Trans. Nonferrous Met. Soc. China 23(2013) 260-265
Thermodynamic behavior and morphology of impurities in metallurgical grade silicon in process of O2 blowing
Ji-jun WU1,2,3, Wen-hui MA1,2,3, Yan-long LI1,3, Bin YANG1,2, Da-chun LIU1,2, Yong-nian DAI1,2,3
1. The National Engineering Laboratory for Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
2. Yunnan Provincial Key Laboratory of Nonferrous Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
3. Engineering Research Center for Silicon Metallurgy and Silicon Materials of Yunnan Provincial Universities, Kunming 650093, China
Received 1 February 2012; accepted 4 May 2012
Abstract: Gas blowing is a valid method to remove the impurities from metallurgical grade silicon (MG-Si) melt. The thermodynamic behavior of impurities Fe, Al, Ca, Ti, Cu, C, B and P in MG-Si was studied in the process of O2 blowing. The removal efficiencies of impurities in MG-Si were investigated using O2 blowing in ladle. It is found that the removal efficiencies are higher than 90% for Ca and Al and nearly 50% for B and Ti. The morphology of inclusions was analyzed and the phases Al3Ni, NiSi2 and Al3Ni were confirmed in MG-Si by X-ray diffraction. It was found that SiB4 exists in Si-B binary system. The chemical composition of inclusions in MG-Si before and after refining was analyzed by SEM-EDS. It is found that the amount of white inclusion reduces for the removal of most Al and Ca in the forms of molten slag inclusion and the contents of Fe, Ni and Mn in inclusion increase for their inertia in silicon melt with O2 blowing.
Key words: metallurgical grade silicon; thermodynamics; O2 blowing; impurities; inclusion; removal efficiency
1 Introduction
Metallurgical grade silicon (MG-Si) is the raw material of solar grade silicon (SoG-Si) and it is produced with silica and carbon materials in an ore furnace. There are usually Al2O3, Fe2O3, CaO, TiO2, MgO, etc in silica and ash of carbonaceous reducing agent and the impurities in MG-Si are mainly Fe, Al and Ca. It was reported that nearly 100%Fe2O3, 50%-55%Al2O3 and 30%MgO would be reduced after smelting [1-3].
Gas blowing is an important step to remove impurities from MG-Si and obtain SoG-Si. Presently, the requirement for SoG-Si is about a purity of 99.9999% and the methods of producing SoG-Si are mainly Siemens and metallurgical process [4,5]. Compared with Siemens, the metallurgical process is becoming a popular method for its low cost, friendly environment, safety and low energy consumption [6-8]. MG-Si melt is usually secondarily refined by gas blowing with O2, H2 H2O and CO2 etc and some of impurities Fe, Al, Ca, B and P can be pre-removed to a certain extent [9,10]. FLAMANT et al [11] made the purification from MG-Si by a solar process with a flow of Ar and H2O and the contents of B and P were reduced from 5.7×10-6 and 9.4×10-6 to 2.1×10-6 and 3.2×10-6, respectively. In this work, the thermodynamic behavior of impurities in MG-Si was investigated and O2 was blown into MG-Si melt in ladle in order to obtain data for scaling up the process. At the same time, the morphologies of impurities in silicon before and after refining were researched through characterization of inclusions by XRD and SEM-EDS, which is helpful to the impurity removal of MG-Si in theory.
2 Experimental
The trail of impurities removal from MG-Si melt was tried out in ladle (800 kg scale) by O2 blowing (flow rate: 8 L/min) at about 1600 °C for 2 h, which is described in Fig. 1. The compressed O2 was blown into the melt through the pipe at the bottom of ladle. The chemical compositions of silicon samples before and after refining were measured by ICP-AES and the effect of O2 blowing on the impurities removal was studied. At the same time, the phases in silicon samples before and after refining were determined with X-ray diffraction apparatus equipped with a position-sensitive detector ranging 10 ° to 100 ° for 2θ.

Fig. 1 Schematic diagram of MG-Si refining using O2 blowing in ladle
Boron is one of the most difficult impurities to remove from silicon. Its content in MG-Si is commonly (10-50)×10-6, so the morphology is difficult to determine for its trace amount in MG-Si. The phase compositions of Si-B binary system were especially investigated by experiment and it was carried out with following steps. The metallurgical grade silicon powder (99%) and boron powder (99.5%) were firstly mixed with the initial composition in mole ratio of Si to B of 9/1. Then, the mixture was pressed into column and melted at 1450 °C in a graphite crucible overlaid Al2O3 coat in an intermediate frequency inductive furnace for 10 h and then quenched at 1300 °C. Finally, the silicon- boron sample was determined by X-ray diffraction. The experimental process is illustrated in Fig. 2.
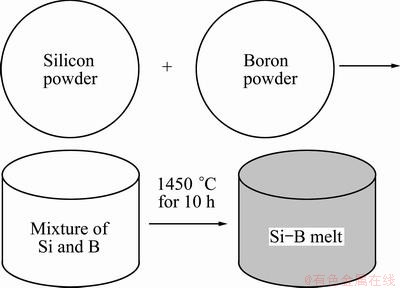
Fig. 2 Schematic diagram of preparing silicon-boron melt sample
3 Results and discussion
3.1 Thermodynamic behavior of impurities in O2 blowing process
Si and other impurities in MG-Si melt will be oxidized into oxides in an oxygen atmosphere and the reactions may be expressed as Eqs. (1)-(3) (Me=Ca, Al, Ti, etc).
[Me]Si+O2→(MexOy) (1)
[Si]+O2→(SiO2) (2)
[Me]Si+(SiO2)→(MexOy)+[Si] (3)
The standard Gibbs free energy changes (ΔGΘ) diagram of oxides for impurities in MG-Si at 1412-1600 °C was obtained and shown in Fig. 3. Though the impurities in MG-Si couldn’t be regarded as pure substance in the process of refining, the order of difficulty for impurities removal might be estimated on the basis of standard Gibbs free energy changes of oxides. It can be concluded that Fe, Cu, V, C and P in MG-Si are difficult to be removed using O2 blowing but it is easy for Ca and Al. It is difficult to forecast the removal of B and Ti because the standard Gibbs free energy changes of their oxides are very approximate to Si.
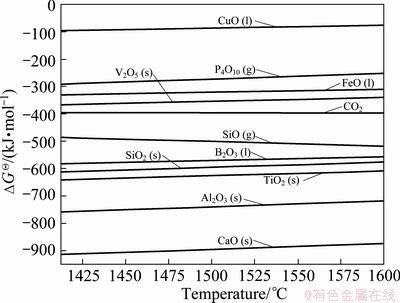
Fig. 3 Standard Gibbs free energy changes of oxides for impurities in MG-Si
3.2 Chemical compositions of MG-Si before and after refining
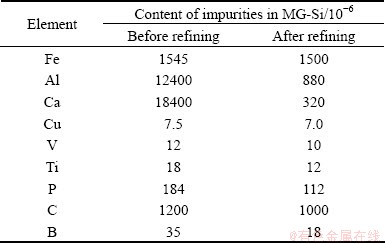
Table 1 shows the chemical compositions of silicon samples before and after refining in ladle. After refining, the contents of impurities Cu, V and C in MG-Si are basically invariable. Although Fe couldn’t be oxidized in silicon in thermodynamics, it also decreases slightly from 1545×10-6 to 1500×10-6, which might be caused by the loss of silicon. Owing to being oxidized strongly, Content of Al and Ca decrease from 1.24% to 880×10-6 and 1.84% to 320×10-6, respectively, and their removal efficiencies are both higher than 90%. P couldn’t be oxidized into P4O10, but its content decreases from 184×10-6 to 112×10-6 for the volatilization of P2 [8]. It is also effective for the removal of B and Ti. Especially, boron content decreases from 35×10-6 to 18×10-6 and it is nearly 50% for removal efficiency. It is found that the experimental results are basically consistent with thermodynamic analysis.
Table 1 Chemical compositions of silicon sample
3.3 Phases in MG-Si
It was reported that the phase structure of impurities in MG-Si had been researched [12,13]. It was thought that the existent morphology of impurities Fe, Al, Ca, Ti, Mn and P in MG-Si has 3 species: 1) Fe-based inclusion, which is consistent with intermetallic compound FeSi in chemical composition, contains 53.3%-56.5%Si and belongs to brittle φ phase; 2) metallic inclusion, which contains Si, Fe, Ti, Mn, V, P and includes approximately 28%Si, 25%Fe, 30%Ti, 0.5%-0.25%Mn, 1%-3%V and 0.5%-1.5%P; 3) molten slag inclusion, which is composed of oxides unreduced in silica and ash in carbonaceous reducing agent. SCHEI et al [14] and GAFFET [15,16] also studied the forms of impurities in MG-Si and the results indicated that Al, Ca and Fe could form the intermetallic compounds FeSi2, CaSi2, AlCaSi2, Al8Fe5Si7 and Al6CaFe4Si8, which will be easily segregated out at the grain boundary during solidification process.
The XRD patterns of silicon samples before and after refining are shown in Fig. 4. It can be seen that Ca exists in the form of CaSi2, which is consistent with SCHEI and MARGARIA’s studies. It is also found that NiSi2 and Al3Ni exist in MG-Si, which are not reported in literatures. Some unknown phases including other impurities have not still been confirmed owing to their low contents in MG-Si. The diffraction peak of SiO2 originating from molten slag inclusion in the refining process is presumably caused by the oxidation of silicon.

Fig. 4 XRD patterns of MG-Si before (a) and after (b) refining using O2 blowing
The Si-B binary phase diagram calculated by the CompuTherm technology (Pandat 7.0) is shown in Fig. 5. There are 6 phases: Liquid, Diamond_Si, SiB3, SiB6, SiBn and β-B in the Si-B binary system. It can be known that the boric phases are Liquid and SiB6 in the range of 1412-1600 °C. The XRD pattern of Si-B melt sample prepared with silicon powder and boron powder shown in Fig. 6 indicates the existence of phase SiB4 in the Si-B binary system.
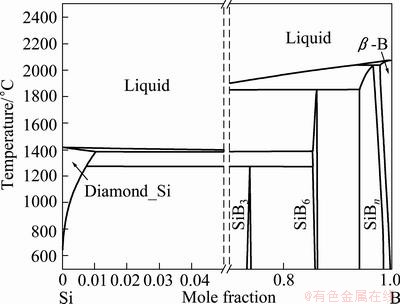
Fig. 5 B-rich and Si-rich sides of Si-B binary system
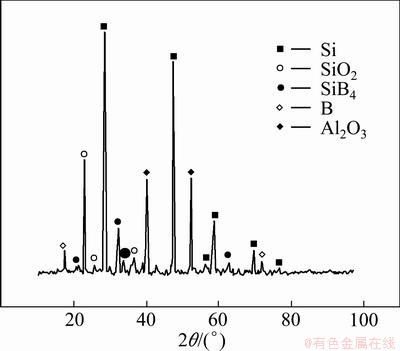
Fig. 6 XRD pattern of Si-B melt sample after quenching
3.4 Morphology and energy spectrum of inclusion in MG-Si
The microstructure indicates that the inclusions in MG-Si might be classified into three species according to colors: 1) light-colored inclusion which has obvious boundary exists at the grain boundary of silicon; 2) dark- colored inclusion which distributes in whole MG-Si is the micro-particles of molten slag; 3) white inclusion which has obvious color feature embeds into the light-colored and dark-colored inclusions. The scanning electron microscopy (SEM) images of sample before and after refining are shown in Fig. 7 and it is indicated that there are a large number of dark, light and white colored inclusions in MG-Si before refining. However, their amounts decrease substantially and the impurities take on light and white colored inclusions after refining while using O2 blowing in ladle.
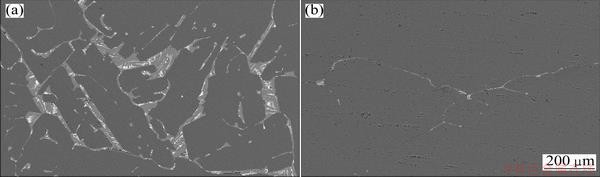
Fig. 7 SEM images of MG-Si samples before refining (a) and after refining (b)

Fig. 8 EDS (a) and element analyses of positions 1 (b), 3 (c), 2 (d), 5 (e) and 4 (f) of inclusions in MG-Si before refining
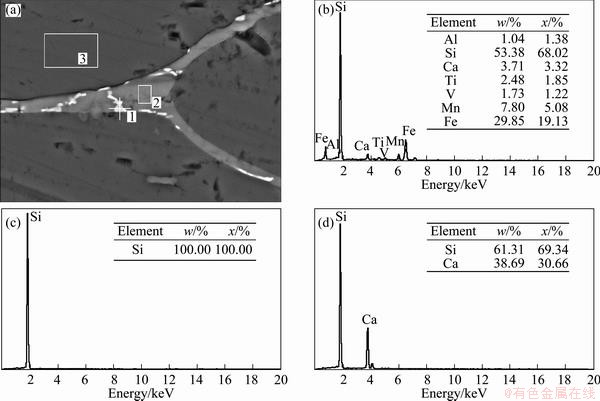
Fig. 9 EDS (a) and element analyses of positions 1 (b), 3 (c) and 2 (d) of inclusions in MG-Si after refining
In order to confirm the chemical compositions of inclusions in MG-Si, the energy dispersive spectra (EDS) of different colored inclusions before and after refining were determined and shown in Fig. 8 and Fig. 9. It can be seen from Fig. 8 that the chemical compositions in white inclusion (position 1) are 41.57%Si, 23.75%Fe, 12.27%Al, 10.6%Ni and a few Ca, Ti, Mn, Ce. The light inclusion (position 2) is composed of 52.25% Si, 14.98% V, 14.56% Ti, 6.64% Fe, 5.21% Mn, 4.22% Al and 2.13% Ca. The white inclusion (position 3) is basically similar to position 1 in composition. The dark inclusion which might be intermetallic compounds is made up of 39.91% Si, 34.35% Al and 25.73% Ca. After refining, the white inclusion (position 1) is less in amount (shown in Fig. 9) but similar in composition compared with before refining (positions 1 and 3) and composed of 53.38%Si, 29.85%Fe, 7.8%Mn and a few Ca, Ti, Al, V. The dark inclusion (position 2) which is probably intermetallic compounds of Si and Ca is composed of 61.31% Si and 38.69% Ca. By comparison, it is found that the white inclusion is mainly Fe-based intermetallic compounds, which contain other impurities Al, Ca, Ni, Mn, etc besides Si and Fe. After refining using O2 blowing, most of Al and Ca are removed by forms of molten slag inclusion, so the amount of white inclusion reduces. The contents of Fe, Ni and Mn in silicon increase for their more positive standard Gibbs free energy changes compared with Si, as shown in Fig. 3. It can be concluded that the light and dark colored inclusions are transformed into white inclusion after refining owing to the removal of Al, Ca, etc.
4 Conclusions
1) The impurity removal from MG-Si is analyzed in thermodynamics and it is obtained by experiments using O2 blowing in ladle. The removal efficiencies are higher than 90% for Ca and Al and nearly 50% for B and Ti. But the removal of Fe, Cu, V and C in MG-Si is basically invalid.
2) The phases NiSi2 and Al3Ni exist in MG-Si. In addition, the phase SiB4 is also found to exist in the Si-B binary system besides SiB3, SiB6, SiBn.
3) The amount of white inclusion reduces for the removal of most Al and Ca by forms of molten slag inclusion and the contents of Fe, Ni and Mn in inclusions increase using O2 blowing in ladle by SEM-EDS determination.
References
[1] Schei A, Tuset J K, Tveit H. Production of high silicon alloys [M]. Trondheim: Tapair Forlag, 1998: 13-16.
[2] Meteleva-Fischer Y V, Yang Y, Boom R, Kraaijveld B, Kuntzel H. Microstructure of metallurgical grade silicon during alloying refining with calcium [J]. Intermetallics, 2012, 25: 9-17.
[3] Liu D H, Ma X D,Du, Y Y, Li T J, Zhang G L. Removal of metallic impurities in metallurgical grade silicon by directional solidification [J]. Materials Research Innovations, 2010, 14: 361-364.
[4] Alemanya C, Trassyb C. Refining of metallurgical-grade silicon by inductive plasma [J]. Solar Energy Materials & Solar Cells, 2002, 72: 41-48.
[5] Koji A, Eichiro O, Hitoshi S, FANG C S, HANSEN K C. Directional solidification of polycrystalline silicon ingots by successive relaxation of supercooling method [J]. Journal of Crystal Growth, 2007, 308(1): 5-9.
[6] Rannveig K,  M, Birgit R. Growth rate and impurity distribution in multicrystalline silicon for solar cells [J]. Materials Science and Engineering A,2005, 413-414: 545-549.
M, Birgit R. Growth rate and impurity distribution in multicrystalline silicon for solar cells [J]. Materials Science and Engineering A,2005, 413-414: 545-549.
[7] Liu L J, Nakano S, Kakimoto K. Carbon concentration and particle precipitation during directional solidification of multicrystalline silicon for solar cells [J]. Journal of Crystal Growth,2008, 310(7-9): 2192-2197.
[8] Miki T, Morita K, Sano N. Thermodynamics of phosphorus in molten silicon [J]. Metallurgical and Materials Transactions B, 1996, 27(12): 937-947.
[9] Nakamura N, Baba H, Sakaguchi Y, Kato Y. Boron removal in molten silicon by a steam-added plasma melting method [J]. Materials Transactions, 2004, 45(3): 858-864.
[10] Morita K, Miki T. Thermodynamics of solar-grade-silicon refining [J]. Intermetallics, 2003, 11(11-12): 1111-1117.
[11] Flamant G, Kurtcuoglu V, Murray J, Steinfeld A. Purification of metallurgical grade silicon by a solar process [J]. Solar Energy Materials and Solar Cells, 2006, 90(14): 2099-2106.
[12] Juneja J M, Mukherjee T K. A study of the purification of metallurgical grade silicon [J]. Hydrometallurgy, 1986, 16(1): 69-75.
[13] Pretorius R. Phase sequence of silicide formation at metal- silicon interfaces [J]. Vacuum, 1990, 41(4-6): 1038-1042.
[14] Schei A, Rong H, Forwald A G. Impurity distribution in silicon, silicon for the chemical industry [M]. Trondheim: Institute of Inorganic Chemistry, 1992: 11-23.
[15] Gaffet E, Malhouroux N, Abdellaoui M. Far from equilibrium phase transition induced by solid-state reaction in the Fe-Si system [J]. Journal of Alloys and Compounds, 1993, 194(2): 339-360.
[16] ABDELLAOUI M, GAFFET E, DJEGA-MARIADASSOU C, BARRADI T. Mossbauer effect evidence for disordering induced by mechanical alloying in the Fe-Si system [J]. J Phys IV France, 1994, 4: 285-290.
冶金级硅吹氧精炼过程中杂质的热力学行为和赋存状态
伍继君1,2,3,马文会1,2,3,李彦龙1,3,杨 斌1,2,刘大春1,2,戴永年1,2,3
1. 昆明理工大学 真空冶金国家工程实验室,昆明 650093;
2. 昆明理工大学 云南省有色金属真空冶金重点实验室,昆明 650093;
3. 昆明理工大学 云南省高校硅冶金与硅材料工程研究中心,昆明 650093
摘 要:吹气精炼是一种从冶金级硅熔体中直接去除杂质的有效方法。研究了冶金级硅中杂质在吹氧过程中的热力学行为,通过与吹氧精炼前的比较,得到了精炼后硅中杂质的去除效率。研究发现,Ca、Al的去除率高于90%,B、Ti的去除率接近50%。通过XRD手段研究了冶金级硅中杂质的赋存状态,并确定了Al3Ni、NiSi2和Al3Ni等金属间化合物的存在。同时实验发现,除了Liquid、Diamond-Si、SiB3、SiB6、SiBn和β-B六个物相外,Si-B二元系中还存在SiB4相。通过SEM-EDS分析了吹O2精炼前、后冶金级硅中夹杂物的化学组成及形态变化。由于精炼过程中大多数Ca、Al以熔渣形式氧化而被去除,硅中白色夹杂的数量大幅度减少,而Fe、Ni、Mn等由于不能氧化而去除,在夹杂物中含量升高。
关键词:冶金级硅;热力学;吹氧;杂质;夹杂;去除率
(Edited by Hua YANG)
Foundation item: Projects (51104080, u1137601) supported by the National Natural Science Foundation of China; Project (2009CD027) supported by the Natural Science Foundation of Yunnan Province, China; Project (14118557) supported by the Personnel Training Foundation of Kunming University of Science and Technology, China
Corresponding authors: Wen-hui MA; E-mail: mwhsilicon@163.com; Ji-jun WU; E-mail: dragon_wu213@126.com
DOI: 10.1016/S1003-6326(13)62454-1