
Solvo-thermal synthesis and characterization of nest-like zinc oxide
LI Yan(李 酽), FENG Hui-yun(凤慧云), ZHANG Nan(张 楠), LIU Chuan-sheng(刘传生)
College of Science, Civil Aviation University of China, Tianjin 300300, China
Received 14 November 2008; accepted 7 April 2009
Abstract: With Zn(NO3)2·6H2O and NaOH as starting materials, a novel nest-like ZnO nanostructure was successfully synthesized by a solvo-thermal method. The products were characterized by X-ray diffraction, photoluminescence and scanning electron microscopy. The growth mechanism of the nest-like ZnO was discussed. The results show that the as-synthesized samples have a wurtzite structure, with a weak UV emission at about 395 nm, a green emission at around 557 nm and a blue-green emission at 453 nm. SEM investigation reveals that the growth route of the nest-like ZnO can be considered involving three stages: nano-sized ZnO sheet can be firstly achieved, then outspreads quickly and increasingly becomes a long fishbone-like strip with many branch sheets, and finally these sheets curl into a nest-like structure.
Key words: ZnO; morphology; solvo-thermal synthesis; growth mechanism; nest-like structure
1 Introduction
Nano-sized ZnO has been recently paid great attention due to its importance in scientific research and potential technological applications[1-3]. Zinc oxide with direct band gap of about 3.37 eV at room temperature is a well known material suitable for generating ultraviolet (UV) light[4]. The exciton binding energy (about 60 meV) of ZnO can ensure an efficient exciton emission at room temperature and low excitation energy[5-6]. Therefore, zinc oxide is widely used in various applications such as photonic devices, transparent conductors, solar cell windows, surface acoustic devices, and gas sensors[7-8]. Because the optical, electrical and magnetic properties of ZnO are markedly influenced by its particle size, morphology, structure, etc[9-11], different solution methods, such as precipitation[12], gas condensation[13], sol-gel method[14], hydrolysis in polyol medium[15], and hydrothermal synthesis[16] have been used to tailor various morphologies of ZnO crystallite, such as nano-rods, nano-wires, nano-belts, and micro- flowers[17-19]. However, the synthesis of ZnO with particular morphology by a simple method under mild conditions still represents a challenge. In this work, a novel nest-like ZnO nanostructure synthesized by a solvo-thermal method was reported. The morphologies, structures and photoluminescence properties of zinc oxide products were characterized with SEM, photoluminescence (PL) and XRD methods.
2 Experimental
2.1 Materials
All of the chemicals including zinc nitrate hexahydrate (Zn(NO3)2·6H2O), sodium hydroxyl (NaOH), sodium chloride (NaCl), ammonia solution (NH3, 25%), absolute alcohol (C2H5OH) and N, N, N-trimethyl-1- hexadecanaminium bromide (CTAB, C19H42BrN) were analytical-grade reagents and purchased from commercial market without further purification.
2.2 Preparation of samples
The ZnO crystalline powder was prepared in the following process. The aqueous solution (0.1 mol/L) of zinc nitrate hydrate and the solution (0.2 mol/L) of sodium hydroxyl were prepared with deionized water, respectively. The sodium hydroxyl solution was slowly added into zinc nitrate solution at room temperature under vigorous stirring, which resulted in the formation of a white suspension. The suspension was then separated with a centrifuge and washed three times with distilled water, and washed with absolute alcohol at last.
The separated powder was dried at 60 ℃ for 24 h in oven to obtain precursor. Subsequently, 3 g precursor materials, 1 g NaCl and 0.01 g CTAB were dispersed into 5 mL ammonia solution and 50 mL absolute alcohol completely, and the mixture was then sealed into a Teflon-lined autoclave with a filling capacity of about 60%. It was maintained at 180 ℃ for 1, 4, 8 and 24 h, respectively. The resulting white precipitate was collected and washed with distilled water and alcohol several times to obtain ZnO crystallites.
2.3 Characterization
The morphology of ZnO particles was observed by 1530VP model field emission scanning electron microscope (SEM) in China National Academy of Nano-technology Engineering. X-ray diffraction (XRD) with Cu Kα radiation (λ=0.154 2 nm) on DX-2000 X-ray diffractometer was used for checking the formation and identification of present compounds in the obtained particles. Photoluminescence spectra of ZnO crystals were measured with a WGY-10 fluorescence spectrophotometer using a Xe lamp (150 mW). The excitation wavelength was 325 nm. The emission spectrum of solid zinc oxide powder samples at room temperature was observed in the wavelength range of 350-600 nm by using a monochrometer and a photomultiplier.
3 Results and discussion
3.1 X-ray diffraction analysis
Fig.1 shows the X-ray diffraction (XRD) patterns of as-obtained ZnO crystallites synthesized solvo-thermally for different time. The obtained ZnO crystals have a wurtzite structure and the diffraction peaks can be well indexed to hexagonal ZnO with lattice parameters of a=0.324 982 nm and c=0.520 661 nm (JCPDS Card No. 36-1451). Although the hydrothermal treatment time is different, the XRD patterns of ZnO samples are similar in shape (in Fig.1). With increasing the hydrothermal treatment time, the diffraction peak intensity of faces (0002),  and
and 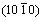 has no obvious change and the peak intensity of polar face
has no obvious change and the peak intensity of polar face 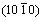 increases accordingly. It is understood that in the beginning of solvo-thermal treatment, the growth rate along direction [0001] is obviously more than other direction and gradually becomes low during the further treatment process until filmy sheets of nano ZnO come into being. Then, these sheets grow quickly along the vertical direction of [0001] and
increases accordingly. It is understood that in the beginning of solvo-thermal treatment, the growth rate along direction [0001] is obviously more than other direction and gradually becomes low during the further treatment process until filmy sheets of nano ZnO come into being. Then, these sheets grow quickly along the vertical direction of [0001] and  , and the plane
, and the plane 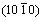 increasingly becomes a long strip (Fig.2(d)) and has a larger area than other planes. As a result of the above phenomena, the diffraction intensity of plane
increasingly becomes a long strip (Fig.2(d)) and has a larger area than other planes. As a result of the above phenomena, the diffraction intensity of plane 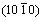 becomes strong with increasing synthesis time (in Fig.1).
becomes strong with increasing synthesis time (in Fig.1).
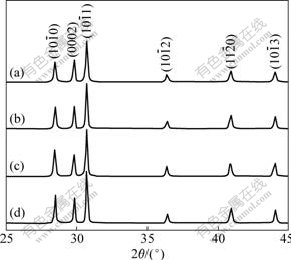
Fig.1 XRD patterns of nest-like ZnO synthesized at 180 ℃ for 1 h (a), 4 h (b), 8 h (c) and 24 h (d)
3.2 Morphology and growth mechanism
SEM images of ZnO samples obtained by solvo-thermal treatment at 180 ℃ for different duration are shown in Fig.2. It can be seen that many ZnO nano-sheets were obtained by solvo-thermal treatment for 1 h in Fig.2(a). The diameter and thickness of these nano-sheets are about 200 nm and 20 nm, respectively. Fig.2(b) shows that not only the diameter of ZnO nano-sheets increased, but also some hexangular stars formed when thermal treatment duration was prolonged to 4 h. We could see clearly that the conglomeration of the ends (plane 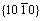 ) of nano-sheets is responsible for the formation of such unique morphology. For this new structure, the direction out of the paper is [0001](in Fig.3), and the polar ZnO nanostructure grows preferentially along [0001] direction (+c axis terminated by zinc) because of the lowest surface energy of (0002) face[20]. Because the growth velocity along the six directions of
) of nano-sheets is responsible for the formation of such unique morphology. For this new structure, the direction out of the paper is [0001](in Fig.3), and the polar ZnO nanostructure grows preferentially along [0001] direction (+c axis terminated by zinc) because of the lowest surface energy of (0002) face[20]. Because the growth velocity along the six directions of 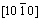 is slower than that along [0001] or
is slower than that along [0001] or 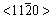 direction, ZnO sheets with a unique structure and geometrical shape of six trigonal sheets can be obtained. With increasing the hydrothermal time to 8 h, ZnO sheet unceasingly grows up and further evolves into curve sheet or coil. Fig.2(d) shows the especial nest-like structure of ZnO synthesized solvo-thermally for 24 h. The magnified view in Fig.2(d) reveals the nest-like structure forming from a long fishbone-like structure in spontaneous coiling behavior caused by ZnO polar. The trunk and branch of the fishbone-like structure are longer strips and short strips prospectively, and the shorter strip integrates with longer strip at angle of about 60?. The wall thickness of the strip is almost uniform, but the length is very different. And many fishbone-like ZnO crystals interlace and congregate together to form a nest-like structure in Fig.2(d).
direction, ZnO sheets with a unique structure and geometrical shape of six trigonal sheets can be obtained. With increasing the hydrothermal time to 8 h, ZnO sheet unceasingly grows up and further evolves into curve sheet or coil. Fig.2(d) shows the especial nest-like structure of ZnO synthesized solvo-thermally for 24 h. The magnified view in Fig.2(d) reveals the nest-like structure forming from a long fishbone-like structure in spontaneous coiling behavior caused by ZnO polar. The trunk and branch of the fishbone-like structure are longer strips and short strips prospectively, and the shorter strip integrates with longer strip at angle of about 60?. The wall thickness of the strip is almost uniform, but the length is very different. And many fishbone-like ZnO crystals interlace and congregate together to form a nest-like structure in Fig.2(d).

Fig.2 SEM images of nest-like ZnO synthesized at 180 ℃ for 1 h (a), 4 h (b), 8 h (c) and 24 h (d)
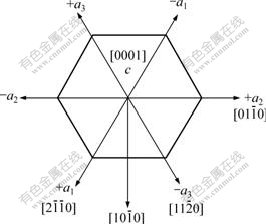
Fig.3 Six á ? orientations of ZnO
? orientations of ZnO
To understand the formation of nest-like morphology, the growth mechanisms in the different stages are studied. ZnO nano-sheets have been synthesized in solution with sodium chloride as structure-directing agent to be adsorbed selectively on ZnO basal planes, and by some negative Cl- ions replacing OH- dangling bond on ZnO positive polar faces 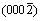 . These replacements hinder splicing growth of Zn(OH)42- along the [0001] and
. These replacements hinder splicing growth of Zn(OH)42- along the [0001] and 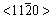 directions, resulting in the formation of zinc oxide nano-sheets. When the growth velocity along c axis is suppressed, the nano-sheets increase in length and width but not in thickness until their final dimensions are reached. When the length of nano-sheet reaches a limit in the process of crystal growth, it curls or integrates to a new structure to reduce the exterior free energy. So, many parallel ramification sheets form in the growth process of the bough sheet. As the ramification sheets occur at both sides of bough sheet, fishbone-like structure can be obtained. According to above analysis, it can be presumed that the growth model of nest-like ZnO can be explained as follows: at first, nano-sized ZnO sheet can be achieved due to the fact that the growth rate along [0001] direction is obviously larger than other directions and gradually becomes low during the further growth. Secondly, ZnO sheet outspreads quickly along the vertical direction of [0001] and
directions, resulting in the formation of zinc oxide nano-sheets. When the growth velocity along c axis is suppressed, the nano-sheets increase in length and width but not in thickness until their final dimensions are reached. When the length of nano-sheet reaches a limit in the process of crystal growth, it curls or integrates to a new structure to reduce the exterior free energy. So, many parallel ramification sheets form in the growth process of the bough sheet. As the ramification sheets occur at both sides of bough sheet, fishbone-like structure can be obtained. According to above analysis, it can be presumed that the growth model of nest-like ZnO can be explained as follows: at first, nano-sized ZnO sheet can be achieved due to the fact that the growth rate along [0001] direction is obviously larger than other directions and gradually becomes low during the further growth. Secondly, ZnO sheet outspreads quickly along the vertical direction of [0001] and 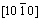 , and ZnO sheet along plane
, and ZnO sheet along plane 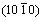 increasingly becomes a long fishbone-like strip with many branch sheets. Finally, these strips curl into a nest-like structure. Meanwhile, the fishbone-like structure tends to interlace together absolutely to reduce the exterior free energy to form a new structure, nest-like morphology.
increasingly becomes a long fishbone-like strip with many branch sheets. Finally, these strips curl into a nest-like structure. Meanwhile, the fishbone-like structure tends to interlace together absolutely to reduce the exterior free energy to form a new structure, nest-like morphology.
3.3 Photoluminescence properties
The photoluminescence from ZnO consists of three emission bands at room temperature, a near-band-edge (UV) emission and two broad, deep-level (visible) emissions. The visible emission is usually considered to be related to various intrinsic defects produced during ZnO preparation and post-treatment. Normally, these defects are located on the surface of the ZnO structure. Fig.4 presents the PL spectra of the as-prepared ZnO crystallites excited by 230 nm UV light from a He-Cd laser at room temperature. For all the samples, a UV emission peak (395 nm) and a green emission peak (557 nm) were observed in the PL spectra. The emission at 395 nm corresponds to the near band-edge emission resulting from the recombination of free excitons; the green emission at 557 nm is commonly referred to the singly ionized oxygen vacancy; and the emission results from the radiative recombination of a photo generated hole with an electron occupying the oxygen vacancy[21]. The visible emission at 453 nm is likely attributed to electron transition, mediated by defect levels in the band gap. With increasing solvo-thermal treatment time, the intensity of the UV peak emission from sample (a) to sample (d) in Fig.4 increased possibly due to the increase of crystallization intensity.

Fig.4 Room temperature photoluminescence spectra of nest-like ZnO synthesized at 180 ℃ for 1 h (a), 4 h (b), 8 h (c) and 24 h (d)
4 Conclusions
1) Nest-like ZnO structure can be successfully synthesized by solvo-thermal method, using C2H5OH as solvent, NaCl and C19H42BrN as the additive, Zn(NO3)2?6H2O and NaOH as the starting materials.
2) The XRD and PL results show that the nest-like ZnO crystal has a wurtzite structure, with UV emission at about 395 nm, a green emission at around 557 nm and a weak emission at about 453 nm.
3) The growth of nest-like ZnO involves three stages: firstly, nano-sized ZnO sheet is achieved; secondly, ZnO sheet outspreads quickly along the vertical directions of [0001] and 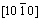 , and evolves into a long fishbone-like strips with many branch sheets; finally, these strips curl into a nest-like structure.
, and evolves into a long fishbone-like strips with many branch sheets; finally, these strips curl into a nest-like structure.
References
[1] WANG X D, SONG J H, WANG Z L. Nanowire and nanobelt arrays of zinc oxide from synthesis to properties and to novel devices [J]. Journal of Materials Chemistry, 2007, 17(8): 711-720.
[2] GYORGY E, SOCOL G, MIHAILESCU I N, SANTISO J, DUCU C, CIUCA S. Pulsed laser deposited zinc oxide thin films for optical gas sensor applications [C]//13th International School on Quantum Electronics: Laser Physics and Applications, Proceedings of the SPIE—The International Society for Optical Engineering. Gourgas: SPIE-Int Soc Opt Eng, 2005(1): 50-54.
[3] BHOSLE V, PRATER J T, FAN Yang, BURK D, FORREST S R, NARAYAN J. Gallium-doped zinc oxide films as transparent electrodes for organic solar cell applications [J]. Journal of Applied Physics, 2007, 102(2): 23501-23505.
[4] MANZOROR U, KIM D K. Synthesis and enhancement of ultraviolet emission by post-thermal treatment of unique zinc oxide comb-shaped dendritic nanostructures [J]. Scripta Materialia, 2006, 54(5): 807-811.
[5] CHEN Z, GAO Q M, RUAN M, SHI J L. Zinc oxide nano-arrays in nano-porous nickel phosphate with a huge blue shift ultraviolet-visible exciton absorption peak [J]. Applied Physics Letters, 2005, 87(9): 093113-093115.
[6] VOROB'EV V A. Investigation of thermally stimulated luminescence of zinc oxide with low-voltage excitation [J]. Journal of Optical Technology, 2005, 72(9): 690-692.
[7] UMAR A, RAHMAN M M, KIM S H, HAHN Y B. Zinc oxide nanonail based chemical sensor for hydrazine detection [J]. Chemical Communications, 2008(2): 166-168.
[8] WAMA R, UTIYAMA M, PLASHNITSA V V, MIURA N. Highly sensitive impedance-based propene sensor using stabilized zirconia and zinc oxide sensing-electrode [J]. Electrochemistry Communications, 2007, 9(12): 2774-2777.
[9] POKROPIVNY V V, KASUMOV M M. Synthesis and growth mechanism of zinc oxide nanostructures in arc discharge [J]. Technical Physics Letters, 2007, 33(1): 44-47.
[10] XIE X X, LI X J, YAN H H. Detonation synthesis of zinc oxide nanometer powders [J]. Materials Letters, 2006, 60(25/26): 3149-3152.
[11] LAZARECK A D, CLOUTIER S G, KUO T F, TAFT B J, KELLEY S O, XU J M. DNA-directed synthesis of zinc oxide nanowires on carbon nanotube tips [J]. Nanotechnology, 2006, 17(10): 2661-2664.
[12] FAN Z Y, LU J G. Zinc oxide nanostructures: Synthesis and properties [J]. Journal of Nanoscience and Nanotechnology, 2005, 5(10): 1561-1573.
[13] WEI J, LEE I K, KOMPCH A, DORFLER U, WINTERER M. Chemical vapor synthesis and characterization of chromium doped zinc oxide nanoparticles [J]. Journal of European Ceramic Society, 2007, 27(13/15): 4333-4337.
[14] GUDKOVA A V, KIENSKAVA K I, NAZAROV V V, KIM V, MUKHTAROVA S E. Synthesis and use of highly dispersed zinc oxide [J]. Russian Journal of Applied Chemistry, 2005, 78(11): 1757-1760.
[15] HUANG X T, LIU J P, DUAN J X, AI H H, TU P H. A low-temperature synthesis of multiwhisker based zinc oxide micron crystals [J]. Materials Letters, 2005, 59(28): 3710-3714.
[16] LU C H, HWANG W J, GODBOLE S V. Microwave-hydrothermal synthesis and photoluminescence characteristics of zinc oxide powders [J]. Journal of Materials Research, 2005, 20(2): 464-471.
[17] SINGH J, TIWARI R S, SRIVASTAXVA O N. Synthesis of zinc oxide nanotetrapods and nanorods by thermal evaporation without catalysis [J]. Journal of Nanoscience and Nanotechnology, 2007, 7(6): 1783-1786.
[18] XU F S, LIU X F, TSE S D, COSANDEY F, KEAR B H. Flame synthesis of zinc oxide nanowires [J]. Chemical Physics Letters, 2007, 449(1/3): 175-181.
[19] SHANG T M, SUN J H, ZHOU Q F, GUAN M Y. Controlled synthesis of various morphologies of nanostructured zinc oxide: flower, nanoplate, and urchin [J]. Crystal Research and Technology, 2007, 42(10): 1002-1006.
[20] HU J Q, LI Q, WONG N B, LEE C S, LEE S T. Synthesis of uniform hexagonal prismatic ZnO whiskers [J]. Chemistry of Materials, 2002, 14(3): 1216-1219.
[21] DAI Y, ZHANG Y, BAI Y Y, WANG Z L. Bicrystalline zinc oxide nanowires [J]. Chemical Physics Letters, 2003, 375(1/2): 96-101.
Foundation item: Projects(09JCYBJC04200, 05yfJMTC 12900) supported by the Natural Science Foundation of Tianjin, China
Corresponding author: LI Yan; Tel: +86-22-24092519; Fax: +86-22-24092514; E-mail: liyan01898@163.com
DOI: 10.1016/S1003-6326(09)60107-2
(Edited by YANG Hua)