Electrochemical performance of LiFePO4/(C+Fe2P) composite cathode material synthesized by sol-gel method
来源期刊:中南大学学报(英文版)2011年第4期
论文作者:陈权启 李小栓 王建明
文章页码:978 - 984
Key words:LiFePO4/(C+Fe2P) composite; sol-gel; sphere-like morphology; electrochemical performance
Abstract:
A LiFePO4/(C+Fe2P) composite cathode material was prepared by a sol-gel method using Fe(NO3)3×9H2O, LiAc·H2O, NH4H2PO4 and citric acid as raw materials, and the physical properties and electrochemical performance of the composite cathode material were investigated by X-ray diffractometry (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and electrochemical tests. The Fe2P content, morphology and electrochemical performance of LiFePO4/(C+Fe2P) composite depend on the calcination temperature. The optimized LiFePO4/(C+Fe2P) composite is prepared at 650 °C and the optimized composite exhibits sphere-like morphology with porous structure and Fe2P content of about 3.2% (mass fraction). The discharge capacity of the optimized LiFePO4/(C+Fe2P) at 0.1C is 156 and 161 mA·h/g at 25 and 55 °C, respectively, and the corresponding capacity retentions are 96% after 30 cycles; while the capacity at 1C is 142 and 149 mA?h/g at 25 and 55 °C, respectively, and the capacity still remains 135 and 142 mA·h/g after 30 cycles at 25 and 55 °C, respectively.
J. Cent. South Univ. Technol. (2011) 18: 978-984
DOI: 10.1007/s11771-011-0790-7![]()
CHEN Quan-qi(陈权启)1, 2, LI Xiao-shuan(李小栓)1, WANG Jian-ming(王建明)2
1. Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education,
School of Chemistry, Xiangtan University, Xiangtan 411105, China;
2. Department of Chemistry, Zhejiang University, Hangzhou 310027, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: A LiFePO4/(C+Fe2P) composite cathode material was prepared by a sol-gel method using Fe(NO3)3×9H2O, LiAc?H2O, NH4H2PO4 and citric acid as raw materials, and the physical properties and electrochemical performance of the composite cathode material were investigated by X-ray diffractometry (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and electrochemical tests. The Fe2P content, morphology and electrochemical performance of LiFePO4/(C+Fe2P) composite depend on the calcination temperature. The optimized LiFePO4/(C+Fe2P) composite is prepared at 650 °C and the optimized composite exhibits sphere-like morphology with porous structure and Fe2P content of about 3.2% (mass fraction). The discharge capacity of the optimized LiFePO4/(C+Fe2P) at 0.1C is 156 and 161 mA?h/g at 25 and 55 °C, respectively, and the corresponding capacity retentions are 96% after 30 cycles; while the capacity at 1C is 142 and 149 mA?h/g at 25 and 55 °C, respectively, and the capacity still remains 135 and 142 mA?h/g after 30 cycles at 25 and 55 °C, respectively.
Key words: LiFePO4/(C+Fe2P) composite; sol-gel; sphere-like morphology; electrochemical performance
1 Introduction
Lithiated transition metal polyanion cathode materials based on ![]() have been attractive for their stable frameworks, relative high operating voltage, good lithium ion transport and large theoretical capacity. Presently, the studied polyanion cathode materials mainly involve LiFePO4 [1-7], Li3V2(PO4)3 [8-10] and LiVPO4F [11-13], while LiFePO4 has been considered as the most promising cathode material because of its relatively low cost and environmental friendliness. However, the intrinsically low electronic conductivity, on the order of 10-9 S/cm, of pristine LiFePO4 degrades its electrochemical performance and impedes its applications. Previous reports have demonstrated that it is an effective method to improve the electrochemical performance of LiFePO4 by enhancing its electronic conductivity. The solutions to improve the electronic conductivity of cathode materials frequently include adding conductive materials [5, 14-15], coating particles with a thin carbon layer [16] and doping heteroatoms [3, 17-19]. Fe2P, a metal phosphide with high electron conductivity of about 10-1 S/cm usually generated by carbothermal reduction at high temperatures, has been considered as an effective conductive additive to significantly improve the electrochemical performance of LiFePO4. More recently, LiFePO4/(C+Fe2P) composite with improved electrochemical performance was prepared by solid-state method using Fe(NO3)3×9H2O [14] and Fe2O3 [15] as iron sources at high temperature. Whereas, the solid-state reaction needs high calcination temperature and results in larger particles of LiFePO4, unfavourable for improving the electrochemical performance of LiFePO4.
have been attractive for their stable frameworks, relative high operating voltage, good lithium ion transport and large theoretical capacity. Presently, the studied polyanion cathode materials mainly involve LiFePO4 [1-7], Li3V2(PO4)3 [8-10] and LiVPO4F [11-13], while LiFePO4 has been considered as the most promising cathode material because of its relatively low cost and environmental friendliness. However, the intrinsically low electronic conductivity, on the order of 10-9 S/cm, of pristine LiFePO4 degrades its electrochemical performance and impedes its applications. Previous reports have demonstrated that it is an effective method to improve the electrochemical performance of LiFePO4 by enhancing its electronic conductivity. The solutions to improve the electronic conductivity of cathode materials frequently include adding conductive materials [5, 14-15], coating particles with a thin carbon layer [16] and doping heteroatoms [3, 17-19]. Fe2P, a metal phosphide with high electron conductivity of about 10-1 S/cm usually generated by carbothermal reduction at high temperatures, has been considered as an effective conductive additive to significantly improve the electrochemical performance of LiFePO4. More recently, LiFePO4/(C+Fe2P) composite with improved electrochemical performance was prepared by solid-state method using Fe(NO3)3×9H2O [14] and Fe2O3 [15] as iron sources at high temperature. Whereas, the solid-state reaction needs high calcination temperature and results in larger particles of LiFePO4, unfavourable for improving the electrochemical performance of LiFePO4.
In this work, sphere-like LiFePO4/(C+Fe2P) was prepared at relatively lower calcination temperature by a sol-gel method using cheap Fe(NO3)3×9H2O, LiAc?H2O, NH4H2PO4 and citric acid as raw materials, and the effects of calcination temperatures on the content of Fe2P and the physical and electrochemical performance of LiFePO4/(C+Fe2P) composite cathode material were investigated.
2 Experimental
Stoichiometric amount of cheap Fe(NO3)3?9H2O, LiAc?H2O, NH4H2PO4 and certain amount of citric acid were dissolved in de-ionized water solution, and then the mixed solution was stirred vigorously at 80 °C in thermostatic bath for 10 h to get the resulted gel. The obtained gel was dried in a vacuum oven at 80 °C for 12 h, and then the precursor was pelletized and heated at 300 °C in a tubular furnace with flowing argon gas for 8 h to allow NH3 and H2O to evolve. After slow cooling to the room temperature, the product was ground and pelletized again, and heated to 600-850 °C at a ramp rate of 2 °C/min under a stream of argon gas for 8 h, then slowly cooled to room temperature to obtain the LiFePO4/(C+Fe2P) composite product.
During the synthesis of LiFePO4/(C+Fe2P) composite material, the citric acid as a chelating agent for iron facilitates the formation of the homogenous precursor gel; moreover, its decomposition at higher temperatures in an inert atmosphere may provide well dispersed carbon which was mainly used as the selective reduction agent for Fe(III) and conductive additive.
Thermogravimetric (TG) analysis and differential scanning calorimetry (DSC) were carried out using a NETZSCHSTA 409 PG/PC thermal analyzer for heating from room temperature to 950 °C at a rate of 10 °C/min in N2 atmosphere. The crystallinity and structure of the samples were determined using a D/Max III X-ray diffractometer (XRD) with Cu Kα radiation (λ=1.541 8 ?). The carbon content of samples was analyzed by a carbon-sulfur analyzer (Mlti EA2000). The surface morphology of the samples was observed using a SIRION-100 (FEI) scanning electron microscope (SEM). The microstructure was examined using a JEM 1010 transmission electron microscope (TEM).
The electrochemical tests of the samples were carried out using two-electrode or three-electrode cells. In all cells, lithium metal served as the counter and reference electrodes, Celguard2300 was used as the separator, and the electrolyte was 1 mol/L LiPF6 solution in a mixture of ethylene carbonate/dimethyl carbonate (1:1 in volume). The cathode was prepared by casting a slurry of the prepared sample, acetylene black and polyvinylidene fluoride (PVDF) in a mass ratio of 80:10:10 on an aluminium foil current collector. After dried at 120 °C in a vacuum oven for 24 h, the resulting electrodes with an active material loading of about 6 mg/cm2 were transferred to an Ar-filled glove box to assemble testing cells. Galvanostatic charge-discharge measurements were carried out in two-electrode cells using a PCBT-143-320 battery programme-control test system (Lixing, Wuhan, China) in the voltage range of 2.5-4.5 V vs Li+/Li at room temperature. Cyclic voltammetry (CV) was conducted in three-electrode cells at a scanning rate of 0.05 mV/s on a PARSTAT 2273 electrochemistry work station.
3 Result and discussion
Figure 1 shows the TG-DSC curves of the dried precursor gel. On the DSC curve near 109.3 °C, there is an obvious endothermic peak, associated with the mass loss on the TG curve, which is related to the fast dehydration of precursor gel. There are three exothermic peaks near 222.9, 489.3, and 556.7 °C, possibly associated with the evolution of NH3 and H2O, decomposition of organic compound and formation of LiFePO4, respectively. When the calcination temperature is higher than 600 °C, TG curve shows that the mass almost remains constant, implying that the formation of LiFePO4 phase begins at this temperature. Based on the above analysis, the calcination temperature for the dried precursor gel is adjusted in the range of 600-850 °C.

Fig.1 TG-DSC curves of dried gel in nitrogen atmosphere
The XRD patterns of samples synthesized at different temperatures are presented in Fig.2. It can be seen that the XRD patterns of all samples except the sample calcined at 600 °C comprise the phases of LiFePO4 and Fe2P, in agreement with the olivine-type LiFePO4 (PDF 83-2092) and Fe2P (PDF 51-0943), respectively. There exists only olivine-type LiFePO4 (PDF 83-2092) in the XRD pattern of sample calcined at 600 °C. The existence of Fe2P with higher electronic conductivity can enhance the electrochemical performance of LiFePO4 [15]. A quantitative analysis of Fe2P is performed by the direct comparison method for the integrated intensity of reflection of LiFePO4 and Fe2P. Quantitative analysis for a particular substance was possible because the intensities of the diffraction lines ascribed to one phase of the mixture depend on the proportion of that phase in the sample [15]. The mass ratio of Fe2P to LiFePO4 is approximately calculated by Jade 5.0, an X-ray diffraction data analysis software system. The carbon content of samples determined using a carbon-sulfur analyser (Mlti EA2000) and the Fe2P contents are listed in Table 1. It is clear that the carbon content decreases with increasing the calcination temperature and the Fe2P content increases with increasing the calcination temperature, suggesting that higher calcination temperature favors the formation of conductive Fe2P. It is found that adequate amount of Fe2P can significantly improve the conductivity of LiFePO4 cathode material, resulting in better electrochemical performance of LiFePO4 [14-15].
Typical SEM images for the samples prepared at 650, 750 and 850 °C are shown in Fig.3. It can be seen that the sample prepared at 650 °C has smaller particle size than the samples prepared at 750 and 850 °C and the surface of the sample calcined at 650 °C is rougher and porous, while the surfaces of samples calcined at 750 and 850 °C are smooth. The porous structure is in favor of penetration of electrolyte and the smaller particle size is helpful for the reduction of lithium ion diffusion pathway in LiFePO4, suggesting that the composite sample prepared at 650 °C may have better electrochemical performance. In order to further understand the microstructure of composite samples prepared at 650, 750 and 850 °C, the microstructures were observed with TEM and the images are presented in Fig.4. Figure 4(c) demonstrates that LiFePO4 and Fe2P phases can be discerned by SAD (selected area diffraction), and Fe2P occurs as solid sphere with a diameter of about 100 nm, similar to the previous report [15]. Figure 4(b) reveals that a few smaller Fe2P particles locate on the surface of the composite synthesized at 750 °C. However, it is difficult to discriminate Fe2P from LiFePO4 in Fig.4(a), and the composite prepared at 650 °C exhibits sphere- like morphology with porous structure, implying that the sphere-like Fe2P particles may be dispersively embedded into the composite and result in better electric contact of particles. The contact of larger highly conductive Fe2P with LiFePO4 nanocrystal just like Fig.4(c) is not effective to improve the electric conductivity of composite. Compared with Figs.4(b) and 4(c), porous structure and dispersive Fe2P could significantly improve the conductivity of the LiFePO4/(C+Fe2P) composite synthesized at 650 °C, resulting in better electrochemical performance.
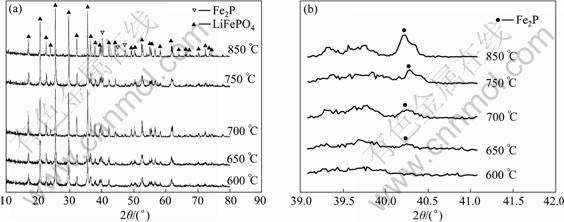
Fig.2 XRD patterns of samples synthesized at different temperatures (a) and magnified XRD patterns of samples synthesized at different temperatures in 2θ range of 39°-41° (b)
Table1 Lattice parameters of LiFePO4, carbon content and Fe2P content of composites prepared at different temperatures
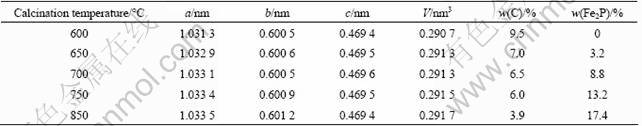
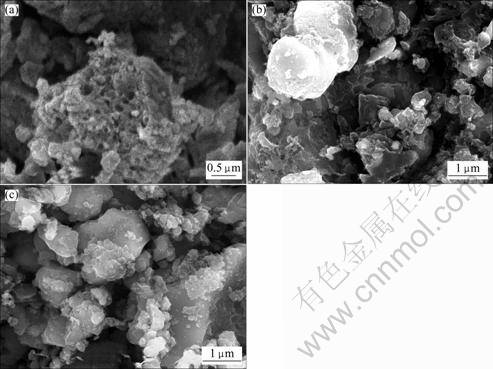
Fig.3 SEM images of samples synthesized at 650 °C (a), 750 °C (b) and 850 °C (c)
Figure 5 illustrates the initial charge-discharge profiles of LiFePO4/(C+Fe2P) composite cathode materials prepared at different calcination temperatures at 0.1C (17 mA/g) in the voltage range of 2.5-4.5 V vs Li+/Li. The capacity of the cathode is calculated based on the mass of LiFePO4 active material. It can be seen that the discharge capacity of the LiFePO4/(C+Fe2P) composites increases with the calcination temperature increasing from 600 to 650 °C, then decreases with the calcination temperature increasing from 700 to 850 °C. The possible reason for this change tendency of discharge capacity is that lower temperature is unfavourable for the growth of LiFePO4 crystal and the formation of highly conductive Fe2P and carbon, while higher sintering temperature facilitates the formation of larger LiFePO4 particles and the larger amount of non-electroactive Fe2P, resulting in the poorer electrochemical performance. The LiFePO4/(C+Fe2P) composite synthesized at 650 °C exhibits the highest discharge capacity of 156 mA?h/g, close to the theoretical capacity of LiFePO4 (170 mA?h/g), higher than that of the LiFePO4/(C+Fe2P) reported by XU et al [14] and KIM et al [15].
Fig.4 TEM images of samples synthesized at 650 °C (a), 750 °C (b) and 850 °C (c) and SAD patterns of Fe2P (d) and LiFePO4 (e)
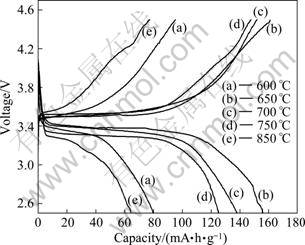
Fig.5 Initial charge-discharge profiles of samples synthesized at different temperatures at 0.1C
The cyclic voltammograms of the LiFePO4/(C+Fe2P) composites prepared at 650, 750 and 850 °C are summarized in Fig.6. All composites exhibit a couple of redox peaks, corresponding to the characteristics of electrochemical lithium insertion/extraction reactions of LiFePO4 [1]. The voltage differences of redox peaks for LiFePO4/(C+Fe2P) composite prepared at 650, 750 and 850 °C are 0.14, 0.22 and 0.3 V, respectively, suggesting that the composite synthesized at 650 °C has better reversibility, resulting in better electrochemical performance. The cycle performances of the LiFePO4/(C+Fe2P) composites synthesized at different temperatures shown in Fig.7 reveal that the composite prepared at 650 °C exhibits the highest discharge capacity of 156 mA?h/g at a rate of 0.1C and a capacity retention rate of 96% after 30 cycles.
In order to investigate the effects of charge/ discharge current rates and environmental temperature on the electrochemical performance of LiFePO4/(C+Fe2P) composite, the composite synthesized at 650 °C was galvanostatically charged/discharged at different rates at 25 and 55 °C, respectively, and the initial charge- discharge profiles and cycle performances of composite cathode are presented in Fig.8 and Fig.9, respectively. Figure 8 shows that the discharge capacity at 25 °C decreases from 156 mA?h/g (0.1C) to 142 mA?h/g (1C) and the capacity at 55 °C declines from 161 mA?h/g (0.1C) to 149 mA?h/g (1C). The reduction of capacity with increasing current rates is resulted from the larger polarization at higher current rate, and the increase of capacity with increasing environmental temperature may be attributed to the fact that elevated temperature can effectively improve the diffusion of lithium-ion in LiFePO4/(C+Fe2P) composite and results in better electrochemical performance.
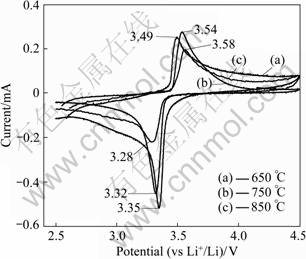
Fig.6 Cyclic voltammograms of LiFePO4/(C+Fe2P) composites synthesized at different temperatures at scanning rate of 0.05 mV/s
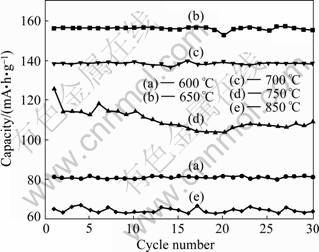
Fig.7 Cycle performances of LiFePO4/(C+Fe2P) composites synthesized at different temperatures at rate of 0.1C
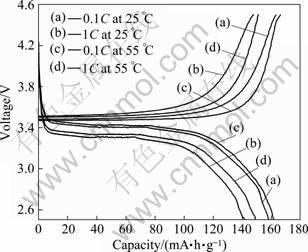
Fig.8 Initial charge-discharge profiles of LiFePO4/(C+ Fe2P) at different rates at 25 and 55 °C

Fig.9 Cycle performances of LiFePO4/(C+ Fe2P) at different rates at 25 and 55 °C
The cycle performances presented in Fig.9 reveal that the LiFePO4/(C+Fe2P) composite has better cycle performance at different environmental temperatures. The capacity retention rates of cathode composite are 96% (0.1C, 25 °C), 96% (0.1C, 55 °C), 95% (1C, 25 °C) and 95% (1C, 55 °C) after 30 cycles, respectively. It is noted that the LiFePO4/(C+Fe2P) composite still remains the capacity at 1C of 135 and 142 mA?h/g after 30 cycles at 25 and 55 °C, respectively, higher than that of the previously reported LiFePO4/(C+Fe2P) [14-15]. From the above results, it can be concluded that the sol-gel method is more effective than the solid-state method to prepare LiFePO4/(C+Fe2P) with better electrochemical performance.
4 Conclusions
1) LiFePO4/(C+Fe2P) composite cathode material can be prepared by a sol-gel method using cheap Fe(NO3)3×9H2O as the iron source and citric acid as the chelating agent and carbon source.
2) The Fe2P content of the LiFePO4/(C+Fe2P) composite increases with the calcination temperature increasing from 600 to 850°C, and the morphology of the LiFePO4/(C+Fe2P) composite changes with the increase of calcination temperature. The porous sphere-like morphology and about 3.2% of non-active Fe2P with high conductivity can significantly improve the electrochemical performance of the composite, which leads to the LiFePO4/(C+Fe2P) composite synthesized at 650 °C to exhibit better electrochemical performance than that of other composites prepared at the other temperatures.
3) Higher environmental temperature favors the improvement of the electrochemical performance of the LiFePO4/(C+Fe2P) composite. At 55 °C, the LiFePO4/ (C+Fe2P) composite delivers capacities of 161 and 148 mA?h/g at 0.1C and 1C, respectively; while at 25 °C, it exhibits capacities of 156 and 142 mA?h/g at 0.1C and 1C, respectively. The LiFePO4/(C+Fe2P) composite shows excellent cycle performances at 25 and 55 °C, and its capacity retention rate is not less than 95% after 30 cycles at the current rates of 0.1C and 1C.
References
[1] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. Journal of the Electrochemical Society, 1997, 144(4): 1188-1194.
[2] BELHAROUAK I, JOHNSON C, AMINE K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4 [J]. Electrochemistry Communications, 2005, 7(10): 983-988.
[3] ZHANG Bao, LI Xin-hai, LUO Wen-bin, WANG Zhi-xing. Electrochemical properties of LiFe1-xMgxPO4 for cathode materials of lithium ion batteries [J]. Journal of Central South University: Science and Technology, 2006, 37(6): 1094-1097. (in Chinese)
[4] GABERSCEK M, DOMINKO R, JAMNIK J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes [J]. Electrochemistry Communications, 2007, 9(12): 2278-2283.
[5] MI C H, CAO Y X, ZHANG X G, ZHAO X B, LI H L. Synthesis and characterization of LiFePO4/(Ag+C) composite cathodes with nano-carbon webs [J]. Powder Technology, 2008, 181(3): 301-306.
[6] CHANG Zhao-Rong, L? Hao-Jie, TANG Hong-Wei, LI Hua-Ji, YUAN Xiao-Zi, WANG Hai-jiang. Synthesis and characterization of high-density LiFePO4/C composites as cathode materials for lithium-ion batteries [J]. Electrochimica Acta, 2009, 54(20): 4595-4599.
[7] DING Y, JIANG Y, XU F, YIN J, REN H, ZHUO Q, LONG Z, ZHANG P. Preparation of nano-structured LiFePO4/graphene composites by co-precipitation method [J]. Electrochemistry Communications, 2010, 12(1): 10-13.
[8] HUANG H, YIN S C, KERR T, TAYLOR N, NAZAR L F. Nanostructured composites: A high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries [J]. Advanced Materials, 2002, 14(21): 1525-1528.
[9] YIN S C, GRONDEY H, STROBEL P, ANNE M, NAZAR L F. Electrochemical property: Structure relationships in monoclinic Li3-yV2(PO4)3 [J]. Journal of the American Chemical Society, 2003, 125(34): 10402-10411.
[10] CHEN Quan-qi, WANG Jian-ming, TANG Zhen, HE Wei-chun, SHAO Hai-bo, ZHANG Jian-qing. Electrochemical performance of the carbon coated Li3V2(PO4)3 cathode material synthesized by a sol-gel method [J]. Electrochimica Acta, 2007, 52(16): 5251-5257.
[11] ZHONG Shen-kui, YIN Zhou-lan, WANG Zhi-xing, CHEN Qi-yuan. Synthesis and characterization of triclinic structural LiVPO4F as possible 4.2 V cathode materials for lithium ion batteries [J]. Journal of Central South University of Technology, 2007, 14(3): 340-343.
[12] BARKER J, SAIDI M Y, SWOYER J L. Electrochemical insertion properties of the novel lithium vanadium fluorophosphate, LiVPO4F [J]. Journal of the Electrochemical Society, 2003, 150(10): A1394-A1398.
[13] BARKER J, GOVER R K B, BURNS P, BRYAN A, SAIDI M Y, SWOYERB J L. Performance evaluation of lithium vanadium fluorophosphate in lithium metal and lithium-ion cells [J]. Journal of the Electrochemical Society, 2005, 152 (9): A1776-A1779.
[14] XU Yan-bin, LU Ying-jun, YAN Lan, YANG Zheng-yin, YANG Ru-dong. Synthesis and effect of forming Fe2P phase on the physics and electrochemical properties of LiFePO4/C materials [J]. Journal of Power Sources, 2006, 160(1): 570-576.
[15] KIM C W, PARK J S, LEE K S. Effect of Fe2P on the electron conductivity and electrochemical performance of LiFePO4 synthesized by mechanical alloying using Fe3+ raw material [J]. Journal of Power Sources, 2006, 163(1): 144-150.
[16] WANG L N, ZHAN X C, ZHANG Z G, ZHANG K L. A soft chemistry synthesis routine for LiFePO4-C using a novel carbon source [J]. Journal of Alloys and Compounds, 2008, 456(1/2): 461-465.
[17] MICHAEL T. An expected conductor [J]. Nature Materials, 2008, 1(2): 81-82.
[18] SURENDRA K M, JUDITH G, ORTAL H, ELLA Z, THIERRY D, JAMES H M, IVAN E, ANDREAS K, BORIS M, AURBACH D. LiMn0.8Fe0.2PO4: An advanced cathode material for rechargeable lithium batteries [J]. Angewandte Chemie International Edition, 2009, 48: 8559-8563.
[19] YIN Xiong-ge, HUANG Ke-long, LIU Su-qin, WANG Hai-yan, WANG Hao. Preparation and characterization of Na-doped LiFePO4/C composites as cathode materials for lithium-ion batteries [J]. Journal of Power Sources, 2010, 195(13): 4308-4312.
(Edited by PENG Chao-qun)
Foundation item: Project(50571091) supported by the National Natural Science Foundation of China; Project(09C947) supported by the Scientific Research Fund of Hunan Provincial Education Department, China
Received date: 2010-06-12; Accepted date: 2010-10-28
Corresponding author: CHEN Quan-qi, Associate Professor, PhD; Tel: +86-731-58292206; E-mail: qqchen@xtu.edu.cn, quanqi.chen@yahoo.com

