Influence of cobalt content on microstructure and corrosion performance of extruded Sn-9Zn solder alloys
来源期刊:中南大学学报(英文版)2020年第3期
论文作者:蔡志勇 江守利 钟剑锋 李佳原 王日初 彭超群
文章页码:711 - 720
Key words:Sn-9Zn solder alloy; cobalt addition; extrusion; microstructure; corrosion performance
Abstract: The Pb-free solders have attracted a great deal of attention recently due to the environmental concerns. The present work focuses on the effect of cobalt content (0, 0.5 and 3.0) on the microstructural characteristics, melting point and corrosion performance of extruded Sn-9Zn solder alloys. The results reveal that the Zn-rich precipitates with spherical or needle-like shape in the Sn-9Zn-xCo alloys are refined remarkably by forming the γ-Co5Zn21 and Co2Sn2Zn Co-contained intermetallic compounds, though the melting point and eutectic reaction temperature decrease slightly. It is suggested that the corrosion property of the extruded Sn-9Zn-xCo alloys is improved significantly by adding the cobalt element, while the content should be controlled reasonably. Combining the corrosion morphology, the influence of cobalt content on the corrosion behavior of the Sn-9Zn-xCo alloys is analyzed in terms of the refined microstructure and the enhanced passive film stability.
Cite this article as: JIANG Shou-li, ZHONG Jian-feng, LI Jia-yuan, WANG Ri-chu, PENG Chao-qun, CAI Zhi-yong. Influence of cobalt content on microstructure and corrosion performance of extruded Sn-9Zn solder alloys [J]. Journal of Central South University, 2020, 27(3): 711-720. DOI: https://doi.org/10.1007/s11771-020-4325-y.

J. Cent. South Univ. (2020) 27: 711-720
DOI: https://doi.org/10.1007/s11771-020-4325-y

JIANG Shou-li(江守利)1, ZHONG Jian-feng(钟剑锋)1, LI Jia-yuan(李佳原)2,
WANG Ri-chu(王日初)2, 3, PENG Chao-qun(彭超群)2, CAI Zhi-yong(蔡志勇)2, 3
1. Nanjing Research Institute of Electronics and Technology, Nanjing 210039, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
3. Key Laboratory of Electronic Packaging and Advanced Functional Materials, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The Pb-free solders have attracted a great deal of attention recently due to the environmental concerns. The present work focuses on the effect of cobalt content (0, 0.5 and 3.0) on the microstructural characteristics, melting point and corrosion performance of extruded Sn-9Zn solder alloys. The results reveal that the Zn-rich precipitates with spherical or needle-like shape in the Sn-9Zn-xCo alloys are refined remarkably by forming the γ-Co5Zn21 and Co2Sn2Zn Co-contained intermetallic compounds, though the melting point and eutectic reaction temperature decrease slightly. It is suggested that the corrosion property of the extruded Sn-9Zn-xCo alloys is improved significantly by adding the cobalt element, while the content should be controlled reasonably. Combining the corrosion morphology, the influence of cobalt content on the corrosion behavior of the Sn-9Zn-xCo alloys is analyzed in terms of the refined microstructure and the enhanced passive film stability.
Key words: Sn-9Zn solder alloy; cobalt addition; extrusion; microstructure; corrosion performance
Cite this article as: JIANG Shou-li, ZHONG Jian-feng, LI Jia-yuan, WANG Ri-chu, PENG Chao-qun, CAI Zhi-yong. Influence of cobalt content on microstructure and corrosion performance of extruded Sn-9Zn solder alloys [J]. Journal of Central South University, 2020, 27(3): 711-720. DOI: https://doi.org/10.1007/s11771-020-4325-y.
1 Introduction
The Sn-Zn alloys have attracted wide attentions in the field of Pb-free solders owing to the environment-friendliness, excellent mechanical properties, and cost-effectiveness. Especially, the melting temperature of eutectic alloy with 9 wt.% Zn (198 °C) is close to the eutectic Sn-38.1 wt.% Pb solder alloy (183 °C) [1-4]. Therefore, the preparation of Sn-Zn alloys requires less revision of the present manufacturing routes, which is favorable for industrial manufacturing.
However, the Sn-9Zn alloy exhibits poor corrosion resistance compared with the Sn-Pb alloy. In a humid environment, the Zn-rich phase dissolves preferentially with a fast corrosion rate [5, 6]. The poor corrosion resistance has a great influence on the preservation and application of such alloy. Additionally, the oxidation and inferior wetting properties of the alloy lead to a relatively poor interfacial bonding [7]. Consequently, the application of the Sn-9Zn alloy needs further investigation on relationship among the composition, microstructure, and properties.
Very recently, the available Pb-free solders modified with minor alloy elements have drawn large attentions due to their better corrosion properties [8-14]. LIU et al [15] reported that the Sn-9Zn alloy added with a trace amount of Ti showed significantly refined microstructure. Consequently, the modified alloy was conducive to form a passive film with higher integrity, and then the corrosion resistance was improved. On the other hand, the addition of the alloying elements forms intermetallic compounds (IMCs) and facilitates the formation of a more uniform and compact protection layer on the surface of the Sn-9Zn alloy. The formed layer enhanced the corrosion resistance in the order of Ag
Unstill now, few works regard the cobalt alloying of the Sn-9Zn solder alloys. The present work focuses on the influence of cobalt content on the microstructural characteristics, corrosion resistance, and corrosion morphology of the extruded Sn-9Zn-xCo solder alloys.
2 Experimental procedures
The raw materials Ni, Sn, and Co with purity of 99.99% and Zn with purity of 99.9% were weighed to prepare the Sn-9Zn-xCo (x=0%, 0.5%, and 3.0%) solder alloys. Firstly, the Sn-9Zn-xCo alloys were carefully prepared by induction melting in quartz tube under the protection of argon. The molten alloys were then cast into Cu mold. For the accuracy and convenience of the experiment, the received Sn-9Zn-xCo specimens were homogenized at 180 °C for 30 min and then extruded to strips with the dimensions of 100 mm×10 mm×2 mm. Secondly, the extruded specimens were homogenized at 100 °C for 10 min. Finally, the Sn-9Zn-xCo alloys were cut into 11 mm×11 mm×2 mm squares and mechanically polished and fixed with copper wires and assembled into working electrodes. The test surface was 10 mm×10 mm square. The chemical composition of the extruded Sn-9Zn-xCo samples is measured using inductively coupled plasma optical emission spectrometer (ICP-OES), and the results are listed in Table 1.
Table 1 Chemical composition of extruded Sn-9Zn-xCo solder alloys prepared by direct melting method (mass fraction, %)

The melting point of as-rolled Sn-9Zn-(Co) alloy was measured by NRC STA449C differential scanning calorimeter. The DSC thermal analysis charts were obtained by repeated measurements of different samples at 10 °C/min heating rate in the range of 50-260 °C.
Before the corrosion tests, the surface of the sample was pre-ground by 600#, 800#, 1000# and 1500# sandpaper, and then washed with anhydrous alcohol and deionized water in an ultrasonic cleaner for 3 min. All electrochemical tests were carried out in an air environment at room temperature of 25 °C. The auxiliary electrode was a Pt electrode, the reference electrode was a saturated dry mercury electrode (SCE), and the etching solution was a 0.5 mol/L NaCl solution. The samples were tested before polarization and soaked for 30 min in the solution to obtain stable data. The stable potential of the sample in NaCl solution was 0.997 V. The cathode polarization range of the sample was from -1.5 to 0.2 V and the scan rate was 2 mV/s. The AC impedance test has a disturbance voltage of 10 mV and a frequency range of 0.01-100 kHz. The data of the EI test and the EIS test were fitted using Cview and Zview softwares, respectively. In the immersion experiment, the samples were kept in a 0.5 mol/L NaCl solution at a constant temperature of 25 °C for 10 d.
The electro-probe microanalysis (EPMA, JXA-8230) samples were prepared by mechanical polishing in the direction normal to the soldering interface. Then, the reaction layers located at these interfaces were detected by X-ray diffraction (XRD, D/max 2550) using Cu Kα radiation (λ=0.15405 nm). These samples were etched using 5%HCl+5%H2O2 solution to remove the extra solders and cleaned with acetone in an ultrasonic washer for 5 min. The etched interfacial microstructure and fractured surface of the joints were observed using field emission scanning electron microscopy (FE-SEM, Sirion200) operating at 20 kV. The composition of intermetallic compounds was detected using an energy dispersive spectrometer (EDS) incorporated in the FE-SEM instrument.
3 Results and discussion
3.1 Microstructures and melting characteristics
Figure 1 shows microstructures of the as-extruded Sn-9Zn-xCo solder alloys. It is observed from Figure 1(a) that the alloy has a typical eutectic characteristic composes of large Zn-rich precipitate in black distributed in the β-Sn matrix in gray. However, the Zn-rich dendrites with different dimensions result in a relatively heterogeneous distribution of Sn-Zn eutectic structure. With the addition of cobalt, it is seen that the coarse Zn-rich precipitates transform into spherical and needle-like shapes, as shown in Figure 1(b). When the content of cobalt increases to 3 wt.%, large primary pine-tree crystals turn into spheroidal particles overall, as shown in Figure 1(c). It can be observed from Figure 1(d) and Table 2 that cobalt consumes zinc atoms to form a Co- containing compound, which is identified as γ-Co5Zn21 phase and Co2Sn2Zn phase [20-22], and they disperse uniformly in the β-Sn matrix. The added cobalt element is uniformly dispersed in the alloy, which hinders the aggregation of Zn atoms and generates a coarse primary phase. On the other hand, during the solidification process, the temperature is lowered, the solubility of the cobalt atom is lowered, and it is precipitated from the solder matrix and distributed in the β-Sn matrix. Therefore, the growth and coarsening of Zn-rich phase in the Sn-9Zn alloy is suppressed significantly by adding the cobalt element.
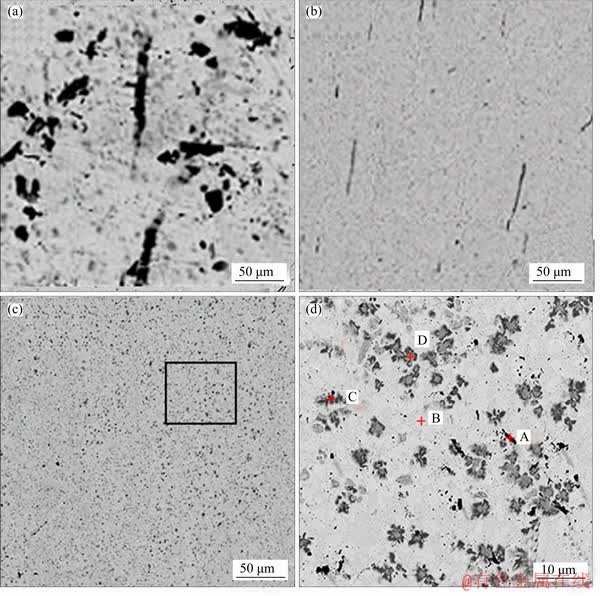
Figure 1 SEM microstructures of as-extruded Sn-9Zn alloy (a), Sn-9Zn-0.5Co alloy (b) and Sn-9Zn-3Co alloy (c, d)
Table 2 SEM-EDS results of as-extruded Sn-9Zn-3Co solder alloy in terms of the positions in Figure 1(d)

The refined microstructure of the extruded Sn-9Zn-xCo alloys is ascribed to the fact that the minor alloying element cobalt with hexagonal closed packed structures could directly affect the solidification process by enhancing the heterogeneous nucleation. During the solidification, cobalt provides a large number of nucleation points for the nucleation of β-Sn matrix and Zn-rich phases simultaneously that refine the microstructure. It can be explained that cobalt atoms hinder the accumulation of active element zinc to form coarse phases as it is often used for metal diffusion barrier to impede the diffusion of atoms [23]. It is known that the Sn-Zn solder with refined Zn-rich precipitates exhibits better corrosion properties than those with coarse secondary phases [22]. Namely, the refined microstructures of the extruded Sn-9Zn-xCo solder alloys are expected to realize better corrosion-resistance properties.
The melting point of as-extruded Sn-9Zn-(Co) alloy shown in Figure 2 was obtained by the DSC thermal analysis diagram. As shown, the melting point of Sn-9Zn alloy is affected limited by the addition of different content of Co. The initial temperature of eutectic reaction decreases slightly with the increase of the addition amount. This indicates that, although the melting point of Co is high, it has little effect on liquidus, solidus temperature and melting temperature range of Sn-9Zn solder. Such effect can be explained as the limited addition of Co.
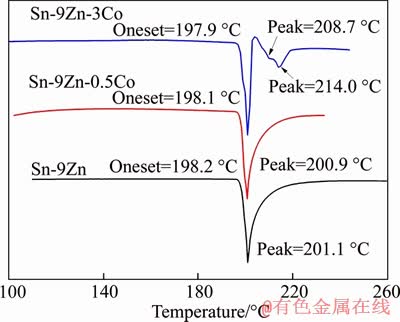
Figure 2 DSC curves of Sn-9Zn-xCo alloy
As shown in Figure 2, the results of heating curve test of Sn-9Zn are close to its theoretical eutectic temperature.Because the equilibrium melting temperature of Sn-9Zn alloy is 198.5 °C [24], the initial eutectic reaction temperature is 198.2 °C, the superheat of Sn-9Zn alloy is 0.3 °C, and the melting interval △T is 1.9 °C. When 0.5% cobalt was added, the heating curve of Sn-9Zn-0.5Co alloy was similar to that of Sn-9Zn alloy. Only one peak curve was observed. The initial eutectic temperature was 198.1 °C, and the melting range was almost unchanged. However, three peaks were observed in the heating curve of Sn-9Zn-3Co. According to the microstructures, Sn-9Zn-3Co alloy undergoes three reactions during heating, and the three reaction temperatures are liquid phase temperature (214.0 °C), univariate reaction temperature (208.7 °C) and eutectic reaction temperature (197.9 °C), which are determined by peak temperature and initial temperature respectively [25].
3.2 Corrosion behavior
3.2.1 Electrochemical impedance spectroscopy (EIS)
The Nyquist and Bode diagrams of the extruded Sn-9Zn-xCo solder alloys are shown in Figure 3. They are the function of the immersion time of the alloys in 0.5 mol/L NaCl solution. It is seen that the Nyquist plot is composed of two concave semicircles. The shape of the Bode spectrum indicates that there is an ideal capacitance deviation in the impedance spectrum with two-time constants over the entire frequency range. The time constant at the first high frequency reveals the dielectric property of the porous reaction layer located at the solution/porous membrane interface. While the low frequency time constant and the second medium are related to the passive reaction layer associated with the charge transfer resistance- blunt film/alloy interface. In addition, it can be seen from the Bode diagram that the maximum value of the two peaks and the corresponding frequency change with the content of cobalt added, and the peak of the Sn-9Zn-3Co is the largest one among them.

Figure 3 Nyquist diagram (a) and Bode diagram (b) of extruded Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution
According to the above-mentioned explanations, the ZIS software was used to fit the measured EIS data of Sn-9Zn-xCo solder alloys. As shown in Figure 4, two equivalent circuits (EC) are obtained, and the constant phase element (CPE) is generally used instead of the pure capacitor. It can better reflect the actual electrochemical behavior. The constant phase element is related to the surface activity, roughness, uniformity, physical adsorption, and electrode porosity of the alloy [23].
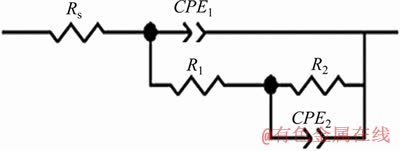
Figure 4 Equivalent circuit of extruded Sn-9Zn-xCo solder alloys in present work
Here, Rs is the solution resistance; R1 and CPE1 are the resistance and capacitance of the porous corrosion product layer, respectively; R2 is the resistance of the passive reaction layer associated with the charge transfer process at the passive film/alloy interface; CPE2 is a double layer capacitor. The results of the EIS spectra of the extruded Sn-9Zn-xCo alloys after immersion in 0.5 mol/L NaCl solution are listed in Table 3. Using the standard deviation X to evaluate the reliability of the fitting results, in all cases it has reached the order of 10-4. It is suggested that the proposed equivalent circuit can evaluate the electrochemical interface behavior quantitatively. In addition, the degree of fitting can be well verified by comparison of the experimental data and the data of fitted curve.
Table 3 EC parameters for Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution
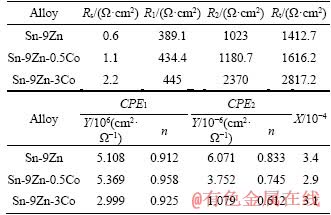
Comparing the results of the three Sn-9Zn-xCo solder alloys, the value of the total resistance increases with the increase of cobalt content. This indicates that the Co-containing alloys have higher corrosion resistance than the cobalt free alloy. It is reported that the total resistance Rt is inversely proportional to the corrosion rate [26-28]. The Rt values of the Sn-9Zn-xCo alloys can be given by Rt=R1+R2+Rs. Rt can reflect the corrosion behavior of the Sn-9Zn-xCo alloys to some extent. The addition of cobalt alloy elements in the Sn-9Zn alloy increases the values of R1 and R2, and the total resistance Rt increases with the addition of cobalt. The Rt value of the Sn-9Zn-3Co alloy is relatively large, approximately 2817.2 Ω·cm2. The Y value also reflects the corrosion resistance to a certain extent, and the increase in the Y value is attributed to an increase in defects (for example, pits) in the passive layer of the passivation film, and accordingly, the thickness of the passivation film is reduced.
3.2.2 Potentiodynamic polarization curves
In the studies of metal passivation, the dynamic method is commonly used to control the potential to measure the polarization curve. Figure 5 shows the measured potentiodynamic polarization curves of the extruded Sn-9Zn-xCo solder alloys. It is seen that the dynamic potential polarization curve is mainly divided into three parts. First, the corrosion potential of the alloys becomes positive and the corrosion current drops monotonically to the lowest point. Second, the corrosion current of the alloys rises rapidly to the highest point. Finally, it falls again to reach a constant value. The three stages are relative to the passivation of the extruded Sn-9Zn-xCo alloys and the growth of passivation film, the passivation film dissolves, and the secondary passivation, respectively. The corrosion resistance of the Co-contained alloys is improved significantly as compared with the cobalt free alloy. The current density of cathode and the anode of polarization curve of the extruded Sn-9Zn-Co alloy is relatively lower, but the corrosion potential is higher. The typical polarization behavior of the extruded Sn-9Zn-xCo alloys can be observed from Figure 4. It can be seen that the working electrode of the alloys is polarized to a more positive potential while the current density remains almost a constant value. Such phenomenon suggests that a passivation film has been formed on the surface of the working electrode. As the potential continues to change, the current density of the alloys increases sharply to about -1.0 V, suggesting that the passivation film begins to dissolve and pitting occurs, and the current density drops again, followed by a platform indicating secondary passivation. The metal anodic polarization process is generally divided into three parts: dissolution, passivation, and self-dissolution (chemical dissolution) of the anode metal. Under a certain external potential, when the electrode potential is more positive than the thermodynamic potential of the anode alloys, the anode alloys dissolve.
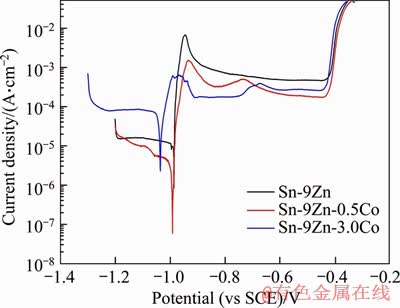
Figure 5 Potentiodynamic polarization curves of extruded Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution
Table 4 illustrates the electrochemical parameters of the polarization of the extruded Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution. Since there is no well-defined experimental anode Tafel region, the corrosion current density (icorr) is calculated by the Tafel extrapolation of the cathodic polarization curve [29]. It is seen from Table 4 that the corrosion current density of the cobalt free alloy is 19.119 μA/cm2. The corrosion current density of the extruded Sn-9Zn-0.5Co alloy is only 1/10 of the cobalt free alloy. Therefore, the corrosion rate of the Sn-Zn-0.5Co solder alloy is remarkably lower than that of the cobalt free alloy. However, the corrosion current density of the extruded Sn-9Zn-3Co alloy is higher than that of the cobalt free alloy. Although the corrosion resistance of the Sn-9Zn-3Co after the second passivation is not as good as that of the Sn-9Zn-0.5Co alloy, it is more resistant to corrosion than the cobalt free alloy. Namely, the addition of cobalt element reduces the corrosion potential of the Sn-9Zn solder. The corrosion potential of the Sn-9Zn-3Co solder is the most negative, about 0.05 V lower than that of the cobalt free, indicating that the cobalt free solder is more susceptible to corrosion than the Sn-9Zn-xCo solder in 0.5 mol/L NaCl solution at room temperature. Summarily, the addition of proper amount of cobalt element leads to the formation of a stable and dense passivation film on the extruded Sn-9Zn-xCo alloys, which is beneficial for reducing the corrosion rate and promotes the corrosion resistance of the alloys.
Table 4 Electrochemical parameters for extruded Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution

The results of potentiodynamic polarization and AC impedance tests show that the extruded Sn-9Zn-xCo solder alloys is less sensitive to pitting than the cobalt free alloy. This phenomenon is attributed to higher impedance values and higher pitting potentials. In other words, the pitting sensitivity of the extruded Sn-9Zn-xCo alloys decreases with increasing the cobalt content, which can be confirmed by the evolution of the Rt value and the pitting potential. The Sn-9Zn-xCo alloy exhibits excellent protection properties and inhibits Cl- transmission to prevent pitting corrosion. The above suggests show that the Sn-9Zn alloy is not easily corroded in 0.5 mol/L NaCl solution at room temperature, and the addition of a proper amount of cobalt is helpful for improving the corrosion resistance to some extent.
By analyzing the microstructure of the extruded Sn-9Zn-xCo alloys, it is found that the addition of an appropriate amount of cobalt alloy element as nucleation point promotes the nucleation of Zn-rich phase at the same cooling rate, hinders the formation of eutectic phase and β-Sn phase in coarse Zn-rich phase. Consequently, the structure of the alloys is more refined, so that the internal defects of the structure are reduced. Therefore, the corrosion resistance of the solder alloys is inevitably improved and the uniformly refined structure provides more nucleation points for forming a passivation film, and then a denser passivation film. However, the addition of 3 wt.% cobalt element causes a large amount of fine γ-Co5Zn21 and Co2Sn2Zn compound to form in the Sn-9Zn alloy, and the rate of passivation is low at the initial stage of polarization. The rate of forming a passivation film is slow, but once the passivation film is formed, which is denser and more stable than that of the cobalt free alloy, the pitting dissolution occurs until the degree of corrosion is less in the process of secondary passivation. Generally, the addition of cobalt is beneficial to optimizing the microstructure of the extruded Sn-9Zn solder alloys, making it more favorable to form a dense and stable passivation film, and the corrosion resistance of the alloys is improved.
3.3 Corrosion morphology
The SEM micrographs of the extruded Sn-9Zn-xCo solder alloys after soaking for 10 d in 0.5 mol/L NaCl solution are shown in Figure 6. As expected, many pits and microcracks are observed in the cobalt free alloy, indicating that the solder without the addition of cobalt element has weaker protective performance. In the process of corrosion, the pits can provide a prioritized route for the transport of the reactants, and then form corrosion products along the pits. However, the cobalt free solder alloy is pitting in the test solution. Compared with the three extruded Sn-Zn-xCo alloys, the surface morphologies of the Sn-9Zn-0.5Co and Sn-9Zn-3Co appear to be more uniform and fewer pits than the cobalt free alloy. From the above analysis, it can be concluded that the extruded Sn-9Zn-xCo solder alloys exhibit better corrosion resistance than the cobalt free alloy. Such result is further confirmed that the EIS result is related to an increase in the Rt value of the alloys.
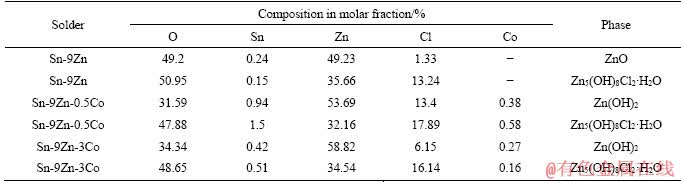
As illustrated in Table 5, EDX analysis shows a large amount of zinc, oxygen and chlorine in the corrosion products of the extruded Sn-9Zn-0.5Co solder alloy. Accordingly, the atomic ratio of zinc, chlorine and oxygen suggests that the corrosion product consists mainly of ZnO, Zn(OH)2 and Zn5(OH)8Cl2·H2O, or the mixture with trace amounts of oxide/chloride. This phenomenon is consistent with the results in Refs. [29, 30].
4 Conclusions
1) The microstructure of the Sn-9Zn solder alloy is refined by adding cobalt, the coarse Zn-rich phase gradually disappears and the shape becomes short needle or even completely converted into fine particles. The γ-Co5Zn21 and Co2Sn2Zn compounds become the main solidified phases that disperse uniformly in the β-Sn matrix. The melting point of Sn-9Zn-3Co alloy is the lowest (197.9 °C), which is 0.4 °C lower than that of the Sn-9Zn alloy.
2) Total resistance Rt of the extruded Sn-9Zn-xCo alloys increases gradually with adding cobalt in the 0.5 mol/L NaCl solution. The Sn-9Zn-3Co alloy has the highest Rt value of 2817.2 Ω·cm2 among them. The corrosion current density decreases first and then increases with increasing the cobalt content, and the corrosion rate of the Sn-Zn-0.5Co alloy is significantly lower than that of the cobalt free alloy.
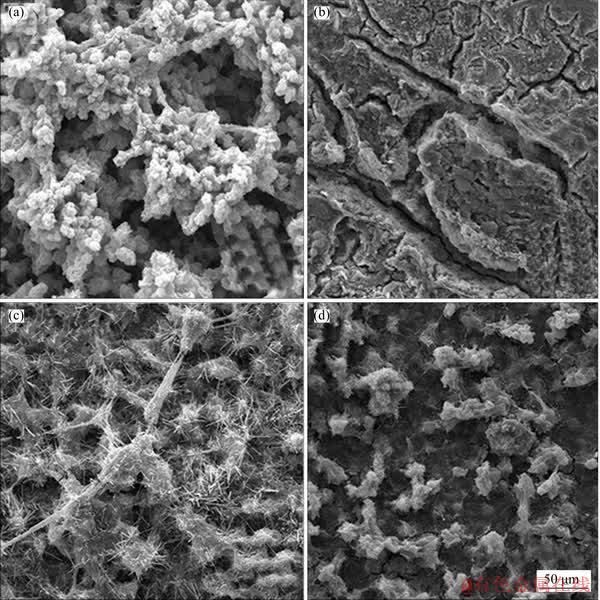
Figure 6 Surface morphologies of extruded Sn-9Zn (a, b), Sn-9Zn-0.5Co (c), and Sn-9Zn-3Co (d) solder alloys immersed in 0.5 mol/L NaCl solution for 10 d
Table 5 EDX compositions of corrosion products of extruded Sn-9Zn-xCo solder alloys in 0.5 mol/L NaCl solution
3) With the addition of cobalt, corrosion resistance of the extruded Sn-9Zn alloys is also improved. The lower corrosion resistance of the cobalt free alloy is ascribed to the existence of coarse Zn-rich phase, which has high defects located at the intergranular boundary between the Zn-rich phase and β-Sn matrix.
References
[1] SUN L, ZHANG L. A review: The wettability and oxidation resistance of Sn-Zn-X lead-free solder joints [C]// International Conference on Power Electronics and Energy Engineering. Hong Kong, 2015. DOI: 10.2991/peee- 15.2015.50.
[2] ZHANG Liang, XUE Song-bai, GAO Li-li, SHENG Zhong, YE Huan, XIAO Zheng-xiang, ZENG Guang, CHEN Yan, YU Sheng-lin. Development of Sn-Zn lead-free solders bearing alloying elements [J]. Journal of Materials Science: Materials in Electronics, 2010, 21(1): 1-15. DOI: 10.1007/s10854-009-0014-1.
[3] CHENG Shun-feng, HUANG Chien-ming, PECHT M. A review of lead-free solders for electronics applications [J]. Microelectronics Reliability, 2017, 75: 77-95. DOI: 10.1016/j.microrel.2017.06.016.
[4] REN Guang, WILDING J, COLLINS M N. Alloying influences on low melt temperature Sn-Zn and Sn-Bi solder alloys for electronic interconnections [J]. Journal of Alloys and Compounds, 2016, 665: 251-260. DOI: 10.1016/j.jallcom. 2016.01.006.
[5] MORANDO C, FORNARO O, GARBELLINI O, PALACIO H. Thermal properties of Sn-based solder alloys [J]. Journal of Materials Science: Materials in Electronics, 2014, 25(8): 3440-3447. DOI: 10.1007/s10854-014-2036-6.
[6] REN Guang, WILDING I J, COLLINS N. Alloying influences on low melt temperature Sn-Zn and Sn-Bi solder alloys for electronic interconnections [J]. Journal of Alloys and Compounds, 2016, 665: 251-260. DOI: 10.1016/ j.jallcom.2016.01.006.
[7] LI De-zhi, CONWAY P P, LIU Chang-qing. Corrosion characterization of tin-lead and lead free solders in 3.5wt.% NaCl solution [J]. Corrosion Science, 2008, 50(4): 995-1004. DOI: 10.1016/j.corsci.2007.11.025.
[8] NAZERI M F M N, MOHANAD A A. Corrosion measurement of Sn-Zn lead-free solders in 6 M KOH solution [J]. Measurement, 2014, 47: 820-826. DOI: 10.1016/ j.measurement.2013.10.002.
[9] WANG Ming-na, WANG Jian-qiu, KE Wei. Effect of microstructure and Ag3Sn intermetallic compounds on corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder [J]. Journal of Materials Science: Materials in Electronics, 2014, 25(12): 5269-5276. DOI: 10.1007/s10854-014-2300-9.
[10] AHMIDO A, SABBAR A, ZOUIHRI H, DAKHSI K, GUEDIRA F, SERGHINI-IDRISSI M, HAJJAJI S. Effect of bismuth and silver on the corrosion behavior of Sn-9Zn alloy in NaCl 3wt.% solution [J]. Materials Science and Engineering B, 2011, 176(13): 1032-1036. DOI: 10.1016/ j.mseb.2011.05.034.
[11] NAZERI M F M, MOHAMAD A A. Corrosion resistance of ternary Sn-9Zn-xIn solder joint in alkaline solution [J]. Journal of Alloys and Compounds, 2016, 661: 516-525. DOI: 10.1016/j.jallcom.2015.11.184.
[12] MOHANTY U S, LIN Kwang-lung. Effect of Al on the electrochemical corrosion behaviour of Pb free Sn-8.5 Zn-0.5Ag-xAl-0.5Ga solder in 3.5% NaCl solution [J]. Applied Surface Science, 2006, 252(16): 5907-5916. DOI: 10.1016/j.apsusc.2005.08.020.
[13] IJAZ M F, ZHUKOVA Y, KONOPATSKE A, DUBINSKIY S, KORBKOVA A, PUSTOV Y, BRAILOVSKI V, PROKOSHIKIN S. Effect of Ta addition on the electrochemical behavior and functional fatigue life of metastable Ti-Zr-Nb based alloy for indwelling implant applications [J]. Journal of Alloys and Compounds, 2018, 748: 51-56. DOI: 10.1016/j.jallcom.2018.03.033.
[14] WANG Ming-na, WANG Jian-qiu, KE Wei. Corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder joints [J]. Microelectronics Reliability, 2017, 73: 69-75. DOI: 10.1016/j.microrel.2017.04.017.
[15] LIU Jian-chun, ZHANG Gong, MA Ju-sheng, KATSUAKI S. Ti addition to enhance corrosion resistance of Sn-Zn solder alloy by tailoring microstructure [J]. Journal of Alloys and Compounds, 2015, 644: 113-118. DOI: 10.1016/j.jallcom.2015.04.168.
[16] LIU Jian-chun, WANG Zheng-hong, XIE Jing-yang, MA Ju-sheng, SHI Qing-yu, ZHANG Gong, SUGANUMA K. Effects of intermetallic-forming element additions on microstructure and corrosion behavior of Sn–Zn solder alloys [J]. Corrosion Science, 2016, 112: 150-159. DOI: 10.1016/j.corsci.2016.07.004.
[17] MENDEZ C M, SCHEIBER V L, ROZICKI R S, KOCIUBCZYK A I, ARES A E. Electrochemical behavior of Sn-Zn alloys with different grain structures in chloride-containing solutions [J]. Arabian Journal of Chemistry, 2018, 11(7): 1084-1096. DOI: 10.1016/ j.arabjc.2016.12.019.
[18] CHO M G, KIM H Y, SEO S K, LEE H M. Enhancement of heterogeneous nucleation of β-Sn phases in Sn-rich solders by adding minor alloying elements with hexagonal closed packed structures [J], Applied Physics Letter, 2009, 95(2): 1530-1325. DOI: 10.1063/1.3177335.
[19] LI Jia-yuan, PENG Jian, WANG Ri-chu, FENG Yan, PENG Chao-qun. Effects of Co addition on shear strength and interfacial microstructure of Sn-Zn-(Co)/Ni joints [J]. Journal of Materials and Science: Materials in Electronics, 2018, 29(23): 19901-19908. DOI: 10.1007/s10854-018- 0120-z.
[20] HUANG Yu-chih, CHEN Sinn-wen. Co alloying and size effects on solidification and interfacial reactions in the Sn-Zn-(Co)/Cu couples [J]. Journal of Materials Research, 2010, 25(12): 2430-2438. DOI: 10.1557/jmr.2010.0314.
[21] WANG Chao-hong, HUANG Sheng-en, LIU Jian-lin. Liquid-state interfacial reactions of Sn-Zn/Co couples at 250 °C [J]. Journal of Electronic Materials, 2012, 41(12): 3259-3265. DOI: 10.1007/s11664-012-2190-7.
[22] WANG Chao-hong, HUANG Sheng-en, HUANG Po-yun. Phase equilibria of the ternary Sn-Zn-Co system at 250 °C and 500 °C [J]. Journal of Electronic Materials, 2015, 44(12): 4907-4919. DOI: 10.1007/s11664-015-4084-y.
[23] GAO F, TAKEMOTO T, NISHIKAWA H. Effects of Co and Ni addition on reactive diffusion between Sn-3.5Ag solder and Cu during soldering and annealing [J]. Materials Science and Engineering A, 2006, 420(1, 2): 39-46. DOI: 10.1016/j.msea.2006.01.032.
[24] HUANG Yu-chih, CHEN Sinn-wen, CHOU Chin-yi, WOJCIECH G. Liquidus projection and thermodynamic modeling of Sn-Zn-Cu ternary system [J]. Journal of Alloys and Compounds, 2009, 477(1, 2): 283-290. DOI: 10.1016/j.jallcom.2008.10.156.
[25] SUGANUMA K, MURATA T, NOGUCHI H, TOYODA Y. Heat resistance of Sn-9Zn solder/Cu interface with or without coating [J]. Journal of Materials Research, 2000, 15(4): 884-891. DOI: 10.1557/jmr.2000.0126.
[26] CHEN Y T, CHAN Y T, CHEN C C. Wettability and interfacial reactions between the molten Sn-3.0wt%Ag-0.5wt%Cu solder (SAC305) and Ni-Co alloys [J]. Journal of Alloys and Compounds, 2010, 507(2): 419-424.
[27] WANG Zheng-hong, CHEN Chuan-tong, LIU Jian-chun, ZHANG Gong, SUGANUMA K. Corrosion mechanism of Zn-30Sn high-temperature, lead-free solder in neutral NaCl solution [J]. Corrosion Science, 2018, 140: 40-50. DOI: 10.1016/j.corsci.2018.06.025.
[28] HEAKAL F E T, FEKRY A M, JIBRIL M A E B. Electrochemical behavior of the Mg alloy AZ91D in borate solutions [J]. Corrosion Science, 2011, 53(4): 1174-1185. DOI: 10.1016/j.corsci.2010.11.040.
[29] LIU Jian-chun, ZHANG Gong, MA Ju-sheng, SUGANUMA K. Ti addition to enhance corrosion resistance of Sn-Zn solder alloy by tailoring microstructure [J]. Journal of Alloys and Compounds, 2015, 644: 113-118. DOI: 10.1016/ j.jallcom.2015.04.168.
[30] LIU Jian-chun, PARK S W, NAGAO S, NOGI M, KOGA H, MA Ju-sheng, ZHANG Gong, SUGANUMA K. The role of Zn precipitates and Cl anions in pitting corrosion of Sn-Zn solder alloys [J]. Corrosion Science, 2015, 92: 263-271. DOI: 10.1016/j.corsci.2014.12.014.
(Edited by FANG Jing-hua)
中文导读
钴含量对挤压Sn-9Zn焊料合金显微组织与腐蚀性能的影响
摘要:环境保护已成为当前发展的重要议题,无铅焊料最近得到广泛的关注。本文主要研究钴含量(0、0.5和3.0)对挤压Sn-9Zn焊料合金的显微组织、熔点和腐蚀性能的影响。结果表明,通过形成γ-Co5Zn21和Co2Sn2Zn金属间化合物,Sn-9Zn-xCo合金中球形或针状富锌析出相得到显著细化,但熔点和共晶反应温度略有降低。通过添加钴元素可明显改善Sn-9Zn-xCo挤压合金的抗腐蚀性能,但应合理控制其含量。结合腐蚀形态,从显微组织细化和钝化膜稳定性增强方面,分析钴含量对Sn-9Zn-xCo合金腐蚀行为的影响。
关键词:Sn-9Zn焊料;钴含量;挤压;显微组织;腐蚀性能
Foundation item: Project(2017YFB0305700) supported by the Ministry of Science and Technology of China; Projects (51490660, 51490664) supported by the National Natural Science Foundation of China; Project(2017YFB0305700) supported by the National Key Research and Development Project of China
Received date: 2019-08-26; Accepted date: 2019-12-26
Corresponding author: CAI Zhi-yong, PhD, Associate Professor; Tel: +86-731-88836638; E-mail: zycaimse@163.com, ORCID: 0000- 0002-4926-8441

