Chemical, physical, thermal, textural and mineralogical studies of natural iron ores from Odisha and Chhattisgarh regions, India
来源期刊:中南大学学报(英文版)2018年第12期
论文作者:Anand Babu KOTTA Swapan Kumar KARAK Mithilesh KUMAR
文章页码:2857 - 2870
Key words:iron ore; chemical analysis; mineralogy analysis; thermal analysis; textural analysis
Abstract: The chemical, physical, thermal and texture properties of iron ores from different regions of Odisha and Chhattisgarh regions, India, have been investigated to understand the compositional variations of Fe, Al2O3, SiO2, S and P. They were analyzed for its susceptibility to meet the industrial requirements, for various iron manufacture techniques. Chemical analysis indicated that the majority of the iron ores is rich in hematite (> 90 wt%), poor in gangue (<4.09 wt% SiO2 and <3.8 wt% Al2O3) and deleterious elements (P<0.065 wt% and S<0.016 wt%) in all these iron ores found to be low. XRD peaks reviled that the gangue is in the form of kaolinite and quartz, and same was observed in Fourier transform infrared (FTIR) spectroscopy in the range of 914 to 1034 cm–1. The iron ores were found to have excellent physical properties exemplify with tumbler index (82 wt%–91 wt%), abrasion index (1.27wt%–4.87 wt%) and shatter index (0.87wt%–1.64 wt%). FTIR and thermal analysis were performed to assimilate the analysis interpolations. It was found that these iron ores exhibit three endothermic reactions, which are dehydration below 447 K with mass loss of 0.13 wt% to 1.7 wt%, dehydroxylation at 525–609 K with mass loss of 1.09 wt%–4.49 wt% and decomposition of aluminosilicates at 597–850 K with mass loss of 0.13 wt%–1.15 wt%. From this study, we can conclude that due to its excellent physico-chemical characteristics, these iron ores are suitable for BF and DRI operations.
Cite this article as: Anand Babu Kotta, Swapan Kumar Karak, Mithilesh Kumar. Chemical, physical, thermal, textural and mineralogical studies of natural iron ores from Odisha and Chhattisgarh regions, India [J]. Journal of Central South University, 2018, 25(12): 2857–2870. DOI: https://doi.org/10.1007/s11771-018-3958-6.

J. Cent. South Univ. (2018) 25: 2857-2870
DOI: https://doi.org/10.1007/s11771-018-3958-6

Anand Babu KOTTA, Swapan Kumar KARAK, Mithilesh KUMAR
Department of Metallurgical and Materials Engineering, National Institute of Technology,Rourkela 769008, Odisha, India
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: The chemical, physical, thermal and texture properties of iron ores from different regions of Odisha and Chhattisgarh regions, India, have been investigated to understand the compositional variations of Fe, Al2O3, SiO2, S and P. They were analyzed for its susceptibility to meet the industrial requirements, for various iron manufacture techniques. Chemical analysis indicated that the majority of the iron ores is rich in hematite (> 90 wt%), poor in gangue (<4.09 wt% SiO2 and <3.8 wt% Al2O3) and deleterious elements (P<0.065 wt% and S<0.016 wt%) in all these iron ores found to be low. XRD peaks reviled that the gangue is in the form of kaolinite and quartz, and same was observed in Fourier transform infrared (FTIR) spectroscopy in the range of 914 to 1034 cm–1. The iron ores were found to have excellent physical properties exemplify with tumbler index (82 wt%–91 wt%), abrasion index (1.27wt%–4.87 wt%) and shatter index (0.87wt%–1.64 wt%). FTIR and thermal analysis were performed to assimilate the analysis interpolations. It was found that these iron ores exhibit three endothermic reactions, which are dehydration below 447 K with mass loss of 0.13 wt% to 1.7 wt%, dehydroxylation at 525–609 K with mass loss of 1.09 wt%–4.49 wt% and decomposition of aluminosilicates at 597–850 K with mass loss of 0.13 wt%–1.15 wt%. From this study, we can conclude that due to its excellent physico-chemical characteristics, these iron ores are suitable for BF and DRI operations.
Key words: iron ore; chemical analysis; mineralogy analysis; thermal analysis; textural analysis
Cite this article as: Anand Babu Kotta, Swapan Kumar Karak, Mithilesh Kumar. Chemical, physical, thermal, textural and mineralogical studies of natural iron ores from Odisha and Chhattisgarh regions, India [J]. Journal of Central South University, 2018, 25(12): 2857–2870. DOI: https://doi.org/10.1007/s11771-018-3958-6.
1 Introduction
The reserves of iron ores are estimated to be 170 billion tons in the world, it contains 81 billion tons of iron content, which is 47.64% (geological survey in 2014). India is the fifth largest iron ore reserve (8100 Mt) and produces 0.1 billion tons of iron, which is 7.06% of world production. The contribution of exports is 14.426 Mt, which is 1.08% of total exports [1]. In India, major deposits of iron ores are available in the states of Odisha (33%), Jharkhand (28%), Chhattisgarh (19%), Karnataka (11%) and Goa (5%) [2]. The quality and quantity of the iron and steel production mainly depends on the chemical, physical, thermal and metallurgical properties of the raw materials [3–7]. Chemical compositions of the iron ore determine the material behavior during the reduction process. The presence of alumina (Al2O3) in high quantity in the ore increases the viscosity which results in deterioration of productivity by 4% and an increase in flux consumption by 30 kg/t during the reduction process, which in turn requires a high coke rate of 2.2% [8–10]. The cold strength of the iron ore provides the material behavior during the handling and feeding into the furnace. Tumbler index gives the sign of susceptibility of the raw material to break due to abrasion during transportation, handling and loading into the blast furnace. Shatter index measures sensitivity to failure by impact loading, unloading and loading into the furnace [11, 12].
Many authors found that the textural properties of the iron ores are likely to be an important factor in the iron making process. The surface area of the material is most important factor in the reduction process and it is directly proportional to the rate of reduction. True specific area of the materials cannot be calculated by geometrical calculations due to its irregular shape and internal porosity, it is measured in atomic level by the adsorption of inert gas method [13, 14]. The chemical composition is not sufficient to study the behaviour of iron ores during sintering and reduction process. Physicochemical and chemical reactions are very important to understand the thermal behaviour of the iron ores. Thermogravimetric (TG) analysis provides the thermo physical behaviour (mass loss or gain) of material with increasing temperature or isothermally as a function of time in an inert atmosphere. It can be helpful to improve the efficiency of the reduction process and fundamental understanding the iron ore behaviour with respect to time such as chemical phenomena decomposition, and solid-gas reactions (e.g., oxidation or reduction) [15].
The present investigation was focused on the micro-mechanism level of five different iron ores, which are obtained from eastern India (Odisha and Chhattisgarh). The analyzed properties of these ores compare to market standards in order to assess its quality and determine the viability for commercial exploitation and to provide sufficient fundamental information to the iron producing industries, particularly those that do not have research and development facilities.
2 Materials and methods
2.1 Materials
Odisha and Chhattisgarh regions are rich in iron ore reserves in India. For this study, hematite iron ore samples were collected from these regions, three samples from the Odisha region (marked as IO1, IO2 and IO3), and two are from Chhattisgarh region (IO4 and IO5). The geological map of Odisha and Chhattisgarh region are shown in Figure 1.
2.2 Chemical composition
The chemical composition of all iron ore samples were determined by using X-ray fluorescence (XRF) technique according to ISO 9516-1 standard [16]. Additionally, loss on ignition (LOI) was also calculated according to ISO standards. The obtained results have been listed in Table 1.
2.3 Moisture content of iron ore concentrate
The moisture content of iron ore has been determined according to international standards (IS: 11690–1986) [17]. According to the standard, 1 kg of iron ore sample (–10 mm size) spread uniformly in the pan and heated in the oven at 378 K for 4 h, then weigh the mass loss of the sample, put back into the oven for 1 h and repeat the procedure until a constant weight of the test sample is attained.
2.4 Determination of physical properties
Tumbler index (TI) and abrasion index (AI) were determined by a standard method according to ISO (IS: 6495–1984) [18]. Shatter index (SI) was determined by a standard method, which is defined by IS: 3963–1981 [19]. The apparent porosity of the iron ore lumps were determined by the method of hot test boiling water (HTBW), which was defined by the standard method IS 1528 (Part 3, 2010) [20].
2.5 Mineralogical analysis
The micro-morphology and mineralogy of the various iron ores were studied using an optical microscope, and scanning electron microscope fitted with an energy dispersive spectrometer (EDS). X-ray diffraction (XRD) analysis was used to detect the various phases of the oxide in the iron ore samples with a 2θ range of 20°–90°.
2.6 Thermal analysis
Thermal analysis was carried out in the Netzsch device (STA409C, Germany). In this analysis, 6–8 g of iron ore sample was heated from room temperature to 1473 K, with a heating rate of 10 K/min under argon atmosphere with a flow rate of 100 mL/min.
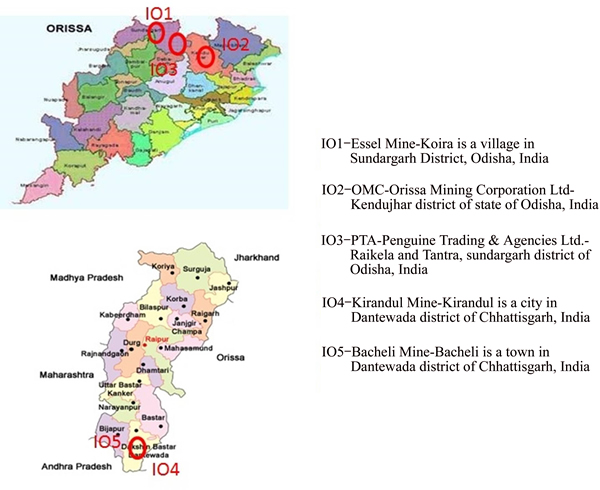
Figure 1 Geological map of Odisha and Chhattisgarh region, India
Table 1 Chemical composition and loss on ignition of iron ores from different mines
2.7 Fourier transform infrared (FTIR) analysis
Fourier transformation infrared was performed on various iron ore samples to show the contained elements and the manner in which they are chemically bound. FTIR spectra were recorded using a spectrum two and operated with Perkin Elmer USA/ RX-I FTIR apparatus in the spectral range of 4000–450 cm–1 with a resolution of 4 cm–1.
2.8 Surface area and pore volume
The Brunauer–Emmett–Teller (BET) technique was universally adopted for measuring the surface area and internal porosity of the materials by adsorption of inert gas (liquid nitrogen) on the surface of the particles and by measuring the amount of adsorbate inert gas corresponding to a monomolecular layer on the surface. It was carried out on a quanta chrome/AUTOSORB-iQMP apparatus to determine the surface area from the nitrogen isotherm (adsorption and desorption) at –196.15 °C (77 K) [13]. Barrett-Joyner-Halenda (BJH) method is most commonly used to determine the pore size distribution of the particles. This test was carried out according to ASTM standard method D4641/87 [21].
3 Results and discussion
3.1 Chemical composition of iron ores
The mineralogical composition of selected hematite iron ores is listed in Table 1. The chemical composition indicates that the gangue minerals in the iron ore are mainly composed of silica and alumina with a negligible amount of deleterious elements sulphur and phosphorus. Figure 2(a) shows the allowable amount of gangue and Fe content in iron ores for various iron production processes. The results also indicate that, the iron ores obtained from Bacheli and Kirandul have very high content of Fe (Fe>65%) and low gangue content (<5%) and thus come under high grade iron ore. OMC and PTA iron ores have Fe and gangue contents in the range of 62%–64% and 5%–7% and hence are medium grade, where as Essel iron ore is low grade because it contains a more than 12% gangue minerals and less than 57% Fe. The ratio between the alumina and the iron (A12O3/Fe) must be below 0.05, and the ratio of alumina and silica (A12O3/SiO2) must be below 1, to achieve good productivity of the blast furnace. The ratios A12O3/Fe varies from 0.02 to 0.13 A12O3/SiO2 in the range of 0.7 to 1.65, all iron ore meet the BF conditions Except IO1. It was also noted that the content of harmful elements such as P (0.04%–0.065%) and S (0.012%–0.016%) are significantly lower in all iron ores compared to the acceptable world trade level for raw iron ores as shown in Figure 2(b).
To study its quality for worldwide viable exploitation, the chemical composition of selected Indian natural iron ores was compared 5 of the world’s major iron ore producing countries and to global market standards for iron ores. Therefore, considering both Fe and gangue contents, it can be concluded that the quality of major iron ores (IO1-IO4) is comparable with the best-exported iron ores from Australia, Brazil, Russia, and China corresponds to the World high-grade hematite ores [21–23].
3.2 Moisture content of iron ore concentrate
The amount of water contained in the samples above the nominal content can be shown on the surface of the sample as physi or chemisorbed water, or the excess water can be captured in the crystal structure. Numerous authors were investigated on the excess of water in the lepidocrocite and maghemite (Boehmite and γ-Al2O3, respectively), the excess water stored as sorbed on the surface, partially in dislocations and interlayer’s [24–26].

Figure 2 Recommended ranges commercial iron ores for blast furnace (BF) and direct reduction (Midrex, HYL III and SL/RN) processes a) Fe Vs Gangue b) P Vs S content
The results are likely to be acceptable for iron ores (iron oxides). Excess water content (moisture) was determined for five iron ore samples, as shown in Figure 3. It can be observed that the moisture content of samples IO1–IO5 ranges from 0.98% to 2.9%. In all samples, the moisture is high for IO1 because of its impurities and its porosity. The moisture content decreases with the increase in hematite content and increases with the A12O3 and SiO2 content. The A12O3 is in the form of fine clay that will absorb moisture, as the amount of A12O3 increases in the ore, the moisture content increases.
3.3 Physical properties of iron ores
The cold strength (TI, AI and SI) of the iron ore was determined from the various deposits shown in Table 2. The TI values of all iron ore samples ranged from 82% to 86% by weight, which is considerably higher than 70%. Therefore, iron ores can be handled, loaded and transported without disintegration into small particles. The AI for iron ores ranges from 1.27% to 4.81% by weight, which is below the acceptable range (<5%), as shown in Figure 4. It indicates that the amount of particles in the form of dust generated during the handling process is within the acceptable range to allow a dust-free handling environment. In iron making process, some impact forces on the iron ore will act in the furnace during charging. As a result, the ore must withstand the impact forces when feeding the furnaces. The SI represents the susceptibility of to break down due to collision during supply into the reduction furnaces. The SI values of iron ores in between 0.87% and 1.64%, IO1 and IO2 samples having the highest SI values (1.64 and 1.32 respectively) due to their larger impurities make the ores susceptible to hairline fracture.The results compared with the commercial samples of ore iron ore from Muko ores in Uganda were between 0.57% and 2.01%, it shows that the SI value in the same range as for these ores. The physical properties of the different iron making process are shown in Figure 4, from the Figure 4, the iron ores are suitable for the blast furnace operations, coal-based reduction and also the midrex process.

Figure 3 Effect of Al2O3 on moisture content
Table 2 Physical properties of iron ores from different mines


Figure 4 Recommended ranges for physical properties for commercial iron ores for blast furnace (BF) and direct reduction (Midrex, HYL III and SL/RN) processes
Porosity:Porosity is the most important property in the reduction process. The reducing gas can flow inside the lump ore. The high porosity represents a significant amount of contact between the reducing gas and ore for this reason, and they can provide a large amount of reducibility. Normally, there is no porosity limit in the process of the DRI for the lumps but confined to iron ore pellets over 20%. In this study, the porosity of iron ore samples is shown in Table 2. Porosity was observed to be between 2.1% and 4.5%, IO1 with a high porosity of 4.5% due to its high content of gangue (7.53% A12O3 and 4.54% SiO2) and a high amount of moisture content.IO2–IO5 have a porosity less than 3%. The resulting porosity values are compared with the literature. It was found that the iron ores are suitable for BF and DRI process.
3.4 Mineralogical phase identification of iron ore samples
3.4.1 X-ray diffraction (XRD) analysis
XRD analysis was performed on iron ore samples (IO1–IO5) to identify the phases present, and same are represented in Figure 5. The XRD pattern revealed that the main phase was hematite for all the samples (IO1–IO5) and appearing at 2θ of 38°, 41° and 63°, as shown in Figure 5. The peaks of Fe2O3 and Fe3O4 overlapped at 2θ of 42° and 73°, it is due to that the Fe2O3 coexists with Fe3O4 at below 843 K in the Fe-O phase diagram. Other mineral phases are silicon oxide and aluminium oxides are present in the form of quartz (SiO2) and kaolinite (Al2Si2O5(OH)4). Kaolinite main peak at 2θ of 24°, the peaks of quartz were observed at 2θ of 38° and 58°. Kaolinite phase was not observed in IO3 and IO5 as shown in Figure 5.

Figure 5 XRD patterns of Indian iron ores
3.4.2 Optical microscopy
Optical microscopy was used for characterization of iron ore samples, in particularly to the study the microstructures and the distribution of impurities in the matrix of iron ores. Optical microstructure analysis shows different textures and formation of hematite structures in iron ores.In iron ore, hematite is a matrix form, and it contains dark inclusions which are considered as an impurity in the iron ores. Although the chemical composition is similar to different deposits of iron ore, the microstructures differ significantly. The micrographs of the various iron ore samples (IO1– IO5) illustrate the variety of forms of hematite grains, as shown in Figure 6. It can be seen that the microstructure of sample IO1 is a microcrystalline hematite matrix with a large amount of inclusions and resembles a fine structure, the impurity inclusions are located between the hematite grains and the mean size is less than 10 μm. Sample IO2 microcrystalline platy structure, dark inclusions are present at boundaries and are less than 20 μm. The microstructure of the sample IO3 has a crystal plate structure, the size of the crystal is larger compared to that in the microstructure IO2. The microstructures of samples IO4 and IO5 are similar to one another, and impurity inclusions are present in the crystalline structures.
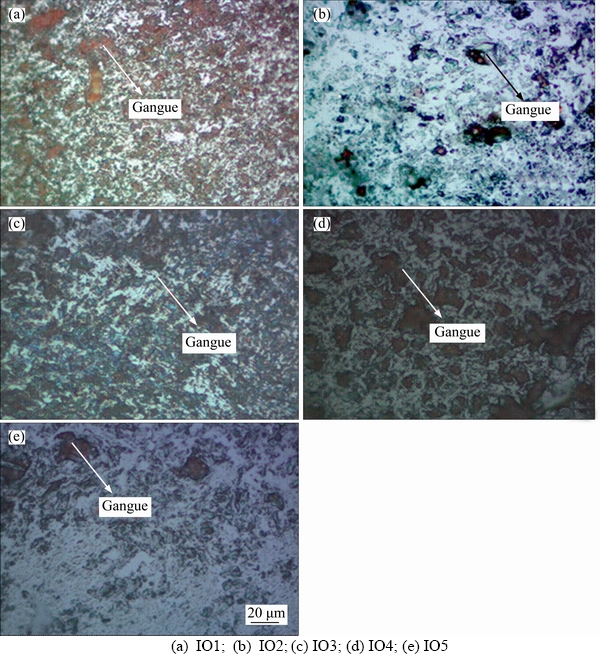
Figure 6 Optical microstructures of Indian iron ores:
3.4.3 SEM study
Mineralogical and micro–morphological examinations were performed using scanning electron microscopy with EDS. This study was carried out on individual particles to identify their micromorphology. The iron ore particle contains a variable amount of gangue with Fe as shown in Figure 7. Figure 7(a) gives the morphology of hematite and main elements distribution in iron ores obtained by SEM-EDS analysis. Apparently, Fe and O showed the good distribution of these particles. The iron-containing particles contain a variable amount of Al and Si as shown in Figures 7(b) and (e). According to the chemical compositions obtained by EDS analysis P2 mainly comprised Fe, Al, and O, which respectively took up about 41.4%, 2.90% and 55.63% of the total mass and P5 indicated that Fe, Si and O in the range of 95.76%, 1.93% and 2.31%. In Figure 7(c), P3 represents the morphology of gangue particle, following their chemical composition is O, Al, Si, and Fe accounted for about 55.27%, 4.42%, 30.40% and 9.92%. The Al and Si have existed in the form of kaolinite. It was confirmed by XRD analysis as shown in Figure 5. Figure 7(g) represents morphology of the gangue along with Fe. P6 revealed that Al, O, and Fe were the main components accounting for about 26.352%, 59.67% and 13.81%.
3.5 Fourier transform infrared (FTIR) analysis
Figure 8 shows FT-IR diffuse reflectance spectra, in the range of 4000–450 cm–1, five different iron ore samples to demonstrate that the spectrum varies significantly with the properties of the sample. The most typical FTIR absorption bands of hematite are present in the low-frequency region (<600 cm–1) as shown in Figure 8. In a total of five samples, the first intense IR absorption band of the hematite was identified in the range of 469 to 472 cm–1, and the second intense range was 539 to 546 cm–1. The presence of quartz can be in the spectral range of 1000–1260 cm–1, two IR bands were observed at the range of 1030–134 cm–1, and 1102–1113 cm–1 are due to Si—O—Si bonding [28]. In the larger wavelength, stretched and sharp bands were observed in the range of 1600 cm–1 and 3100–3700 cm–1 due to O—H groups and bonded water associated with these iron ore mineral as shown in Figure 8 [29]. Kaolinite is also present and identified by the presence of peaks at 1006–1012 cm–1 for Si–O–Al, and Al–O–H bonds were observed at 799–808 cm–1 and 905–912 cm–1 in all iron ore samples and they are stretched in IO5.
3.6 Textural properties
Figure 9 shows the nitrogen (N2) isotherms at 77 K for various iron ore samples (IO1–IO5). The isotherms of all iron ore samples are similar to the isotherms of type IV, corresponding to the mesoporous particles and parallel from 0.2 to 0.98 of the relative pressure. All samples show a hysteresis loop type H4 according to IUPAC classification [21]. The surface area of the iron ore samples was calculated by using the BET equation, and it follows that the N2 isotherms fit very well into the BET equation. The total pore volume present in the samples corresponds to the amount of N2 absorbed in the isotherm at a given P/P0 value, the absorption increasing with increasing pressure, as shown in Figure 9. The BET surface area of the iron ore powders are presented in Table 3. It was observed that the range of 8.364–12.588 m2/g. The large surface area was found in an IO1 sample which was 12.588 m2/g, it is due to the presence of high amount porosity, as shown in Table 2. The mean diameter and the pore size distribution were calculated from the BJH curves. The pore size distribution was determined from the N2 absorption in isotherms of the samples, as shown in Figure 10 which shows a good relationship between the pore size distribution and the pore diameter. The pore mean diameters of the iron ore samples were in the range of 3.1 to 4.7 nm, as shown in Table 3, indicating that it is a mesoporous material [30].
3.7 Thermal analysis
The thermal analysis experiments can explain the behavior of raw materials during heating processes. Thus, the TG/DSC were performed on iron ore samples to investigate its behavior when subjected to heating.The obtained results are shown in Figure 11. It can be seen in DSC analysis, a series of endothermic reactions occur during heating, and they are due to the loss of moisture,hydroxial bonds and decomposition of volatile substances/matter in the iron ore. The reactions are related to the mass changes of iron ore over temperatures as shown in Table 4. The first endothermic reaction was observed at the lower temperature due to the hydration of hygroscopic moisture, it was completed at 447 K. From the TG analysis, the highest moisture loss was found in IO1, IO2 (1.70%, 1.46%), less than 1% in the IO3 sample, and the remaining samples (IO4 and IO5) are less than 0.3%. Second endothermic reactions were observed, it is due to the release of hydroxyl bonds in the iron oxyhydroxides and the loss of the bounded water in the iron ore, also referred to as dehydroxylation. In iron ores, Fe was chemically bonded with hydrogen (OH) and form some hydroxyl iron minerals, such as goethite (FeOOH), hydrohematite (Fe2O3·nH2O), limonite (FeOOH·nH2O), ferrihydrates ((Fe3+)2O3·0.5H2O), iron(II) hydroxide Fe(OH)2 and bernalite (Fe(OH)3). When raising the temperature, the hydroxyl minerals involved in dehydroxylation reactions, the following equations shows the dehydroxylation reactions [31].
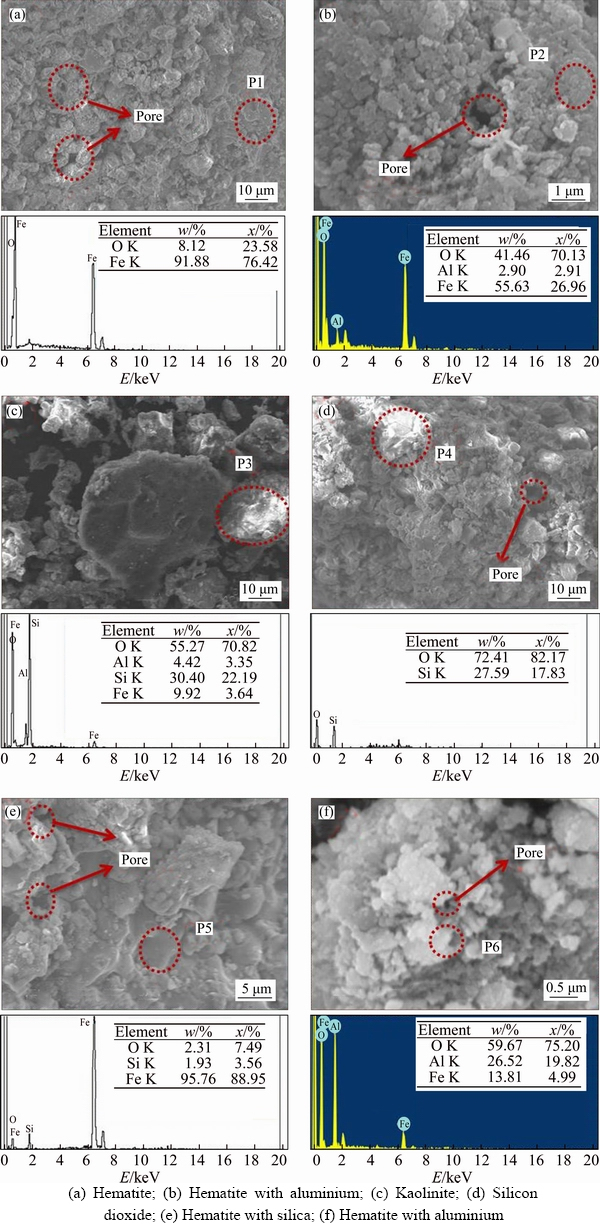
Figure 7 SEM photomicrograph with EDS:
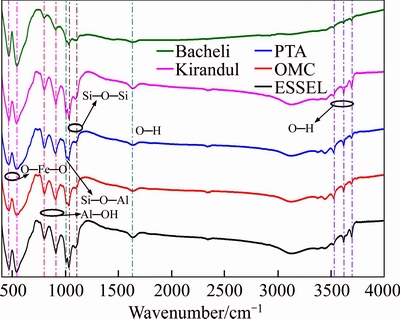
Figure 8 FTIR spectra of different iron ore samples
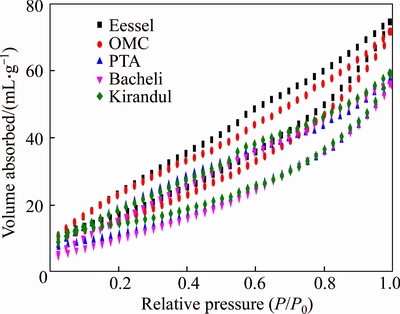
Figure 9 Nitrogen adsorption and desorption isotherms for iron ore samples
Table 3 Textural properties of iron ores


Figure 10 BJH method pore size distribution curves from Nitrogen sorption data
2FeOOH→Fe2O3 (1)
2FeOOH·nH2O→Fe2O3+(n+1)H2O (2)
Fe(OH)2→FeO+H2O (3)
2Fe(OH)3→Fe2O3+H2O (4)
Al2Si2O5(OH)4→Al2O3+SiO2+H2O (5)
Sample IO1 shows the pronounced effect of heating, and an endothermic reaction was observed in between 530 and 612 K. The same endothermic reaction was observed in IO2–IO5 and faintly varying to the previous sample as shown in Figure 11. The amount of mass changes in dehydroxylation was seen through TG analysis as shown in Table5. The highest mass loss was found in IO1 and IO2, which were 4.49% and 4.19%. In the case of sample IO3, it is smaller than the previous sample, which is 2.88%, nearly 1% in IO4 and IO5 samples. The further endothermic reaction has occurred at a higher temperature in the range of 597–850 K. In sample IO1, this reaction was found with the temperature peaking at 771 K, it is due to Figure 11 TG and DSC curves of different iron ores:the decomposition of the clay matter as shown in Eq. (5) [32]. During heating, the amount of mass loss was similar in IO1, IO2 and IO4 sample; it could be due to the presence of high amount of Al2O3 in the form of kaolinite, it was confirmed through the XRD analysis. The remaining samples have a very less amount of mass loss which is less than 1%, and it is due to the absence of kaolinite.

Figure 11 TG and DSC curves of different iron ores:
Table 4 Mass loss (wt%) and corresponding heat of reactions for individual thermal regions of iron ores

3.8 Possible prospects of using natural iron ore for iron production process
A block diagram depicts the different iron making routes, in which significant amount of iron was produced from Blast furnace (BF), basic oxygen furnace (BOF) and direct reduction (DR) process, as shown in Figure 12. BF/BOF process requires a high-grade raw material (iron ore and coke) with high levels of physical and metallurgical characteristics, in these process large amount of CO2 emissions is present, because these emissions auxiliary plants (environmental control systems) are necessary. In decades ago, electric arc furnace (EAF) route was introduced, it produced the 26.6% in 1988 and 33.8% in 2010. The operating cost has increased due to the melting of raw materials and the shortage of feedstock. To overcome these drawbacks, they find out a way, i.e., sponge iron (direct reduction), which is a suitable material for use in the raw material of EAF. DRI was introduced in the late 1960s and early 1970s, and it is a most successful implementation. Many DRI process are introduced, which are Midrex and HYL III (gas based) and SL/RN(coal based), now the Midrex processes produce the major role in DRI process [33–36]. The requirements of the lump iron ore for the production of iron in the different process are shown in the Table 5. According to the table, the iron ores (IO2–IO5) meet the chemical requirements as feed material for blast furnace (BF). The comparison of physical properties of iron ores with standard ranges, the all lump ore are suitable for BF and DRI operations (Midrex IO2–IO5, SL/RN and HYL III-IO2 and IO4). The content of Fe, Al2O3 and SiO2 in the IO1 sample falls short of the given requirements, but it satisfies the physical properties. Therefore, the iron ore IO1 deposit can also be used as a feed material for production of pellets and sinter route or beneficiation with higher quality ores for DR (EAF/sponge iron) process.
4 Conclusions
From the present research work, the following conclusions have been drawn:
1) Chemical analyses reports indicated the highest iron content in Chhattisgarh hematite iron ores followed by hematite iron ores of Odisha of OMC, PTA and Essel mines.
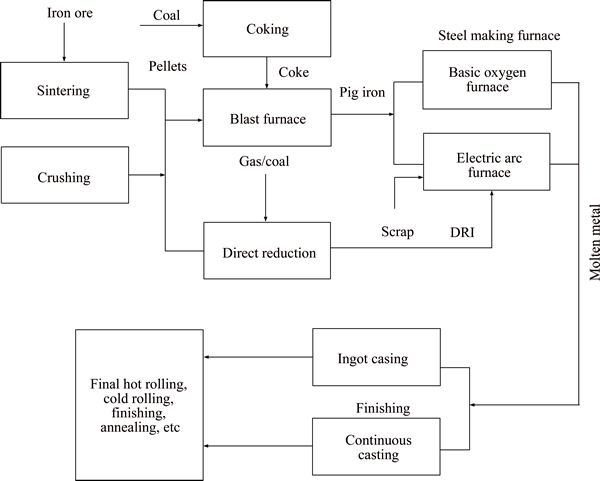
Figure 12 Schematic representations of main routes of iron production techniques
Table 5 Characteristics of iron ore lumps used for different iron making techniques and characteristics of Indian iron ores

2) Except hematite iron ore of Essel mine, others have been found to have gauge (Al2O3 + SiO2) contents less than 8.0% and negligible amount of deleterious elements P (< 0.065%) and S (< 0.012%).
3) The tumbler, abrasion, and shatter indices of all these studied iron ores were found to be in the range 82%–91%, 1.27%–4.87%, and 0.87%–1.64%. These values were acceptable for iron making process.
4) Thermal analysis (TG/DSC) results of all the studied hematite iron ores showed a series of endothermic reactions at various temperatures. The first endothermic reactions in all the cases were observed at a temperature below 447 K, it is due to dehydration of hygroscopic moisture (≤1.7 wt%), and followed by a second ones in the temperature range of 525–609 K due to decomposition of oxyhydroxides from iron ores. After that, other endothermic peaks were detected in the range of 597–850 K due to decomposition of OH groups in the iron ores.
5) As confirmed by SEM-EDS, XRD and FTIR analyses, the gauge minerals in all the studied hematite iron ores were present in the form of aluminosilicates that is kaolinite.
6) The results obtained through BET technique indicated surface areas of the studied hematite iron ores to be in the range of 8.364–12.588 m2/g and the mean pore diameters in the range of 3.14 to 4.7 nm.
References
[1] GOLDRING D C. Iron ore categorisation for the iron and steel industry [J]. Applied Earth Science, 2003, 112(1): 5–17. DOI: https://doi.org/10.1179/0371745032501162.
[2] UPADHYAY R K, VENKATESH A S, ROY S. Mineralogical characteristics of iron ores in Joda and Khondbond areas in Eastern India with implications on beneficiation [J]. Resource Geology, 2010, 60(2): 203–211. DOI: https://doi.org/10.1111/j.1751-3928.2010.00126.x.
[3] UPADHYAY R K, VENKATESH A S. Current strategies and future challenges on exploration, beneficiation and value addition of iron ore resources with special emphasis on iron ores from eastern India [J]. Applied Earth Science, 2006, 115(4): 187–95. DOI: https://doi.org/10.1179/174327506X13 8922.
[4] SINHA M, NISTALA S H, CHANDRA S, MANKHAND T R. Mineralogy of iron ores of different alumina levels from Singhbhum belt and their implication on sintering process [J]. Journal of Minerals and Materials Characterization and Engineering, 2015, 3: 180–193. DOI: http://dx.doi.org/ 10.4236/jmmce.2015.33021.
[5] MUWANGUZI A J, KARASEV A V, BYARUHANGA J K, J NSSON P G. Characterization of chemical composition and microstructure of natural iron ore from Muko deposits [J]. ISRN Materials Science, 2012, 3: 1–9. DOI: https://doi.org/10.5402/2012/147420.
NSSON P G. Characterization of chemical composition and microstructure of natural iron ore from Muko deposits [J]. ISRN Materials Science, 2012, 3: 1–9. DOI: https://doi.org/10.5402/2012/147420.
[6] KUMAR M, JENA S, PATEL S K. Characterization of properties and reduction behavior of iron ores for application in sponge ironmaking [J]. Mineral Processing and Extractive Metallurgy Review, 2007, 29(2): 118–29. DOI: https:// doi.org/10.1080/08827500701421896.
[7] CHOKSHI Y, LIMAYE M A, DUTTA S K, LODHARI D R. Mineralogical studies of low-grade iron ore from jharkhand–orissa region, india [J]. Transactions of the Indian Institute of Metals, 2016, 69(1): 151–155. DOI: https:// doi.org/10.1007/s12666-015-0740-4.
[8] RAO D S, KUMAR T V, RAO S S, PRABHAKAR S, RAJU G B. Mineralogy and geochemistry of a low grade iron ore sample from Bellary-hospet sector, India and their implications on beneficiation [J]. Journal of Minerals and Materials Characterization and Engineering, 2009, 8(2): 115–131. DOI: 10.4236/jmmce.2009.82011.
[9] MAHIUDDIN S, BONDYOPADHWAY S, BARUAH J N. A study on the beneficiation of Indian iron-ore fines and slime using chemical additives [J]. International Journal of Mineral Processing, 1989, 26(3, 4): 285–296. DOI: https://doi.org/ 10.1016/0301-7516(89)90034-3.
[10] LU L. Effects of alumina on sintering performance of hematite iron ores [J]. ISIJ International, 2007, 47(3): 349–358. DOI: https://doi.org/10.2355/isijinternational.47. 349.
[11] CLOUT J M, MANUEL J R. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores [J]. Powder Technology, 2003, 130(1): 393–399. DOI: https://doi.org/ 10.1016/S0032-5910(02)00241-3.
[12] MAO Hong-xia, ZHANG Ren-de, LV Xue-wen, BAI Cheng-guang, HUANG Xiao-bo. Effect of surface properties of iron ores on their granulation behavior [J]. ISIJ International, 2013, 53(9): 1491–1496. DOI: https://doi.org/10.2355/isijinternational. 53.1491.
[13] LADAVOS A K, KATSOULIDIS A P, IOSIFIDIS A, TRIANTAFYLLIDIS K S, PINNAVAIA T J, POMONIS P J. The BET equation, the inflection points of N2 adsorption isotherms and the estimation of specific surface area of porous solids [J]. Microporous and Mesoporous Materials, 2012, 151: 126–133. DOI: https://doi.org/10.1016/ j.micromeso.2011.11.005.
[14] LEOFANTI G, PADOVAN M, TOZZOLA G, VENTURELLI B. Surface area and pore texture of catalysts [J]. Catalysis Today, 1998, 41(1): 207–219. DOI: https://doi.org/10.1016/ S0920-5861(98)00050-9.
[15] DWECK J. Qualitative and quantitative characterization of Brazilian natural and organophilic clays by thermal analysis [J]. Journal of Thermal Analysis and Calorimetry, 2008, 92(1): 129–135. DOI: https://doi.org/10.1007/s10973-007- 8751-y.
[16] BOUCHARD M, MILLIARD A, RIVARD S, NESS S. ISO 9516-1 simplified borate fusion/WDXRF analytical method for iron ore including total iron analysis: Part 2. [J]. Powder Diffraction, 2014, 29(2): 170–175. DOI: https://doi.org/ 10.1017/S0885715614000323.
[17] Indian Standard IS: 11690. Method of moisture determination of iron ore lot [S]. Bureau of Indian Standards, 1986: 1–12. DOI: https://archive.org/details/gov.in.is.11690. 1986.
[18] Indian Standard IS: 6495. Method of tumbler test for iron oxides: Lump ores, sinter and pellet [S]. Bureau of Indian Standards, 1984: 1–8. DOI: https://archive.org/details/ gov.in.is.6495.1984.
[19] Indian Standard IS: 9963. Method of determination of shatter index of iron ore lumps, sinter and Pellets [S]. Bureau of Indian Standards, 1981: 1–6. DOI: https://archive.org/details/ gov.in.is.9963.1981.
[20] Indian Standard IS: 1528. Methods of sampling and physical tests for refractory materials [S]. Bureau of Indian Standards, 2010: 1–3. DOI: https://archive.org/details/gov. in.is.1528.7. 2010.
[21] KANEKO K. Determination of pore size and pore size distribution: 1. Adsorbents and catalysts [J]. Journal of Membrane Science, 1994, 96(1, 2): 59–89. DOI: https:// doi.org/10.1016/0376-7388(94)00126-X.
[22] MCCANN G, STREZOV V, LUCAS J A, EVANS T, STREZOV L. Iron ore characterisation during high temperature thermal processing [J]. Asia-Pacific Journal of Chemical Engineering, 2004, 12(3, 4): 369–382. DOI: https://doi.org/10.1002/apj.5500120412.
[23] FORMOSO A, MORO A, FERN NDEZ P G, MEN
NDEZ P G, MEN NDEZ J L, MUNIZ M, CORES A. Influence of nature and particle size distribution on granulation of iron ore mixtures used in a sinter strand [J]. Ironmaking & Steelmaking. 2003, 30(6): 447–60. DOI: https://doi.org/10.1179/030192303225004187.
NDEZ J L, MUNIZ M, CORES A. Influence of nature and particle size distribution on granulation of iron ore mixtures used in a sinter strand [J]. Ironmaking & Steelmaking. 2003, 30(6): 447–60. DOI: https://doi.org/10.1179/030192303225004187.
[24] CLOUT J M F, MANUEL J R. Iron Ore: Mineralogy, processing and environmental sustainability [M]. Sawston, Cambridge: Woodhead Publishing, 2015: 45–84.
[25] TSUKADA T, SEGAWA H, YASUMORI A, OKADA K. Crystallinity of boehmite and its effect on the phase transition temperature of alumina [J]. Journal of Materials Chemistry, 1999, 9(2): 549–53. DOI: 10.1039/A806728G.
[26] BELLOTTO M, REBOURS B, EUZEN P. Mechanism of pseudo-boehmite dehydration: Influence of reagent structure and reaction kinetics on the transformation sequence [J]. Materials Science Forum, 1998, 278: 572–577. DOI: https://doi.org/10.4028/www.scientific.net/MSF.278-281.572.
[27] CORES A, BABICH A, MU IZ M, ISIDRO A, FERREIRA S, MART
IZ M, ISIDRO A, FERREIRA S, MART N R. Iron ores, fluxes and tuyere injected coals used in the blast furnace [J]. Ironmaking & Steelmaking, 2007, 34(3): 231–40. DOI: https://doi.org/10.1179/1743 28107X168066.
N R. Iron ores, fluxes and tuyere injected coals used in the blast furnace [J]. Ironmaking & Steelmaking, 2007, 34(3): 231–40. DOI: https://doi.org/10.1179/1743 28107X168066.
[28] ULLAH R, DEB B K, MOLLAH M Y. Synthesis and characterization of silica coated iron-oxide composites of different ratios [J]. International Journal of Composite Materials, 2014, 4(2): 135–45. DOI: https://doi.org/10.5923/ j.cmaterials.20140402.13.
[29] SALAMA W, AREF M E L, GAUPP R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, M ssbauer spectroscopy and thermoanalyses [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 136: 1816–1826. DOI: https://doi.org/10.1016/j.saa.2014.10.090.
ssbauer spectroscopy and thermoanalyses [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 136: 1816–1826. DOI: https://doi.org/10.1016/j.saa.2014.10.090.
[30] SING K S. Adsorption methods for the characterization of porous materials [J]. Advances in colloid and interface science, 1998, 76: 3–11. DOI: https://doi.org/10.1016/s0001- 8686(98)00038-4.
[31] WEISSENBORN P K, DUNN J G, WARREN L J. Quantitative thermogravimetric analysis of haematite, goethite and kaolinite in Western Australian iron ores [J]. Thermochimica Acta, 1994, 239: 147–156. DOI: https:// doi.org/10.1016/0040-6031(94)87063-2.
[32] STREZOV V, ZIOLKOWSKI A, EVANS T J, NELSON P F. Assessment of evolution of loss on ignition matter during heating of iron ores [J]. Journal of Thermal Analysis and Calorimetry, 2010, 100(3): 901–907. DOI: https://doi.org/ 10.1007/ s10973-009-0398-4.
[33] ZERVAS T, MCMULLAN J T, WILLIAMS B C. Developments in iron and steel making [J]. International journal of energy research, 1996, 20(1): 69–91. DOI: https:// doi.org/10.1002/(SICI)1099-114X(199601)20:1<69::AID- ER241>3.0.CO;2-3.
[34] DE LIMA L C, DUARTE J B, VEZIROGLU T N. A proposal of an alternative route for the reduction of iron ore in the eastern Amazonia [J]. International Journal of Hydrogen Energy, 2004, 29(6): 659–661. DOI: https:// doi.org/10.1016/S0360-3199(03)00053-3.
[35] MICHISHITA H, TANAKA H. Prospects for coal-based direct reduction process [J]. Kobelco Technology Review, 2010, 29: 69–76. DOI: http://www.kobelco.co.jp/english/ktr/ pdf/ktr_29/whole.pdf.
[36] BEDARKAR S S, SINGH R. Removal of phosphorous from steel produced by melting sponge iron in induction furnace [J]. Transactions of the Indian Institute of Metals, 2013, 66(3): 207–211. DOI: https://doi.org/10.1007/s12666-013- 0244-z.
(Edited by HE Yun-bin)
中文导读
印度奥里萨邦和恰蒂斯加尔邦天然铁矿石的化学、物理、热、结构和矿物学研究
摘要:研究了印度奥里萨邦和恰蒂斯加尔邦不同地区铁矿石的化学、物理、热性能和结构特性,了解了Fe、Al2O3、SiO2、S和P的组成变化,分析了其敏感性,以满足各种铁制造工业技术要求。化学分析结果表明,大部分铁矿石中赤铁矿含量丰富(> 90 wt%),矸石含量低(<4.09 wt% SiO2,<3.8 wt% Al2O3),所有铁矿中有害元素含量低(P<0.065 wt%,S<0.016 wt%)。XRD衍射表明矸石以高岭石和石英的形式存在,其傅里叶变换红外光谱(FTIR)在914~1034 cm–1范围内。该铁矿具有优良的物理性能,例如转塔指数(82 wt%~91 wt%)、磨损指数(1.27 wt%~4.87 wt%)和破碎指数(0.87 wt%~1.64 wt%)。采用红外光谱和热分析方法对样品进行同化差值分析。研究表明,这些铁矿石具有三个吸热反应,即低于447 K的脱水反应,质量损失为0.13 wt%~1.7 wt%, 525~609 K的脱羟基反应,质量损失为1.09 wt%~4.49 wt%,597~850 K的铝硅酸盐分解,质量损失为0.13 wt%~1.15 wt%。这些铁矿具有优良的物理化学特性,适合高炉和DRI作业。
关键词:铁矿石;化学分析;矿物学分析;热分析;织构分析
Foundation item: Project supported by the National Institute of Technology, Rourkela, India
Received date: 2017-08-04; Accepted date: 2018-04-05
Corresponding author: Swapan Kumar Karak, PhD, Assistant Professor; Tel: +91-0661-2462570; E-mail: skkakark@gmail.com

