
A novel process for sorting fine-sized sulphide minerals by biocoagulation
H. Z. KUYUMCU, J. PINKA, T. BIELIG
Department of Mechanical Process Engineering and Solids Processing,
Technical University of Berlin, Sekr. BH 11, Strasse des 17. Juni 135, 10623 Berlin, Germany
Received 20 September 2008; accepted 5 November 2008
Abstract: Based on a process design idea, investigations at Technical University of Berlin confirm that the biocoagulation of microorganisms and solid particles would be a new method to generate coarser particles suitable for sorting. The procedure of selective biocoagulation of microorganisms, e.g. yeasts like Saccharomyces cerevisiae and Yarrowia lipolytica respectively, and micro-dispersed solids, e.g. minerals like galena and sphalerite, has been analyzed as a basis for a novel sorting process. Therefore, especially the characteristics of the cell surface of the microorganisms, e.g. the electrostatic charge and the composition of extracellular polymeric substances, as well as their influence on the selective biocoagulation were studied. Experimental investigations show that the microorganisms and the sulphide particles below 10 ?m coagulate effectively. Furthermore, the flotation is suitable for the separation of the selectively formed biocoagulates. With the designed column flotation, satisfying recovery rates are reached.
Key words: sulphide mineral; sorting; biocoagulation
1 Introduction
The idea for the process development follows from identified technological limits of the classic mineral processing. The technical relevant sorting processes, like density, magnetic or electrical separation and flotation respectively, require a narrow particle-size range for a sufficient selectivity in order to eliminate overlapping effects. Because of the rapid decrease of the mass forces with declining particle size, the well-known sorting processes are not applicable to an effective separation of particle sizes smaller than 10 ?m.
For obtaining a selective enrichment and recovery respectively of sorting products in that particle size range, usually chemical dissolving or leaching process is used, which often leads to higher processing costs and negative environmental effects. Moreover, the necessity of sorting processes for finely dispersed solid systems is increasing. Additionally, new sorting processes are useful in the cleaning of wastewater to remove a variety of suspended particles[1].
The approach of using the charge of cell walls of microorganisms for selective coagulation of solid particles on microbes is novel. The project targets differ from well-known biotechnological separation processes like biosorption and bioaccumulation of dissolved substances. Therefore, the new micro-process, named as biocoagulation, aims to attach valuable particles to microorganisms producing larger biocoagulates, which can be separated much easily by applying physical processes (Fig.1).
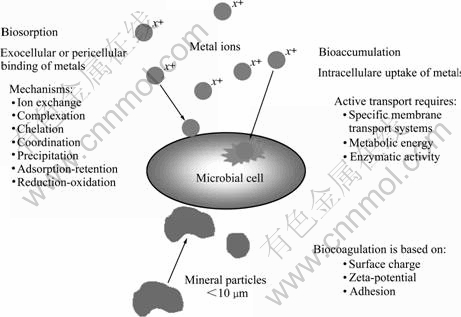
Fig.1 Mechanisms of microbially-mediated transformations
Biosorption and bioaccumulation are based on interactions of microbial cells and soluble metals and metalloids. Microbial cell surface and organelles offer a large number of active functional groups and possible physicochemical mechanisms of interaction. The result is the immobilization of the metals and metalloids on the microbial biomass. Sometimes, the binding is reversible. Biosorption is defined as selective or non selective sequestering of dissolved metals by microbial cells and refers mostly to passive physicochemical mechanisms of inactive (non-metabolizing) metal uptake by microbial biomass. Various mechanisms may occur, e.g. complexation, coordination, chelation, ion exchange, adsorption, microprecipitation and reduction-oxidation [2].
Bioaccumulation is referred to as active absorption (as opposed to the passive physicochemical adsorption- retention assigned to biosorption). Passive adsorption is rapid and independent of the presence of specific nutrients, whereas active absorption is slow and nutrient dependent. Bioaccumulation of metals into a cell generally requires metabolic energy, enzymatic activity and specific transport systems to move material through a cell membrane. It also depends on the tolerance of the microorganisms to the concentration of possibly toxic elements in the intracellular cytoplasm and other subcellular components but may be dependent or independent of metabolism[3].
The design idea to realize a biocoagulation based novel sorting process is composed of three sub processes (Fig.2): 1) selective biocoagulation of microorganisms and fine-sized mineral particles, 2) separation of the biocoagulates out of the slurry, and 3) detachment of microorganisms and particles, aiming to recover and recycle the biomass.
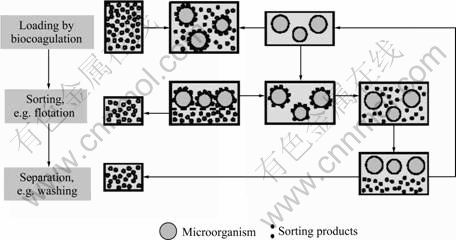
Fig.2 Assumed process design idea
2 Mechanism of biocoagulation
The biocoagulation is based on the interface behaviour of particles, i.e. minerals and microbes, within electrolytes. Commonly, a transition of charge carriers (electrons, ions) takes place between the particle surface and the electrolyte. Thereby, the charge carriers can migrate into the liquid phase or contrarily, they can be adsorbed at the solid phase. Because of the enrichment of charge carriers, an electrochemical bilayer consists of a relatively fixed layer (Stern Layer) and a more diffuse layer (Gouy-Chapman-Layer). The potential existing between the two layers is called Zeta-potential. The pH-value at which the Zeta-potential equals zero is called the isoelectric point(IEP).
Difference in the zeta-potential at the surfaces of minerals and microbes is a measure for the different electric charges that cause the different particles to connect. Therefore, the optimal ambient conditions have to be found in terms of pH-value, ionic strength and composition of the electrolyte.
The essential groups of eukaryotic cell walls are the hydroxyl-, carbonyl-, carboxyl-, amino- and phosphate groups. In dependency on the environmental conditions, these groups have an influence on the electrochemical charge of the cell wall[4-5].
Additionally, the microbes produce extracellular polymeric substances(EPS) that may have an influence on the environmental conditions. They are mostly composed of polysaccharides, proteins, nucleic acids and lipids.
Flotation is a suitable process for the separation of microorganisms. Depending on the type of microorganisms, an attachment between air bubbles and biocoagulates can be expected through natural hydrophoby of microorganisms and biocoagulates respectively without adding any surfactant.
3 Materials and methods
Preliminary tests were carried out with different solid particles and microorganisms. The results presented here are based on the use of the sulphide minerals galena (PbS) and sphalerite (ZnS). Pure minerals were used as test materials. The basic modules of both mineral structures are ions. Both lattices belong to the AB-lattice-type. First of all, the minerals were crushed and milled. Afterwards, the fraction less than 10 ?m was separated.
As microorganisms the yeasts Saccharomyces cerevisiae and Yarrowia lipolytica were chosen. The following growing media were used: 1) Sabouraud- glucose-broth(SGB)-medium for Saccharomyces cerevisiae. The medium consisted of peptones of meat and milk proteins (casein, nitrogen and carbon source) and glucose (energy source, 2%). The pH-value of the used medium was 5.7±0.2. 2) Yeast-extract-peptone- glucose(YEP)-medium for Yarrowia lipolytica. The medium consisted of a mixture of 1% yeast-extract, 2% peptone from casein and 2% glucose.
Microscopic investigations and Zeta-potential analysis were done immediately after adjustment of the defined condition (pH-value, electrical conductivity) for the yeasts, the prepared minerals and the biocoagulates. For those purposes, a light-scattering microscope (Jenoptik) and a Malvern Zeta-sizer were used.
The Malvern Zeta-sizer “Nano Z” uses a combination of electrophoresis and Laser Doppler Velocimetry to determine the Zeta-potential. The principle is the measurement of the particle velocity in a liquid of known ionic strength when applying an electrical field. The relation between velocity and electrical strength is called electrophoretic mobility and can be used to calculate the Zeta-potential. The Zeta- potential of the single minerals, yeasts as well as the Zeta-potential of the biocoagulates at a given pH-value are obtained from the maximum value of the measured values distribution from several tests. Fig.3 shows an example of a Zeta-potential measurement for Yarrowia lipolytica at a pH-value of 2.
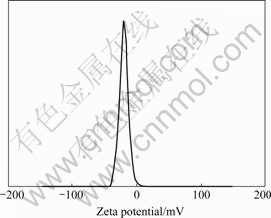
Fig.3 Zeta-potential of Yarrowia lipolytica at pH 2 in 0.03 mol potassium chloride
For separating the biocoagulates from the pulp, column flotation has been used. Column flotation is especially suited to sort the very small biocoagulates, considering low shearing forces between the sulphides and the microorganisms. For the flotation tests, a lab-scale flotation column was built and used for batch tests as well as for continuous flotation tests. The experimental setup is shown in Fig.4.
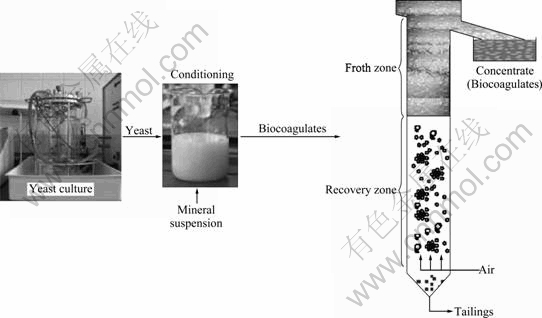
Fig.4 Experimental setup of column flotation
The experimental work was carried out as follows: 1) breeding of the test yeast in an external fermenter, 2) desistance of the yeast and addition of the test mineral, 3) conditioning of the yeast-mineral-suspension to form biocoagulates, 4) feeding the suspension into the flotation column (batch-wise/continuous), 5) flotation by generating air bubbles without adding surfactants, 6) sampling of the concentrate in time intervals, 7) analyses of the concentrates and tailings, and 8) interpretation of the results.
4 Results and discussion
Before the beginning of coagulation tests, growth yeasts. The optimal conditions for the coagulation exponential growth phase, when 108 cells per milliliter medium were counted. At that pint of time, the yeasts were harvested.
At “Biofilm Centre” of University of Duisburg- Essen, the extracellular polymeric substances(EPS) of the yeasts Saccharomyces cerevisiae and Yarrowia lipolytica were analysed. The results are summarized in Table 1.
Table 1 EPS of yeasts Saccharomyces cerevisiae and Yarrowia lipolytica

Hence, Zeta-potential measurements of the single yeasts and biocoagulates at different pH-values were made in addition to measurements of the single sulphide minerals. The isoelectric point of each material used could be determined. Fig.5 shows the results with Yarrowia lipolytica and galena and sphalerite respectively[6].

Fig.5 Zeta-potential of Yarrowia lipolytica, minerals and biocoagulates
The different negative charge at the cell wall allows the coagulation process with the minerals, which was validated by microscopic images (Figs.6 and 7).

Fig.6 Microscopic images of biocoagulates: (a) Saccharomyces cerevisiae and sphalerite (ZnS); (b) Saccharomyces cerevisiae and galenite (PbS); (c) Yarrowia lipolytica and sphalerite (ZnS)
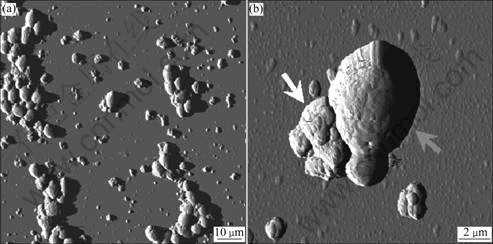
Fig.7 AFM of Saccharomyces cerevisiae and sphalerite
Fig.7 shows images of single biocoagulates taken by means of an atomic force microscope(AFM) provided by the “Biofilm Centre” of University of Duisburg- Essen.
The microscopic analyses show differences between both yeasts. The examinations with sphalerite demonstrate that Saccharomyces cerevisiae adhere at first to larger particles, whereas smaller ones are attached later on. Afterwards, large coagulates and flocks are formed. For Yarrowia lipolytica, it was observed that stress during the coagulation process leads to the forma- tion of hyphae. The optimum results of coagulation were observed in an acid environment. Investigations with mixtures of sphalerite and quartz, galena and quartz, respectively, showed the selectivity of the coagulation process (Fig.8)[7].
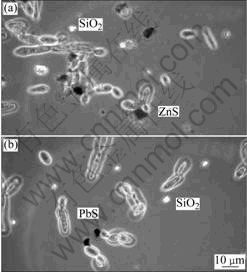
Fig.8 Selective biocoagulation: (a) Yarrowia lipolytica and sphalerite, quartz; (b) Yarrowia lipolytica and galenite, quartz
The results of the flotation tests show that the designed column flotation, depending on the operating conditions, can lead to a recovery in the concentrates up to more than 90% without using any additional chemicals, e.g. collectors. Without additional chemicals, a pH-value of 5 was reached. Fig.9 exemplary shows under these conditions with a constant solids concentration of PbS and a process time of 60 min, a PbS-recovery of 96% with Saccharomyces cerevisiae and of 78% with Yarrowia lipolytica. Thus further tests were carried out with Saccharomyces cerevisiae.

Fig.9 Cumulative recovery of PbS with both yeasts (pH value of 5)
In batch tests the influence of different process parameters on the flotation results, i.e. the recovery of mineral concentrate, was investigated using pure minerals as test material. Fig.10 exemplary shows the cumulative recovery of mineral concentrate and the flotation velocity (?R/?t) under variation of the pH-value and the ratio of mineral mass to cell concentration in the pulp.
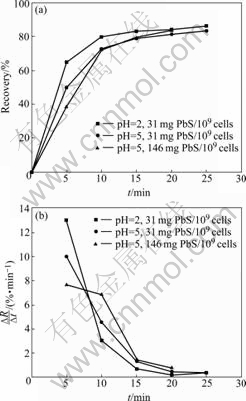
Fig.10 Cumulative recovery of PbS (a) and flotation velocity (?R/?t) (b) in batch tests with different pH-values and varied ratios of mineral mass to cell concentration
Basically, a pH-value of 2 is more suitable for the flotation of the biocoagulates. The flotation results at a pH-value of 5 show no significant decline of the recovery of mineral concentrate (Fig.10). Due to the advantages
regarding the process conditions the continuous, flotation tests were carried out at a higher pH-value of 5.
Fig.11 exemplary shows the cumulative recovery of mineral concentrate by variation of the mineral mass to cell concentration ratio in the pulp. The PbS-recovery can reach up to 94% depending on this ratio. The higher the ratio of mineral mass to cell concentration, the higher the flotation velocity (?R/?t) is as seen in Fig.11. The maximum value of the velocity was found to be after approximately 25 min.

Fig.11 Cumulative recovery of PbS (a) and flotation velocity (?R/?t) (b) with varied ratio of mineral mass to cell concentration
like Saccharomyces cerevisiae and Yarrowia lipolytica respectively, and micro-dispersed solids, e.g. minerals like galena and sphalerite, has been analysed as a basis for a novel sorting process. Therefore, especially the characteristics of the cell surface of the microorganisms, e.g. the electrostatic charge and the composition of extracellular polymeric substances, as well as their influence on the selective biocoagulation were studied.
5 Conclusions
1) Investigations at Technical University of Berlin confirm that the biocoagulation of microorganisms and solid particles would be a new method to generate coarser particles suitable for sorting. The procedure of selective biocoagulation of microorganisms, e.g. yeasts
2) Experimental investigations show that the microorganisms and the sulphide particles below 10 ?m coagulate effectively. Furthermore, flotation is suitable for the separation of the selectively formed biocoagulates. With the designed column flotation, satisfying recovery rates are reached.
Acknowledgement
The research work has been executed as a part of the Integrated EC Project “Biotechnology for Metal Bearing Materials in Europe (BioMinE)”. The authors thank the European Commission for the financial support of the work.
References
[1] GLOMBITZA F, KUYUMCU H Z. Mikrobielles Sortieren. Tagungsband zum 1. Kolloquium Sortieren, Innovationen und Anwendungen, 1999, Berlin: 1-19. (in German)
[2] TSEZOS M. Biohydrometallurgy: From the single cell to the environment [C]// Proceedings of the 17th International Biohydrometallurgy Symposium. Frankfurt am Main, 2007: 589-596.
[3] ROSSI G. Biohydrometallurgy [M]. Hamburg; New York: McGraw-Hill, 1990.
[4] DENGIS P B, ROUXHET P G. Surface properties of top- and bottom-fermenting yeast [J]. Yeast, 1997, 13(10): 931-943.
[5] LIN D Q, BRIXIUS P J, HUBBUCH J J, THOMMES J, KULA M R. Biomass/adsorbent electrostatic interactions in expanded bed adsorption: A Zeta potential study [J]. Biotechnol Bioeng, 2003, 83(2): 149-157.
[6] PINKA J, KUYUMCU H Z, GLOMBITZA F. Untersuchungen zur Sortierung durch Biokoagulation. Tagungsband zum 4. Kolloquium Sortieren, Innovationen und Anwendungen, 2005, Berlin: 113-122. (in German)
[7] KUYUMCU H Z, PINKA J, BIELIG T. Investigations on the sorting of very fine particles by biocoagulation [C]// Proceedings of the 17th International Biohydrometallurgy Symposium. Frankfurt am Main, 2007: 337-340.
Corresponding author: H. Z. KUYUMCU; E-mail: kuyumcu@aufbereitung.tu-berlin.de
(Edited by YANG Bing)