
Reaction process of monazite and bastnaesite mixed rare earth minerals calcined by CaO-NaCl-CaCl2
WU Wen-yuan(吴文远), BIAN Xue(边 雪),
WU Zhi-ying(吴志颖), SUN Shu-chen(孙树臣), TU Gan-feng(涂赣峰)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 13 October 2006; accepted 4 June 2007
Abstract: The decomposition reactions of monazite and bastnaesite mixed rare earth minerals calcined by CaO-NaCl-CaCl2 were studied by means of TG-DTA and XRD. The results show that the process of the minerals decomposed by CaO involves two steps. The first step occurs in the temperature range of 425-540 ℃, and the main reactions are bastnaesite decomposition, i.e. REOF reacts with CaO to produce RE2O3 and CaF2, and Ce2O3 is oxidized to CeO2. During this step, CaCO3 is formed at about 500 ℃. The second step takes place in the temperature range of 610-700 ℃, and the reactions are monazite decomposition into RE2O3, Ca5F(PO4)3 and Ca3(PO4)2 by CaO and CaF2. In this process, the decomposition ability is improved because CaO from CaCO3 decomposing has high chemical activity. In calcining process, the new formed Ca5F(PO4)3 restrains fluorine that can escape in form of gaseous compound. The decomposition ratio of the mixed rare earth minerals reaches 90.8% at 700 ℃.
Key words: mixed rare earth concentrate; decomposition reaction; CaO; NaCl; CaCl2
1 Introduction
The minerals mixed with monazite and bastnaesite containing phosphorus and fluorine are important rare earth resources, and they account for 40% of the world’s important ore of rare earth industry minerals. And these minerals are difficult to decompose because of existence of monazite. At present, the technology of sulfuric acid calcining is mainly applied to industrial production. The important problem of the technique is that the calcined off-gases of SO2 and HF are difficult to recycle by economical and feasible method. If this method is not applied correctly, the environment will be polluted.
The compounds of alkali metal such as NaOH, MgO, and CaO can decompose rare earth minerals containing monazite [1-2]. And it is regarded that this decomposition process does not produce off-gas by many researchers. ZHANG and LINCOLN[3] studied the decomposition process of monazite in the presence of CaO and CaCl2 by mechanical ball milling. The experiments were carried out in Ar atmosphere. The results showed that monazite could effectively decompose, and the decomposition products were chlorine oxygen compound, ThO2 and Ca5F(PO4)3.
HIKICHI et al [4] studied the chemical reaction process of SiO2, Al2O3, CaO with rare earth phosphate. The results showed that rare earth phosphate reacted with CaO at 700 ℃. But WU et al [5-8] found that the reaction of CaO with rare earth phosphate was solid reaction, and the decomposition ratio was very low. In the presence of NaCl, the reaction can be carried out effectively. When the calcined temperature was above 800 ℃, the decomposition ratio reached 78%. In the calcining process, Ca5F(PO4)3 formed. Fluorine existed as solid materials, so the gas production of fluorine was restrained. The main component of calcined off-gas was CO2, and it could be expelled by simple method. The decomposition of CaO-NaCl requires high calcining temperature, and energy consumption was high, so this method was hardly used in industry. In the present work, the molten salt of NaCl-CaCl2 with low melting point was used to reduce the decomposing temperature to meet the industrial requirement.
2 Experimental
2.1 Experimental materials
Monazite and bastnaesite mixed rare earth minerals were supplied by the Concentration Plant of Baotou Steel-Industry. The granularity of the mineral is less than 74 μm, and the chemical composition is listed in Table 1. All reagents in the experiment were in analytically pure grade.
Table 1 Composition of mixed rare earth concentrate (mass fraction, %)

2.2 Experimental process
The calcination experiments were carried out in a box-shaped heating furnace, and the process was as follows. First, the mixed minerals, CaO and NaCl-CaCl2 were mixed homogeneously in the mortar according to the scheme of regression-orthogonal designing. Secondly, the mixed materials were put in a crucible and calcined for 1 h in the furnace. BT119 controlled temperature instrument was used to control the temperature with the error of ±0.2 ℃.
The decomposition ratio was determined by analyzing the calcinated products. Its principle was that Ce in the mixed rare earth concentrate exists in Ce3+, and Ce was oxidized to Ce4+ during the decomposition of CePO4 and CeFCO3. The decomposition ratio(%)=m(Ce4+)/ [m(Ce3+)+m(Ce4+)]. XRD analysis was carried out on a D/max 2400 diffractometer analyzer operated with the Cu Kα radiation. TG/DTA experiment was performed on an SDTQ600 thermal analyzer, with a heating speed of 10℃/min and a temperature range from room- temperature to 1 000 ℃.
3 Results and discussion
3.1 DTA-TG analysis of calcined process
In order to lower the decomposition temperature of the mixed minerals by CaO, the mixture of NaCl and CaCl2 with the melting point of 525 ℃ was added as the assistant agent. The DTA-TG cuves of the mixed minerals added with CaO, NaCl and CaCl2 (m(mineral)? m(CaO)?m(NaCl)?m(CaCl2)=1?0.15?0.04?0.06) are shown in Fig.1.

Fig.1 DTA-TG curves of mixed minerals added with CaO- NaCl-CaCl2
There are three endothermic peaks on the DTA curve, and they are in the temperature ranges of 425-510 ℃, 510-560 ℃ and 630-700 ℃, respectively. The TG curve shows that continuous mass loss happens. Compared with the process of bastnaesite decomposition [9-12] and that of the mixed minerals calcined by CaO-NaCl[8], it is indicated that REFCO3 is decomposed with product of CO2, which causes mass loss in the temperature range of 425-560 ℃.
3.2 XRD analysis of calcination products
In order to investigate the decomposition reactions of the calcined process, XRD analysis was perpormed to study the phase constitutions of the products at different calcined temperatures (Fig.2). The calcined temperatures were selected to be 510, 560 and 700 ℃. All samples were calcined in the resistance furnace for 1 h.
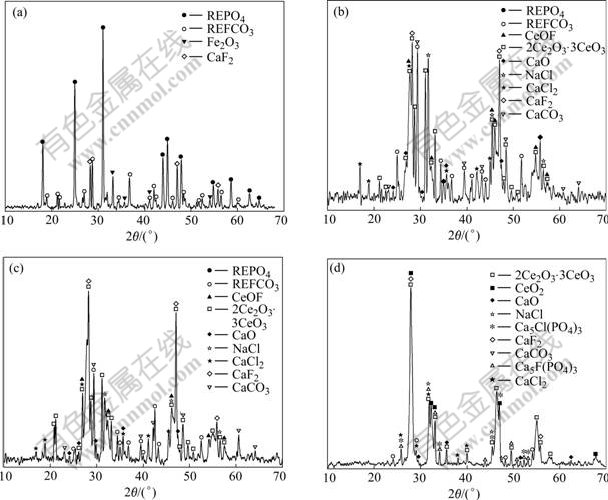
Fig.2 XRD patterns of mixed minerals before and after calcinations: (a) Uncalcined mixed minerals; (b) Products calcined at 510 ℃; (c) Products calcined at 560 ℃; (d) Products calcined at 700 ℃
Fig.2(b) shows that bastnaesite disappears and monazite still exists in the calcined products at 510 ℃, which indicates that bastnaesite is decomposed by CaO into rare earth oxides and CaF2, but monazite is not decomposed. The strength of CaCO3 peaks is intensified at 560 ℃ (Fig.2(c)). This is probably due to the reaction of bastneasite with CaO.
The XRD pattern of the mixed minerals calcined at 700 ℃ shows that Ca3(PO4)2 and Ca5F(PO4)3 appear, CaCO3 disappears, and the diffraction intensity of monazite is decreased. This shows that monazite reacts with CaO and CaF2 to produce rare earth oxides, Ca3(PO4)2 and Ca5F(PO4)3 in the temperature range of 630-700 ℃.
3.3 Decomposition reactions during calcined process
It is known from the analysis of DTA-TG and XRD that the decomposition process of the mixed minerals by CaO-NaCl-CaCl2 can be divided into two steps. The first step is that bastnaesite is decomposed in the temperature range of 425-560 ℃, and the reactions are as follows:
REFCO3=REOF+CO2 (1)
CaO+REFCO3=REOF+CaCO3 (2)
Ce2O3+1/2O2=2CeO2 (3)
CaO+2REOF=RE2O3+CaF2 (4)
The second step is that monazite is decomposed in the temperature range of 630-700 ℃, and the decomposition reactions are
3CaO+2REPO4=RE2O3+Ca3(PO4)2 (5)
9CaO+CaF2+6REPO4=3RE2O3+2Ca5F(PO4)3 (6)
In this step, CaO takes part in reactions (5) and (6), inducing the decomposition product of CaCO3.
In order to further estimate the possibility of calcined reaction, the thermodynamic principle was used to calculate the standard Gibbs free energy change of monazite and bastnaesite calcined by CaO. The calculating process was divided into two steps. The first step was that the integral equations (7) and (8) of standard enthalpy of 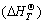 and standard entropy
and standard entropy 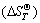 were used to obtain the relation equations between standard generating Gibbs free energy and temperature
were used to obtain the relation equations between standard generating Gibbs free energy and temperature 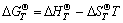 of the products and the reactants, where
of the products and the reactants, where  and
and 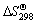 are the standard phase change heat and standard entropy at 298 K, respectively, and a, b, c, and d are temperature coefficients of heat capacity. The calculated results are listed in Table 2.
are the standard phase change heat and standard entropy at 298 K, respectively, and a, b, c, and d are temperature coefficients of heat capacity. The calculated results are listed in Table 2.
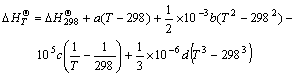 (7)
(7)
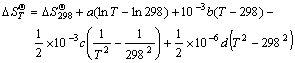 (8)
(8)
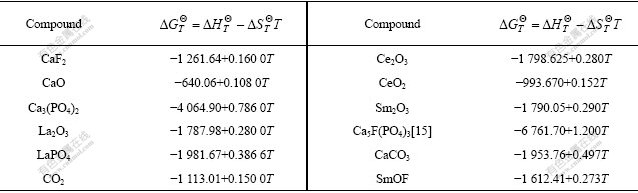
Table 2 Thermodynamic data of reactants and products[13-14]
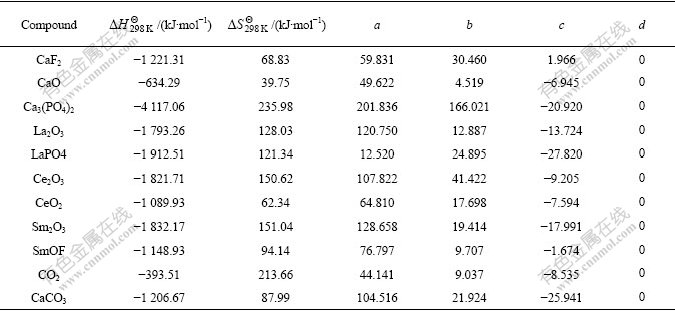
The relations between the standard generating free energy and the temperature of the reactants and the products are listed in Table 3 by using the data in Table 2.
Table 3 Relations between standard generating Gibbs free energy and temperature of reactants and products
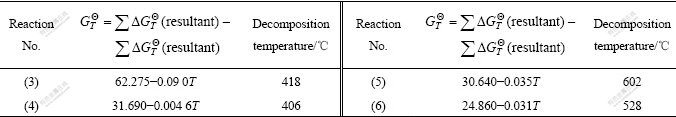
In the second step, the reaction equations of standard Gibbs free energy change and temperature were calculated according to the relation equations in Table 3, and then the decomposition temperature of each reaction was calculated, as listed in Table 4.
Table 4 Relations between standard Gibbs free energy change and temperature and starting decomposing temperature of every decomposition reaction
Because the thermodynamic data about REFCO3 are not available, its decomposition temperature is supposed in the temperature range of 425-510 ℃. If 470 ℃ (this temperature is the top point of the first peak in Fig.1) is selected, the standard generating Gibbs free energy of REFCO3 will be estimated as 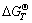 =-2440.7 kJ/mol according to the data in Table 3. So the standard Gibbs free energy change of reaction (2) at 470 ℃ is
=-2440.7 kJ/mol according to the data in Table 3. So the standard Gibbs free energy change of reaction (2) at 470 ℃ is 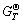 = -39.844 kJ/mol. This result shows that reaction (2) satisfies thermodynamic condition, and it can take place.
= -39.844 kJ/mol. This result shows that reaction (2) satisfies thermodynamic condition, and it can take place.
The calculation results in Table 4 agree well with DTA-TG and XRD ones, therefore, the reactions (1)-(6) in the calined process will occur.
3.4 Effect of NaCl and CaCl2
In order to study the effect of NaCl and CaCl2 on the decomposition process of the mixed minerals by CaO, different mixture ratios of NaCl and CaCl2 were selected, and the calcination experiments were carried out at 700 and 800 ℃. The experiment results are shown in Table 5.
Table 5 Experiment conditions and results
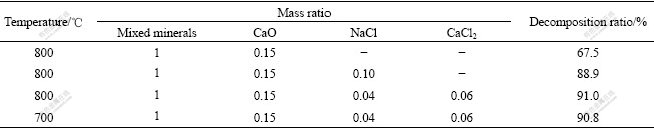
It is known from Table 5 that NaCl and CaCl2 influence the decomposition of the mixed minerals. The reason is that the molten salt offers liquid for the reaction system, which improves the mass transfer of solid reactions. Compared with the melting point of NaCl (800 ℃), that of NaCl-CaCl2 is lower. Therefore, CaO-NaCl-CaCl2 can make the decomposition ratio of mixed minerals at 700 ℃ reach 90.8%.
4 Conclusions
1) The decomposition process of the mixed rare earth minerals containing monazite and bastnaesite by CaO-NaCl-CaCl2 is composed of two steps. The first step is the decomposition process of bastnaesite. In this step, RE2O3 and CaF2 are formed by reacting REOF with CaO, and Ce2O3 is oxidized to CeO2 in the temperature range of 425-540 ℃. In the second step, the monazite is decomposed by CaO and CaF2, and RE2O3, Ca5F(PO4)3 and Ca3(PO4)2 are formed in the temperature range of 610-700 ℃.
2) The free energy change and decomposition temperature of monazite and bastnaesite by CaO are calculated by thermodynamic theory. The feasibility of reactions is testified, and the starting decomposition temperatures are the same as the results of DTA-TG.
3) The different mixture ratios of NaCl and CaCl2 are selected, and the decomposition ratio of mixed minerals calcined by CaO is studied. The results show that NaCl and CaCl2 have high promotion to the decomposing reactions. The decomposition ratio of the mixed minerals by CaO-NaCl-CaCl2 at 700 ℃ can reach 90.8%.
References
[1] MERRITT R R. High temperature methods for processing monazite [J]. Journal of the Less-common Metals, 1990, 166(2): 197-219.
[2] SHI Wen-zhong, ZHU Guo-cai, HUA Jie, XU Sheng-ming, CHI Ruan. Recovery of RE from rare earth concentrate with ammonium chloride roasting [J]. Journal of Henan University: Natural Science, 2002, 22(4): 45-49. (in Chinese)
[3] ZHANG Jin-ping, LINCOLN F J. The decomposition of monazite by mechanical milling with calcium oxide and calcium chloride [J]. Journal of Alloys and Compounds, 1994, 205: 69-75.
[4] HIKICHI Y, HUKUO K, SHIOKAWA J. Solid state reaction between rare earth orthophosphate and oxide [J]. Bull Chemsoc Jpn, 1980, 53: 1455-1456.
[5] WU Wen-yuan, SUN Shun-chen, TU Gan-fen, FEN Hai-dong. Reaction mechanism of synthetic monazite decomposed by CaO [J]. Journal of Northeastern University: Natural Science, 2002, 23(12): 1158-1161.
[6] CHEN Xu-dong, WU Wen-yuan, SUN Shu-chen, HU Guang-yong, TU Gan-feng. Study of roasting decomposition of mixed rare earth concentrate in CaO-NaCl [J]. Chinese Rare Earths, 2004, 25(1): 32-35. (in Chinese)
[7] WU Wen-yuan, CHEN Xu-dong, CHEN Jie, SUN Shu-chen, TU Gan-feng. Study of roasting decomposition of monazite in CaO-NaCl [J]. Chinese Rare Earths, 2004, 25(2): 16-19. (in Chinese)
[8] WU Wen-yuan, HU Guang-yong, SUN Shu-chen, CHEN Xu-dong, TU Gan-feng. Decomposition reaction of mixed rare earth concentrate and roasted with CaO and NaCl [J]. Journal of Rare Earth, 2004, 22(2): 210-214. (in Chinese)
[9] TU Gan-feng, ZHANG Shi-rong, REN Cun-zhi, XING Peng-fei, ZHANG Cheng-xiang. Thermal decomposition reaction kinetics model of powdered bastnaesite [J]. Journal of Rare Earth, 2000, 18(1): 265-267. (in Chinese)
[10] ZHANG Shi-rong, TU Gan-feng, REN Cun-zhi, ZHANG Cheng-xiang, LI Chun-cai. Study on the decomposition behavior of bastnasite [J]. Chinese Journal of Rare Metals, 1998, 22(3): 185-187. (in Chinese)
[11] WU Wen-yuan, CHEN Jie, SUN Shu-chen, TU Gan-feng. Behavior of thermal decomposition of bastnaesite with rare earth nitrate added to it [J]. Journal of Northeastern University: Natural Science, 2004, 25(4): 378-381. (in Chinese)
[12] LIU Shao-gang, CHANG Shu, WEI Xu-jun, XU Xiu-zhi, FENG Fa-lun. Study on the roast reaction of bastnasite [J]. Chinese Rare Earths, 1997, 18(4): 15-18. (in Chinese)
[13] LIANG Ying-jiao, CHE Yin-chang. Thermodynamic data manual of inorganic matter [M]. Shenyang: Northeastern University Press, 1993. (in Chinese)
[14] BARIN I, KNACKE O. Thermochemical properties of inorganic substances [M]. Berlin: Springer-Verlag, 1973.
[15] LIN Chuan-xian. Thermodynamic data manual of minerals and relational compound [M]. Beijing: Science Press, 1985. (in Chinese)
Foundation item: Project(50574031) supported by the National Natural Science Foundation of China; Project(20030145015) supported by the Scientific Research Special Foundation of Doctoral Subject of Chinese University
Corresponding author: WU Wen-yuan; Tel: +86-13840050394; E-mail: wwy030501@yahoo.com.cn
(Edited by CHEN Wei-ping)