Adsorption and desorption properties of D318 resin for Cr(Ⅵ)
来源期刊:中南大学学报(英文版)2009年第3期
论文作者:舒增年 熊春华
文章页码:405 - 409
Key words:Cr(Ⅵ); D318 resin; adsorption; desorption; dynamics; thermodynamics
Abstract: The adsorption capability of D318 resin for Cr(Ⅵ) was investigated by chemistry analysis. Experimental results show that D318 resin has the best adsorption ability for Cr(Ⅵ) at pH=3.16 in HAc-NaAc medium. The statically saturated adsorption capacity of the resin is 265.4 mg/g. The thermodynamic adsorption parameters, enthalpy change ΔH and free energy change ΔG298 of the adsorption reaction are 4.81 and -5.16 kJ/mol, respectively. The apparent activation energy Ea is 22.4 kJ/mol. The adsorption behavior obeys the Freundlich isotherm. The molar coordination ratio of the functional group of resin to Cr(Ⅵ) is 3:2. Cr(Ⅵ) adsorbed on D318 resin can be eluted by 5%NaOH-5%NaCl quantitatively.
基金信息:the Natural Science Foundation of Zhejiang Province, China
J. Cent. South Univ. Technol. (2009) 16: 0405-0409
DOI: 10.1007/s11771-009-0068-5
![]()
SHU Zeng-nian (舒增年)1, XIONG Chun-hua (熊春华)2
(1. Department of Chemistry, Lishui University, Lishui 323000, China;
2. College of Food Science, Biotechnology and Environmental Engineering, Zhejiang Gongshang University,
Hangzhou 310035, China)
Abstract: The adsorption capability of D318 resin for Cr(Ⅵ) was investigated by chemistry analysis. Experimental results show that D318 resin has the best adsorption ability for Cr(Ⅵ) at pH=3.16 in HAc-NaAc medium. The statically saturated adsorption capacity of the resin is 265.4 mg/g. The thermodynamic adsorption parameters, enthalpy change ΔH and free energy change ΔG298 of the adsorption reaction are 4.81 and -5.16 kJ/mol, respectively. The apparent activation energy Ea is 22.4 kJ/mol. The adsorption behavior obeys the Freundlich isotherm. The molar coordination ratio of the functional group of resin to Cr(Ⅵ) is 3?2. Cr(Ⅵ) adsorbed on D318 resin can be eluted by 5%NaOH-5%NaCl quantitatively.
Key words: Cr(Ⅵ); D318 resin; adsorption; desorption; dynamics; thermodynamics
1 Introduction
Cr(Ⅵ) is of severe toxicity, and can be gathered in water, living creatures and farm crops. It can also be absorbed by the human body through the food chain and accumulated inside the body. Cr(Ⅵ) mainly comes from industrial production such as electroplating, tanning, mining and dyestuff. The continuous aggravating pollution of Cr(Ⅵ) will do harm to both environment and mankind health. So Cr(Ⅵ)-contained wastewater is nowadays recognized as one of the most serious harmful effects and has been listed as focal point of controlling by the National Environmental Protection Bureau of China, and how to effectively treat Cr(Ⅵ)-contained wastewater is always a burning question to industrial production. Currently, there are mainly two major types of methods to treat the Cr(Ⅵ)-contained wastewater. One is reduction method, which creates great quantity of dirty mire in the process and easily causes secondary pollution. The other is direct processing of Cr(Ⅵ), and the ion exchange method is one of this type of methods. In recent years, the research on dealing with wastewater containing Cr(Ⅵ) by resin has been very active [1-3], but there are some deficiencies in low adsorption capacity and elusion ratio, and there has not been report that D318 resin adsorbs Cr(Ⅵ). In this work, the adsorption behavior and mechanism of D318 resin adsorbing Cr(Ⅵ) were studied, and the basic adsorption parameters were determined.
2 Experimental
2.1 Materials and instruments
Perkin-Elmer 683 FT-IR analyzer, Sartorius PB-20 pH meter, elemental analyzer EA1110, THZ-82A temperature constant shaking machine and 722 spectrophotometer were used.
D318 resin was provided by Xi’an Electric Power Resin Factory, China. Standard solution of Cr(Ⅵ) was prepared from potassium dichromate(A.R.), and 0.1% diphenyl carbohydrazide solution was used as a developer.
2.2 Adsorption and analytical method of resin
A desired amount of D318 resin was weighed and added into an iodine flask, and then a desired volume of HAc-NaAc buffer solution was added. After 12 h, a required amount of standard solution of Cr(Ⅵ) was added, and the then flask was shaken in a shaker at constant temperature until the concentration of Cr(Ⅵ) maintained invariable. The amount of Cr(Ⅵ) was measured by the specteophotometric determination of diphenyl carbohydrazide. According to concentration change of Cr(Ⅵ) solution adsorbed, the adsorption amount Q, the adsorption distribution coefficient D and adsorption rate E were calculated respectively as follows:
Q=(ρ0-ρe)V/m (1)
D=Q/ρe (2)
![]() (3)
(3)
where Q is the adsorption amount of resin to Cr(Ⅵ) in the equilibrium state, mg/g; ρ0 is the initial concentration of Cr(Ⅵ) in solution, mg/mL; ρe is the equilibrium concentration of Cr(Ⅵ) in solution, mg/mL; m is the resin mass, g; and V is total volume of solution, mL.
3 Results and discussion
3.1 Effect of pH on adsorption rate
Seven parts of 30.0 mg resins were weighed accurately and put into iodine flasks respectively. Under the experimental conditions of T=298 K and ρ0= 0.29 mg/mL, HAc-NaAc buffer solutions of pH=2.5-6.5 were added respectively, and shaken to equilibrium intermittently. The effect of pH on adsorption rate is shown in Fig.1. The adsorption rate is the highest at pH=3.16. So all the following experiments were performed at pH=3.16 in HAc-NaAc buffer solutions. Cr(Ⅵ) exists in the form of CrO42- , HCrO4- and Cr2O72- in aqueous solution, and in the form of CrO42- in basic solution. With the decrease of pH value, CrO42- in solution is transformed into Cr2O72- constantly. Cr(Ⅵ) is transformed into Cr2O72- dominantly when pH=3-6 in the acidic solution. On the other hand, the amino group of resin absorbs H+ when pH is decreased because it carries comparatively strong positive charge that is helpful to absorb Cr2O72-. The Cr(Ⅵ) in the solution is mainly in the form of HCrO4- at pH<3, which consequently decreases the absorption quantity.
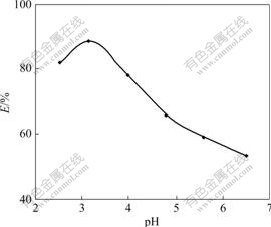
Fig.1 Effect of pH on adsorption rate of Cr(Ⅵ)
3.2 Effect of SO42- and Cl- on resin adsorption of Cr(Ⅵ)
Several parts of 50.0 mg resins were weighed accurately, according to the experimental conditions of T=298 K, ρ0=0.20 mg/mL. NaCl and Na2SO4 solutions with varied concentrations were added, and static sorption equilibrium experiment was carried out. According to Formula (3), different adsorption rates of resin to Cr(Ⅵ) can be obtained (Fig.2). The results show that due to the competitive adsorption of coexistence ions to Cr(Ⅵ), the presence of Cl- and SO42- can block the adsorption of resin to Cr(Ⅵ). With the increase of Cl- and SO42- concentrations, the adsorption rate of resin to Cr(Ⅵ) decreases, and SO42- affects adsorption of resin to Cr(Ⅵ) more greatly than Cl-.
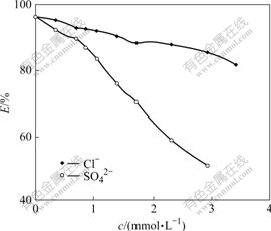
Fig.2 Effect of SO42- and Cl- on resin adsorption ratio of Cr(Ⅵ)
3.3 Adsorption dynamics
3.3.1 Control of adsorption rate process and determination of adsorption rate constant
40.0 mg resin was weighed accurately. According to the experimental conditions of T=298 K and ρ0= 0.24 mg/mL, a part of the upper layer clear solution was taken out at intervals for the determination of remaining concentrations until it reached equilibrium. According to Formula (1), the adsorption capacity of resin can be figured out. After the remains were kept constant and the volume was corrected, a series of data were obtained (Fig.3). When the adsorption amount is half of that at
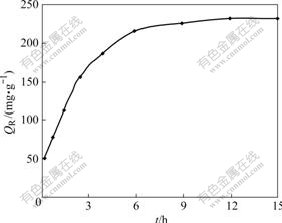
Fig.3 Adsorption kinetic curve of Cr(Ⅵ)
equilibrium, the required time t1/2 is about 2.0 h. The required time of the adsorption equilibrium is 12 h. BOYD et al [4], who studied ion exchange adsorption process in sorbent, deemed that exchange adsorption rate was controlled by a slow process of liquid film diffusion, particle diffusion and chemical reaction.
Particle diffusion equation:
![]() (4)
(4)
Liquid film diffusion equation:
![]() (5)
(5)
Chemical reaction equation:
![]() (6)
(6)
where F=Qt/Q∞, Qt and Q∞ are the resin adsorption amounts of every gram resin at adsorption time t and at equilibrium, respectively; R=3Dl/ro?rok, Dl is the film diffusion constant, ro, the particle radius, ?ro, the film thickness, and k, the distribution constant; B=π2Di/ro2, and Di is the internal diffusion coefficient; S is the mass action constant.
The distinction between Formulae (5) and (6) is only constant term R and S. If the curve of -lg (1-F) vs t shows a linear relationship, a judgment must be worked out by the results of assistant experiments. For example, the ball radius of sorbent affects the liquid film diffusion, while the reagent concentration and adsorption temperature affect chemical reaction.
If the graph of Bt vs t shows a linear relationship, the exchange rate will be controlled by particle diffusion. If the graph of -lg (1-F) vs t is linear, the exchange rate will be controlled by liquid film diffusion or the chemical reaction. Fig.4 shows that the plot of Bt vs t is linear, which indicates that particle diffusion of the absorption of D318 resin to Cr(Ⅵ) is the control phrase of the absorption rate in the initial stage process. The relationship between -lg(1-F) and t is linear (Fig.5),

Fig.4 Plot of Bt vs time
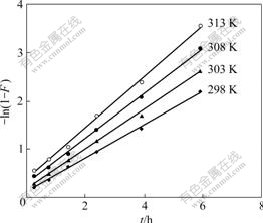
Fig.5 Determination of adsorption rate constant
which shows that liquid film diffusion or chemical reaction has an substantial effect on the absorption rate of D318 resin to Cr(Ⅵ) [4-5]. According to Fig.5, the apparent adsorption rate constant k298 is 9.472×10-5 s-1, and the correlation coefficient r is 0.998 6 at 298 K.
3.3.2 Determination of adsorption activation energy
The experiments were carried out by using the above-mentioned method (Section 3.3.1), and adsorption conditions of resin to Cr(Ⅵ) were determined at different temperatures. A straight line was obtained by plotting -ln(1-F) vs t (Fig.5). According to the slope of the straight line, the adsorption rate constants were also determined at different temperatures (298-313 K), which are k303=1.126×10-4s-1, k308=1.298×10-4s-1, and k313= 1.463×10-4s-1, respectively. According to the formula of Arrhenius ln k=-Ea/(RT)+ln A, a straight line was made by plotting lg k vs 1/T (Fig.6). The slope of straight line in Fig.6 is k′=-1.167. The apparent activation energy (Ea) of the adsorption reaction is Ea=22.4 kJ/mol. It can be known from the adsorption rate constant that the adsorption speed can be increased when the temperature is raised within the scope of experimental temperature [6-9].
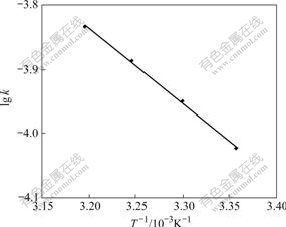
Fig.6 Relationship between lg k and 1/T
3.3.3 Isotherm adsorption curve
25.0, 30.0, 35.0 and 40.0 mg of resins were weighed respectively and adsorption experiments were carried out at T =298 K and ρ0=0.20 mg/mL. When the adsorption equilibrium reached, the equilibrium concentration ρe was determined and the corresponding adsorption capacity of resin Qe was calculated according to Formula (1). The adsorption isotherm is correlated to the well-known Freundlich equation:
Qe=aρe1/b namely lg Qe=1/blg ρe+lg a (7)
where a and b are Freundlich constants.
The straight line was obtained by plotting lg Q vs lg ρe (Fig.7), and the correlation coefficient of the straight line (r=0.998 1) was achieved, which showed that there was a good linear relationship. Calculated by equation Qe=947.8ρe1/3.08 fitting, the result shows that the adsorption behavior of D318 resin for Cr(Ⅵ) obeys the Freundlich equation. Parameter b is between 2 and 10. From the perspective of adsorption dynamics, under such experimental condition, the adsorption of D318 resin for Cr(Ⅵ) was easy to carry out [10-13].

Fig.7 Relationship between lg Qe and lg ρe
3.4 Effect of adsorption temperature on adsorption percentage and determination of thermodynamic parameter
Five parts of 50.0 mg resins were weighed and put into iodine flask individually at ρ0=0.30 mg/mL, the distribution coeffient of the resin for Cr(Ⅵ) was determined at temperatures of 285, 293, 301, 309 and 317 K. According to Formula (2), the corresponding distribution coffient was figured out. From the result shown in Fig.8, it can be seen that increasing the adsorption temperature leads to better adsorption. This means that the adsorption process is an endothermic process. Therefore, the adsorption reaction is a chemical adsorption. Since the adsorption processes in buffer solution, according to lg D=-?H/(2.303RT)+?S/R [14-15], the straight line was obtained by plotting lg D vs 1/T (Fig.8). From the slope of the straight line, ?H of 4.81 kJ/mol is obtained. ?S can be obtained from the intercept of the straight line, which is 33.5 J/(mol·K). At T=298 K, ?G298=?H-T?S=-5.16 kJ/mol. The thermo dynamic results of ?H>0, ?G<0 in the adsorption process indicate that the adsorption process is an endothermic process [16-18].

Fig.8 Effect of temperature on distribution coefficient
3.5 Determination of complex ratio
3.5.1 Saturated capacity method
50.0 mg of resin was weighed accurately. At T= 298 K and ρ0=0.30 mg/mL, the experiments were carried out by using the above-mentioned method (Section 3.3.1). The saturated adsorption capacity of the resin for Cr(Ⅵ) is 265.4 mg/g, and the amount of resin functional group is 7.06 mmol/g by elementary analysis. Therefore, the molar ratio of the resin functional group to Cr(Ⅵ) is 3?2.
3.5.2 Equimolar method
Eight parts of different amounts of resins were accurately weighed and added into different iodine flasks, and then mixed with different amounts of Cr(Ⅵ). The total mole numbers of resin functional group and Cr(Ⅵ) were kept at 248.3 ?mol whatever the molar ratio might be. The experiments were carried out with the same method mentioned previously. The curve of adsorption amount Q vs c(Cr(Ⅵ))/c(Cr(Ⅵ))+c(R) is constructed (unit of formula is mmol, R is functional group of D318 resin). The expected adsorption amount is the largest where the molar fraction of Cr(Ⅵ) is 0.41. This means that the complex molar ratio of the functional group to Cr(Ⅵ) is about 3?2. This result is consistent with that obtained from the saturated capacity method.
3.6 Desorption and recovery of resin
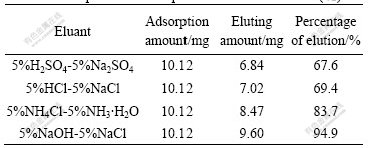
250.0 mg resin was weighed accurately and added into different conical flasks at T=298 K and ρ0= 0.30 mg/mL. The flasks were shaken until adsorption equilibriums reached, and then the saturated adsorption amount was calculated. The upper layers of clear solution were taken out and the resin was dried for analysis. Four parts of 40.0 mg resin adsorbed Cr(Ⅵ) were weighed accurately, and 40 mL 5%NaOH-5%NaCl, 5%NH4Cl-5%NH3·H2O, 5%HCl-5%NaCl and 5%H2SO4-5%Na2SO4 were added into the flasks respectively as eluants followed by shaking the flasks until the adsorption equilibriums reached. The content of Cr(Ⅵ) in aqueous phase was determined (Table 1). Experimental results show that the system of 5%NaOH-5%NaCl is the most efficient, and the percentage of desorption elution is up to 94.9% initially. Therefore, 5%NaOH-5%NaCl can be used as eluant to collect Cr(Ⅵ). D318 resin is a resin of the best application foreground in dealing with waste liquid of chrome.
Table 1 Adsorption and desorption of D318 resin for Cr(Ⅵ)
4 Conclusions
(1) Results of the adsorption experiment show that Cr(Ⅵ) can be optimally adsorbed on the resin at pH=3.16, and the statically saturated adsorption capacity is 265.4 mg/g. Cr(Ⅵ) can be eluted by using 5%NaOH-5%NaCl quantitatively, and the percentage of elution is up to 94.9% initially. Compared with the adsorption resin D201×4, 4-APR and XSD-296, the resin has advantages such as large adsorption capacity and easy recovery.
(2) The exchange adsorption rate of resin for Cr(Ⅵ) is controlled by a process of particle diffusion, liquid film diffusion and chemical reaction. The adsorption behavior for Cr(Ⅵ) obeys the Freundlich isotherm.
(3) The results of ΔH>0, ΔG<0 in the adsorption process show that the adsorption process is a spontaneous endothermic process.
References
[1] FAN Juan, ZHAN Huai-yu, LIU Meng-ru. Adsorption of Cr3+ and Cr2O72- on the spherical lignin-based ion exchange resin [J]. Transactions of China Pulp and Paper, 2005, 20(1): 111-113. (in Chinese)
[2] SHEN Qiou-xian, SHU Zeng-nian, WANG Yong-jiang, XIONG Chun-hua. Studies on the adsorption of D201×4 resin for Cr(Ⅵ) [J]. Chemical Research and Application, 2002, 14(4): 463-466. (in Chinese)
[3] Gode F, Pehlivan E. Adsorption of Cr(Ⅲ) ions by Turkish brown coals [J]. Fuel Processing Technology, 2005, 86(8): 875-884.
[4] Boyd G E, Adamson A W, Myers L S. The exchange adsorption of ions from aqueous solutions by organic zeolites Ⅱ kinetics [J]. Journal of the American Chemical Society, 1947, 69: 2836-2848.
[5] YANG Chao-xiong, WU Jin-yuan. Studies on cellulose-based magnetic polyamidoxime adsorbents [J]. Acta Polymerica Sinica, 1998(1): 43-48. (in Chinese)
[6] Shu Zeng-nian, Xiong Chun-hua, Wang Xu. Adsorption behavior and mechanism of amino methylene phosphonic acid resin for Ag(Ⅰ) [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 700-704.
[7] Xiong Chun-hua, Wu Xiang-mei. Study on the adsorption of iminodiacetic acid resin for yttrium(Ⅲ) [J]. Chinese Journal of Inorganic Chemistry, 2003, 19(12): 1356-1360.
[8] Kiran B, Kaushik A, Kaushik C P. Response surface methodological approach for optimizing removal of Cr(Ⅵ) from aqueous solution using immobilized cyanobacterium [J]. Chemical Engineering Journal, 2007, 126(2/3): 147-153.
[9] Brykina G D, Marchak T V, Krysina L S, Belyavskaya T A. Sorption-photometric determination of copper by using AV-17 anion exchanger modified with 1-(2-thiazolyl-azo)-2-naphthol 3,6-disulphonic acid [J]. Zhurnal Analiticheskoi Khimii, 1980, 35(12): 2294-2299.
[10] Shu Zeng-nian, Xiong Chun-hua, Shen Qiu-xian, YAO Cai-ping, GU Zhen-yu. Adsorption behavior and mechanism of D113 resin for lanthnum [J]. Rare Metals, 2007, 26(6): 601-606.
[11] Jin Yi-zhong, Xu Hao, Xie Yu-tan. Adsorption of benzene and toluene vapour on activated carbon [J]. Journal of Chemical Engineering of Chinese Universities, 2004, 18(2): 258-263. (in Chinese)
[12] CHEN Yong-gui, ZHANG Ke-neng, ZOU Yin-sheng, DENG Fei-yue. Removal of Pb2+ and Cd2+ by adsorption on clay-solidified grouting curtain for waste landfills [J]. Journal of Central South University of Technology, 2006, 13(2): 166-169.
[13] hiro K, renichirou S. Foundation and design of adsorptions [M]. Beijing: Chemical Industry Press, 1983: 33-36.
[14] ZHANG Ke-neng, CHEN Yong-gui, DENG Fei-yue, TIAN Qing-yu. Retention of clay-solidified grouting curtain to Cd2+, Pb2+ and Hg2+ in landfill of municipal solid waste [J]. Journal of Central South University of Technology, 2004, 11(4): 419-422.
[15] Si Gong-min, Wu Qi-hua, Zhang Le-qin. Study on the sorption behaviors and mechanism of chromium(Ⅵ) by macro-porous D252 resin [J]. Ion Exchange and Adsorption, 1990, 6(1): 1-8. (in Chinese)
[16] Ghazy S E, Ragab A H. Removal of lead from water samples by sorption onto powdered limestone [J]. Separation Science and Technology, 2007, 42(3): 653-667.
[17] Bhatnagar A, Minocha A K, Jeon B H, Park J M. Adsorptive removal of cobalt from aqueous solutions by utilizing industrial waste and its cement fixation [J]. Separation Science and Technology, 2007, 42(6): 1255-1266.
[18] Bhattacharyya K G, Gupta S S. Adsorption of chromium(Ⅵ) from water by clays [J]. Industrial and Engineering Chemistry Research, 2006, 45(21): 7232-7240.
(Edited by CHEN Wei-ping)
Foundation item: Project (Y304121) supported by the Natural Science Foundation of Zhejiang Province, China
Received date: 2008-08-25; Accepted date: 2008-10-30
Corresponding author: SHU Zeng-nian, Associate professor; Tel: +86-578-2271338; E-mail: zengnianshu@yahoo.com.cn

