Mechanism of crud formation in copper solvent extraction
来源期刊:中南大学学报(英文版)2002年第3期
论文作者:柳建设 蓝卓越 邱冠周 王淀佐
文章页码:169 - 172
Key words:copper; extraction; crud; mechanism; Zeta potential
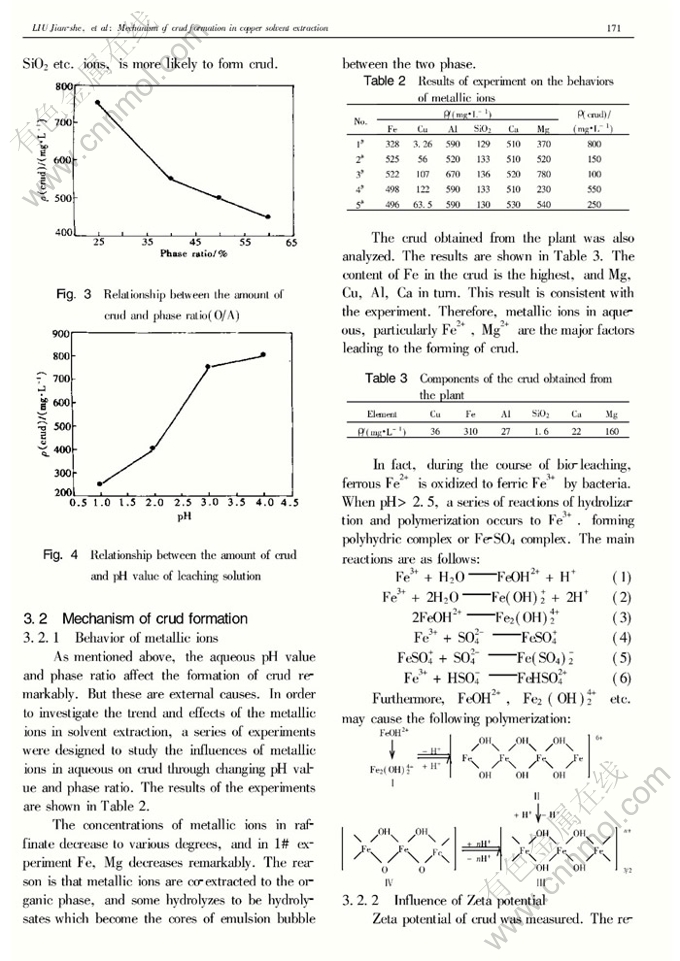
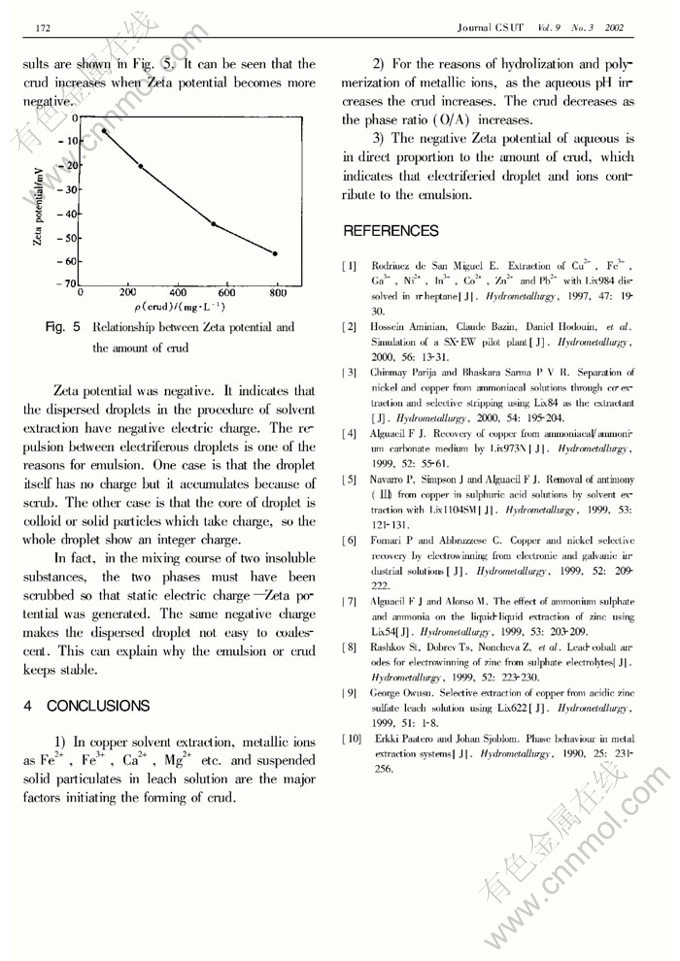
Abstract: The authors investigated the mechanism of crud formation in copper solvent extraction. It is indicated that pH value of solution and the phase ratio (O/A) are the main factors affecting crud formation in solvent extraction. The amount of crud extraction increases with aqueous pH value increase, and reduces with the increase of the phase ratio. Fe3+, Mg2+, fine air bubble and suspended particulates in leaching solution contribute to crud formation. One case is that a series of reactions of hydrolization and polymerization occurs for Fe3+, while pH>2.5, polyhydric complex or Fe-SO4complex are formed. Then the complex-ions of FeOH2+, Fe2(OH)4+2cause poly-reaction, which is likely to lead emulsion. The study on Zeta potential indicates the repulsion between electriferous droplets in solvent extraction prevents phase coalescence, which is one of the major reasons for emulsion.