article ID 975946. DOI: 10.1155/2014/ 975946.
[19] DHINDSA R A, PLUMB D P, THORPE T A. Leaf senescence: Correlated with increased permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase [J]. Journal of Experimental Botany, 1981, 126: 93–101. DOI: 10.1093/jxb/32.1.93.
[20] AEBI H. Catalase in vitro [J]. Methods in Enzymology, 1984, 105: 121–126. DOI: 10.1016/ S0076-6879(84)05016-3.
[21] CASTILLO F I, PENEL I, GREPPIN H. Peroxidase release induced by ozone in Redum album leaves [J]. Plant Physiology, 1984, 74: 846–851.
[22] BEAUCHAMP C, FRIDOVICH I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels [J]. Analysis Biochemistry, 1971, 44: 276–287. DOI: 10.1016/0003-2697(71)90370-8.
[23] VERMA S, DUBEY R S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants [J]. Plant Science, 2003, 164: 645–655. DOI: 10.1016/s0168-9452(03)00022-0.
[24] ANDERSON D, PRASAD K, STEWART R. Changes in isozyme Profiles of catalase, peroxidase and Glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings [J]. Plant Physiology, 1995, 109: 1247–1257. DOI: 10.2307/4276926.
[25] BRADFORD M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding [J]. Analysis of Biochemistry, 1976, 44: 276–287. DOI: 10.1016/0003- 2697(76)90527-3.
[26] DAVENPORT S B, GALLEGO S M, BENAVIDES M P, TOMAROW M L. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annus L [J]. Plant Cell Growth Regulator, 2003, 40: 81–88. DOI: 10.1023/A: 1023060211546.
[27] MICHAEL P I, KRISHNASWAMY M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings [J]. Environmental and Experimental Botany, 2011, 74: 171–177. DOI: 10.1016/j.envexpbot.2011.05.016.
[28] SERGIEV I, ALXIEVA V, KARANOV E. Effect of sperm one, atrazine and combination between them on some endogenous protective systems and stress markers in plants [J]. Comptes Rendus de Academie Bulgare des Sciences, 1997, 51: 121–124. DOI: 10.1016/j.envexpbot.2011.05.016.
[29] MALLICK S, SINAM G, MISHRA RK, SINHA S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L [J]. Ecotoxicology and Environmental Safety, 2010, 73: 987–995. DOI: 10.1016/j.ecoenv.2010.03.004.
[30] SHAHIDA M, SHAMSHAD S, RAFIQ M, KHALID S, BIBI I, NIAZI NK, DUMAT C, RASHID M I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review [J]. Chemosphere, 2017, 178: 513–533. DOI: 10.1016/j.chemosphere.2017.03.074.
[31] RASHAD M B, MUHAMMAD A S, CHRISTOPHER V, LICOLN Z, GUODONG L, NEIL S M, BALA R, JUAN J M N, FRANCISCO G S. Kinnow mandarin plants grafted on tetraploid roots tocks are more tolerant to Cr-toxicity than those grafted on its diploids one [J]. Environmental and Experimental Botany, 2017, 140: 8–18. DOI: 10.1016/j.envexpbot.2017.05.011.
[32] SEN A K, MONDAL N G, MONDAL S. Studies of uptake and toxic effects of Cr (VI) on Pisita stratioites [J]. Water Science and Technology, 1987, 19: 119–127. DOI: 10.1007/978-90-481-2666-8_53.
[33] SIEGEL H. Metal ion in biological systems [M]. New York: Marcel Dekker, 1973: 2.
[34] SINHA V, PAKSHIRAJAN K, CHATURVEDI R. Chromium (VI) accumulation and tolerance by Tradescantia pallid: Biochemical and antioxidant study [J]. Applied Biochemistry and Biotechnology, 2014, 173: 2297–2306. DOI: 10.1007/ s12010-014-1035-7.
[35] SAMARDAKIEWICZ S, WOZNY A. The distribution of lead in duckweed (Lemna minor L.) root tip [J]. Plant Soil, 2000, 226: 107–111. DOI: 10.1023/a: 1026440730839.
[36] SINHA S, SAXENA R, SINGH S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes [J]. Chemosphere, 2005, 58: 595–604. DOI: 10.1016/j.chemosphere.2004.08. 071.
[37] PANDEY V, DIXIT V, SHYAM R. Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts [J]. Protoplasma, 2009, 236: 85–95. DOI: 10.1007/s00709-009-0061-8.
[38] DHIR B, SHARMILA P, PARDHA SARADHI P, NASIM S A. Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich waste water [J]. Ecotoxicology and Environmental Safety, 2009, 72: 1790–1797. DOI: 10.1016/j.ecoenv.2009.03.015.
[39] ELSTNER E F. Oxygen activation and oxygen toxicity [J]. Annual Review of Plant Physiology, 1983, 33: 73–96. DOI: 10.1146/annurev.pp.33.060182.000445.
[40] MILONE M T, SGHERRI C, CLIJSTERS H, NAVARI IZZO F. Antioxidative responses of wheat treated with realistic concentration of cadmium [J]. Environmental and Experimental Botany, 2003, 50: 265–276. DOI: 10.1016/ S0098-8472(03)00037-6.
[41] SUPARNA P, RITA K. Study of metal resistance potential of the Cd, Cr tolerant Alligator Weed [J]. Journal of Stress Physiology and Biochemistry, 2014, 10: 1244–1261.
[42] PLATA S J, ORTEGA VILLASANTE C, FLORES CACERES M L, ESCOBAR C, DEL CAMPO F F, HERNANDEZ L E. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in Alfalfa [J]. Chemosphere, 2009, 77: 946–954. DOI: 10.1016/j.chemosphere.2009.08.007.
[43] MALAR S, MANIKANDAN R, PAULO J C F, SAHI S V, VENKATACHALAM P. Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: A potential plant for phytoremediation [J]. Ecotoxicology and Environmental Safety, 2014, 108: 249–257. DOI: 10.1016/j.ecoenv. 2014.05.018.
[44] YU Xiao-zhang, LIN Yu-juan, FAN Wei-jia, LU Ming-rui. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr (VI) [J]. International Biodeterioration and Biodegradation, 2017, 123: 106–112. DOI: 10.1016/j.ibiod.2017.06.010.
[45] LIU Chun-hsin, CHAO Yun-yang, KAO Ching-huei. Abscisic acid is an inducer of hydrogen peroxide production in leaves of rice seedlings grown under potassium deficiency [J]. Botany Studies, 2012, 53: 229–237. DOI: 10.1007/s12600-012-0219-3.
[46] DAT J F, VANDENBEELE S, VRANOVA E, van MONTAGU M, INZE D, van BREUSEGEM F. Dual action of the active oxygen species during plant stress responses [J]. Cellular and Molecular Life Science, 2000, 57: 779–795. DOI: 10.1007/s000180050041.
(Edited by YANG Hua)
中文导读
铬解毒机制诱导香根草的生长和抗氧化反应
摘要:本文研究了铬(Cr)对香根草(Chrysopogon zizanioides)生长以及抗氧化防御酶活性的影响,探讨其对Cr的解毒机制。在50 mg/L Cr处理条件下,植物地上部分和地下部分的生长量分别降低了36.8%和45.0%;Cr在植物地下部分的积累量(9807 μg/g干重)大于地上部分的积累量(8730 μg/g干重)。随着Cr添加量的增加,光合色素和丙二醛含量增加,在30 mg/L Cr处理条件下含量达到最高,之后,随着Cr处理浓度的增加略有下降;同时,抗氧化防御酶(超氧化物歧化酶、过氧化氢酶和过氧化物酶)的活性显著提高,在较高浓度Cr处理下略有下降。同工酶结果显示在高浓度Cr处理下,超氧化物歧化酶、过氧化氢酶和过氧化物酶的条带强度降低。目前研究结果表明,高浓度Cr导致活性氧过量产生,从而增加了对香根草的氧化损伤,而光合色素、丙二醛、抗氧化酶含量的增加表明香根草对Cr有潜在的耐性机制。结果表明,香根草具有对Cr的解毒机制,并可以积累高浓度的Cr。本文为更深入地研究根草对Cr的解毒机制提供了依据。
关键词:铬;Chrysopogon zizanioides;解毒机制;抗氧化酶;植物修复
Foundation item: Project(41771512) supported by the National Natural Science Foundation of China; Project(2018RS3004) supported by Hunan Science &Technology Innovation Program, China
Received date: 2018-10-25; Accepted date: 2018-12-12
Corresponding author: Venkatachalam PERUMAL, PhD, Professor; E-mail: pvenkat67@yahoo.com; WU Chuan, PhD, Professor; E-mail: wuchuan@ csu.edu.cn; ORCID: 0000-0002- 5259-3130

