
Thermodynamics and technology of extracting gold from low-grade
gold ore in system of NH4Cl-NH3-H2O
JU Shao-hua(巨少华), TANG Mo-tang(唐谟堂), YANG Sheng-hai(杨声海)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 13 May 2005; accepted 29 August 2005
Abstract: According to the principles of simultaneous chemical equilibrium and electronic charge neutrality, the thermodynamics of Au-NH4Cl-NH3-H2O system was studied by using the exponential computation method and through MATLAB programming, and the solid figure of potential-c(NH4Cl)-c(NH4OH) was drawn. The results show that when the sum concentration of Au+ and Au3+ is equal to 5×10-5 mol/L, φ(Au+/Au) is about -0.2 V; when the sum comes up to 0.5 mol/L, the value of φ(Au+/Au) increases to 0.2 V. In this case, φ(O2/OH-) is as high as 0.7 V. This means that it is feasible to extract gold in this system. In addition, to predict the feasibility of reducing gold from the Au(I)-NH4Cl-NH3-H2O system with copper or zinc powder, the solid figures of potential-c(NH4Cl)-c(NH4OH) for both systems of Cu-NH4Cl-NH3-H2O and Zn-NH4Cl-NH3-H2O were also drawn. The results indicate that both copper and zinc powders can reduce Au+ into metal gold, and zinc powder can also reduce H2O into H2, while copper powder can not. The leaching results of a cuprous gold ore show that the extraction of gold can reach 80% in this system. The preliminary results of reduction with copper and zinc powders show that with deoxygenizing, the reduction effects are relatively good.
Key words: gold; gold extraction; NH4Cl-NH3-H2O; hydrometallurgy; thermodynamics; ammonium chloride
1 Introduction
Hydrometallurgy with ammoniacal ammonium chloride as extracting system is a new developing field with many merits. Above all, NH3 and Cl- ions existing in the system have relatively high complex ability with some heavy or noble metal ions. In addition, the pH value in this system varies from 5.0 to 12.0, so it has better selectivity than the sulfate acid system. Further more, the medium is free of toxicity.
In our previous work, the basic theory of extracting zinc in the system was studied. On this basis, we developed a new process for producing high purity zinc directly from complicated zinc oxide materials[1-4]. WANG[5, 6] also developed a new process of heap leaching and solvent extracting copper from copper oxide ore in NH4Cl-NH3-H2O system.
At present time, the methods for treating gold ores are mainly cyanogens, thiourea, thiosulfate, and halogen approach, among which, the cyanogens approach has the advantages of low cost, high efficiency, and now is the main method for producing gold. However, the effect of leaching complicated ores with this method is very poor, due to its low leaching speed and easy effect by elements such as Cu, Fe, Pb, Zn, S and As. In addition, cyanogens is virulent, and because of the strict requirements of environmental protection by the governments around the world, its prospect is rather faint. For the thiourea approach, it has some advantages such as fast dissolving speed, low toxicity, good selectivity. But its drawbacks, i.e. low stability of thiourea, high cost infeasibility of treating the ores with relatively high alkalinity limit its uses. The thiosulfate approach has the advantages of fast dissolving speed, low toxicity, low sensibility with impurities and high extraction of gold. But due to its high consumption of thiosulfate, difficulty in reutilization, strict operating conditions, high leaching temperature, and pollution by high dosage ammonia, the approach is used seldom. The halogen approach is only used in treating complicated gold ores, which also has the drawbacks such as high cost, high toxicity and high pollution to environment[7-12].
In this paper, according to the principles of simultaneous chemical equilibrium and electronic charge neutrality, the thermodynamics of the system of Au-NH4Cl-NH3-H2O was studied by using exponential computation method proposed by TANG and through MATLAB programming, and the solid figure of potential-c(NH4Cl)-c(NH4OH) was drawn. In addition, to predict the feasibility of reducing gold from the system of Au(I)-NH4Cl-NH3-H2O system with copper or zinc powders, the solid figures of potential-c(NH4Cl)-c(NH4OH) of both systems of Cu-NH4Cl-NH3-H2O and Zn-NH4Cl-NH3-H2O were drawn. Finally, a series of leaching and reducing experiments were conducted.
2 Thermodynamic analysis and model estab- lishment
There are altogether 22 species in the system of Au-NH3-NH4Cl-H2O such as Au(NH3) +, 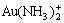 , Au(NH3)3+,
, Au(NH3)3+, 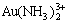 ,
, 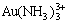 ,
, 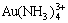 , AuCl(aq),
, AuCl(aq), 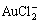 , AuCl2+,
, AuCl2+, 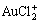 , AuCl3(aq),
, AuCl3(aq), 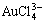 ,
, 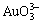 , Au(OH)(s), Au(OH)3(s), Au+, Au3+, Cl-, NH3(aq), NH4+, H+ and OH-.
, Au(OH)(s), Au(OH)3(s), Au+, Au3+, Cl-, NH3(aq), NH4+, H+ and OH-.
Actually, the species such as Au(OH)(s) and Au(OH)3(s) can only be produced when the pH value is very high. In this system, the pH value is kept in the range of 5.0-12.5. So these two species are ignored in this thermodynamic model. While for the species such as Au(NH3), Au(NH3)3+, 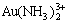 ,
, 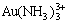 , AuCl(aq) and AuCl3(aq), their stable constants are very small in the ammonia systems, so these species are also ignored in our thermodynamic model. In addition, due to the fact of being hard to exist solely in this system, Au+ and Au3+ are also ignored.
, AuCl(aq) and AuCl3(aq), their stable constants are very small in the ammonia systems, so these species are also ignored in our thermodynamic model. In addition, due to the fact of being hard to exist solely in this system, Au+ and Au3+ are also ignored.
On the basis of the simultaneous equilibrium principle, every aurous complex is in equilibrium with elemental gold in the presence of metallic gold in the system.
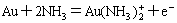 (1)
(1)
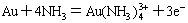 (2)
(2)
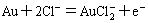 (3)
(3)
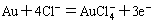 (4)
(4)
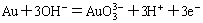 (5)
(5)
According to the exponential computation method proposed by TANG et al[13, 14] and supposition that the activity of each species is equal to its mole concentration, the concentration of these species can be expressed as

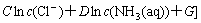 (6)
(6)
where 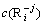 is the concentration of the ions to be calculated; ME =FE/(RT); E is the value of potential; Np=P·ln10; P is the value of pH; c(Cl-) is the concentration of free Cl- ligand in the solution, c(NH3(aq)) is the concentration of free NH3(aq) ligand in the solution; A, B are the numbers of electron and proton obtained or lost by a
is the concentration of the ions to be calculated; ME =FE/(RT); E is the value of potential; Np=P·ln10; P is the value of pH; c(Cl-) is the concentration of free Cl- ligand in the solution, c(NH3(aq)) is the concentration of free NH3(aq) ligand in the solution; A, B are the numbers of electron and proton obtained or lost by a 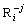 ; C is the number of Cl- ligand; D is the number of NH3(aq) ligand;
; C is the number of Cl- ligand; D is the number of NH3(aq) ligand; 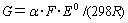 .
.
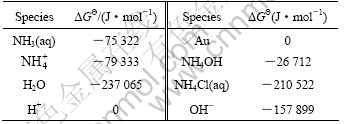
The critical stability constants of aurous complexes, presented in Table 1, were chosen from Ref.[15].The thermodynamic data, presented in Table 2, were chosen from Ref.[16].
Table 1 Critical stability constants of main aurous coordination compounds at 298 K
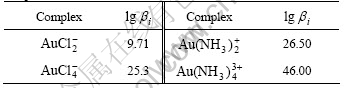
Table 2 Gibbs free energy of related species at 298 K
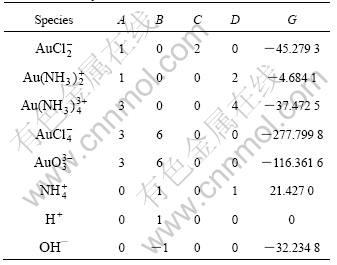
By use of the data presented in Tables 1 and 2, the values of A, B, C, D, G in Eqn.(6) can be calculated as listed in Table 3.
Table 3 Constants in Eqn.(6) for calculating species concentration in system of Au-NH3-NH4Cl-H2O
The sum concentration of aurous, ammonia and chloride can be expressed as Eqns.(7), (8) and (9), respectively.
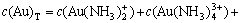
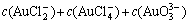 (7)
(7)
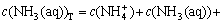
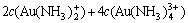 (8)
(8)
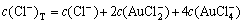 (9)
(9)
According to the principle of electronic charge neutrality, the equation of electronic charge equilibrium can be expressed as
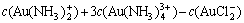 -
-
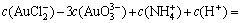
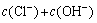 (10)
(10)
Then the model of this system can be set up as a combination of Eqns.(7)-(10).
3 Model solution with MATLAB and discussion
The relation among the seven variables of E, c(Au)T, c(Cl-)T, c(NH3(aq))T, c(Cl-), c(NH3(aq)) and pH is confined by the above model. If three of them are given, the other four variables may be obtained from the above mentioned simultaneous equations by the computation program with MATLAB language compiled by ourselves.
During the actual calculating process, as the values of c(Cl-)T and c(NH3(aq))T are determined by the concentration of the leaching reagent:
c(Cl-)T=c(NH4Cl) (11)
c(NH3(aq))T=c(NH4Cl)+c(NH4OH) (12)
so it is preferable to specify these two values. In addition, if the value of c(Au)T is given, the value of potential which is needed to dissolve c(Au)T can be figured out.
According to the principle above, we worked out the values of potential, when the values of c(Cl-)T and c(NH3(aq))T both vary from 0 to 5.0 mol/L, and the value of c(Au)T is 5.0×10-5 and 0.5 mol/L respectively. Then the solid figure of φ-c(NH4Cl)-c(NH4OH) is drawn as Figs.1 and 2.
In order to see the figure more clearly, Fig.1 is rotated as Fig.2.
It is shown in Figs.1 and 2 that, 1) without ammonium and chloride ions, the potential required for obtaining 5.0×10-5 mol/L aurous Au is very high, while there exist ammonium and chloride ions, the potential decreases to -0.2 V, and as the concen- tration of ammonia increases, the value declines slow- ly; 2) when the concentration of NH4OH is lower than 0.6 mol/L, whatever high the concentration of potential is, the total concentration of aurous Au in the solution can not reach 0.5 mol/L; 3) when the concentration of NH4OH is
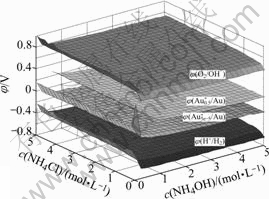
Fig.1 Solid figure of φ-c(NH4Cl)-c(NH4OH) in system of Au- NH3-NH4Cl-H2O:  /Au) is the value of potential needed for obtaining 5×10-5mol/L aurous Au;
/Au) is the value of potential needed for obtaining 5×10-5mol/L aurous Au; 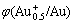 is the value of potential needed for obtaining 0.5×10-5mol/L aurous Au (Note: the first left curve of this figure is got when c(NH4OH)=0.05 mol·L-1, and the same on the following figure.)
is the value of potential needed for obtaining 0.5×10-5mol/L aurous Au (Note: the first left curve of this figure is got when c(NH4OH)=0.05 mol·L-1, and the same on the following figure.)
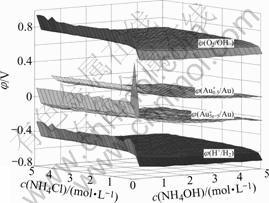
Fig.2 Rotated figure of φ-c(NH4Cl)-c(NH4OH) in system of Au-NH3-NH4Cl-H2O
higher than or equal to 0.6 mol/L, to obtain 0.5 mol/L aurous Au in the solution, the potential is only 0-0.2 V; 4) φ(O2/OH-) is as high as 0.7 V, which means that it is feasible to extract gold in this system.
4 Feasibility of reducing aurous with zinc powder or copper powder
4.1 Thermodynamic analysis of Zn-NH4Cl-NH3-
H2O system
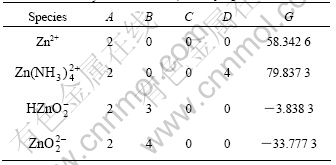
There are altogether more than 20 species in the system of Zn-NH4Cl-NH3-H2O. The four most important species, i.e. Zn2+, 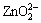 ,
, 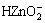 and
and  are taken into consideration, while the other species are omitted. The thermodynamic data for calculation are found out and listed in Table 4.
are taken into consideration, while the other species are omitted. The thermodynamic data for calculation are found out and listed in Table 4.
Table 4 Critical stability constants of main Zn coordination compounds at 298 K and Gibbs free energy of related species at 298 K
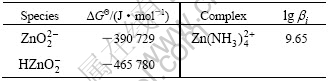
The data of other species needed such as NH3(aq), 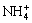 , H2O, OH- and H+ are shown in Table 2.
, H2O, OH- and H+ are shown in Table 2.
By using the data in Table 4, the values of A, B, C, D and G for this system in Eqn.(6) are calculated and listed in Table 5.
Table 5 Constants in Eqn.(6) for calculating species concentration in system of Zn-NH4Cl-NH3-H2O
The sum concentrations of zinc, ammonia and chloride can be expressed as
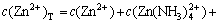
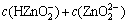 (11)
(11)
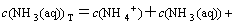
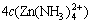 (12)
(12)
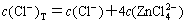 (13)
(13)
According to the principle of electronic charge neutrality, the equation of electronic charge equilibrium can be expressed as
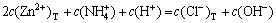 (14)
(14)
Then the model of this system can be set up as a combination of Eqns.(11)-(14).
4.2 Thermodynamic analysis of Cu-NH4Cl-NH3- H2O system
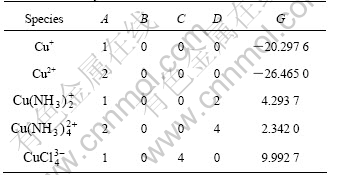
There are altogether more than 26 species in the system of Cu-NH4Cl-NH3-H2O. The five most important species, i.e. Cu+, Cu2+,  ,
, 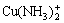 and
and 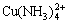 are take into consideration, while other species are omitted. The thermodynamic data for calculation are found out and listed in Table 6.
are take into consideration, while other species are omitted. The thermodynamic data for calculation are found out and listed in Table 6.
The data of other species such as NH3(aq), 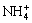 , H2O, OH- and H+ are already listed in Table 2.
, H2O, OH- and H+ are already listed in Table 2.
By using the data in Table 4, the values of A, B, C, D and G for this system in Eqn.(6) are calculated and listed in Table 7.
Table 6 Critical stability constants of main Cu coordination compounds at 298 K and Gibbs free energy of related species at 298 K
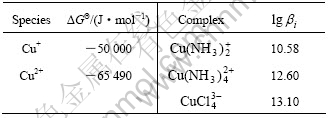
Table 7 Constants in Eqn.(6) for calculating species concentration in system of Cu-NH4Cl-NH4-H2O
The sum concentrations of copper, ammonia and chloride can be expressed as
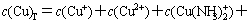
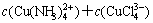 (15)
(15)
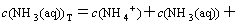
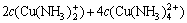 (16)
(16)
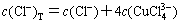 (17)
(17)
According to the principle of electronic charge neutrality, the equation of electronic charge equilibrium can be expressed as
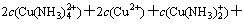
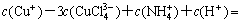
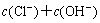 (18)
(18)
Then the model of this system can be set up as a combination of Eqns.(15)-(18).
4.3 Solution of two models with MATLAB and discussion
Supposing the values of c(NH4Cl) and c(NH4OH) both varying from 0 to 5.0 mol/L, and specifying c(Cu)T and c(Zn2+)T both equal to 0.01 mol/L, the potential of solution can be figured out with MATLAB programming. The results are shown in Figs.3 and 4.
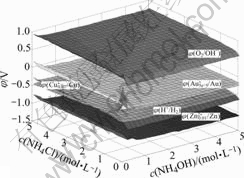
Fig.3 φ-c(NH4Cl)-c(NH4OH) in system of Au-Cu-Zn-NH3- NH4Cl-H2O
It is shown in Figs.3 and 4 that, in this system, φ(Cu+/Cu) is about -0.5 V; φ(Zn2+/Zn) is about -1.0 V; φ(O2/OH-) is about 0.7 V; φ(H+/H2) is about -0.5 V. Thus, both copper and zinc powders can reduce Au+ into metal gold, and zinc powder can also reduce H2O into H2, while copper powder can not. In addition, because the potential of oxygen is much higher than those of Au+, Cu+, or H2. This means that it is better to deoxygenize at the beginning of reduction.
5 Experiments
5.1 Result of leaching experiments
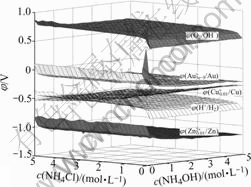
Fig.4 Rotated figure of φ-c(NH4Cl)-c(NH4OH) in system of Au- Cu-Zn-NH3-NH4Cl-H2O
A low grade cupreous gold ore taken from Gansu Province was used in the following leaching experiments. Its chemical composition is shown in Table 8.
The phase composition analysis show that, the copper exists in a mixture of oxide and sulfide ore in the ore, and gold exists as tiny particles wrapping in gangues.
The leaching results are shown in Table 9.
5.2 Preliminary result of reduction experiments
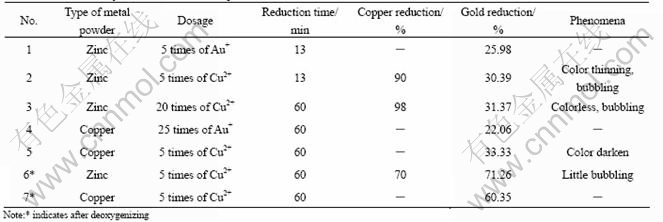
Using the solution gained from the above experiments, whose composition is listed in Table 10, the reduction with copper powder and zinc powder were conducted and the results are shown in Table 11.
From Table 11, it is shown that the reduction of Au+ is very low. This is mainly because that the oxygen dissolved in the solution is competitive with Au+ in the reduction process. So, deoxygenizing is of great importance in this reduction process.
Table 8 Chemical composition of low grade cupreous gold ore (mass fraction, %)

Table 9 Leaching results of low grade gold ore in system of NH4Cl-NH3-H2O
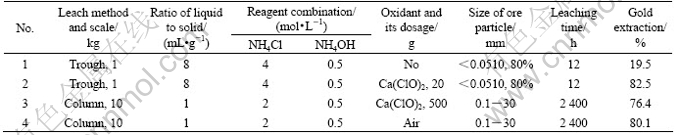
Table 10 Ion concentration of solution used in reduction experiments (mg·L-1)

Table 11 Preliminary results of reduction experiments
6 Conclusions
1) The thermodynamics study of the system Au- NH3-NH4Cl-H2O shows that when the solution potential is higher than -0.2 V, the equilibrium con- centration of Au in solution can reach 5×10-5 mol/L (about 10 mg/L); when the concentration of NH4OH is larger than 0.6 mol/L, and the potential of solution is greater than 0.2 V, the equilibrium concentration of Au in solution can reach 0.5 mol/L; φ(O2/OH-1) in this system is about 0.7 V, which ensures that oxygen can easily oxidize element Au into solution. Therefore, a low cost and non cyanogens process for extracting gold from low grade ores is proposed.
2) The thermodynamic study of the system of Au-Zn-Cu-NH3-NH4Cl-H2O shows that when c(Cu)T and c(Zn2+)T are both equal to 0.01 mol/L, φ(Cu+/Cu) is about -0.5 V; φ(Zn2+/Zn) is about -1.0 V; φ(O2/OH-) is about 0.7 V; φ(H+/H2) is about -0.5 V. Thus, both copper and zinc powders can reduce Au+ into metal gold, and zinc powder can also reduce H2O into H2, while copper powder can not.
3) The leaching results of a cuprous gold ore show that the extraction of gold can reach 80% in this system. The preliminary results of reduction with copper and zinc powders show that deoxygenizing is of great importance for the reduction process.
References
[1] YANG Sheng-hai, TAN Mo-tang. Thermodynamics of Zn(Ⅱ)-NH3- NH4Cl-H2O system[J]. Trans Nonferrous Met Soc China, 2000, 10(6): 830-833.
[2] YANG Sheng-hai, TANG Mo-tang, HE Jing, YAO Wei-yi. Preparation of high-purity zinc from zinc calcine by an ammonia process[J]. Journal of Jishou University (Natural Science Edition), 2003, 24 (3): 45-49. (in Chinese)
[3] YANG Sheng-hai, TANG Mo-tang, DENG Chang-xun, ZHANG Shun-ying. Making high purity zinc from zinc oxide fume dusts[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(6): 1110-1113.(in Chinese)
[4] YANG Sheng-hai, TANG Mo-tang, CHEN Yi-feng, TANG Chao-bo, HE Jing. Anodic reaction kinetics of electrowinning system of Zn(Ⅱ)-NH3 -NH4Cl-H2O[J]. Trans Nonferrous Met Soc China, 2004, 14(3): 626-630.
[5] WANG Cheng-yan. Exploitation of rebellious low grade copper [J]. Mining & Metallurgy, 2001, 10(4): 49-53.(in Chinese)
[6] WANG Cheng-yan. Solvent extraction study on high alkali and low grade copper oxide ore [J]. Nonferrous Metals (Metallurgy part), 2003(3): 2-7. (in Chinese)
[7] SUN Chuan-yao, QIAO Fan-sheng, ZHAO Yong-quan. Directory for Process of Gold Producing [M]. Beijing: Geology Press, 2001. 75-81. (in Chinese)
[8] JIANG Tao. Chemistry of Extractive Metallurgy of Gold [M]. Hunan: Hunan Science & Technology Press, 1998. (in Chinese)
[9] Fujita T, Kejun, L, Shibayama A, et al. Gold leaching by using ammonium thiosulfate solution and gold recovery by solvent extraction and cementation[A]. Yazawa International Symposium. Metallurgical and Materials Processing: Principles and Technolo- gies[C]. San Diego: TMS. 2003. 293-305.
[10] LIU K J, YEN W T, SHIBAYAMA A, MIYAZAKI T, FUJITA T, ALGUACIL F J. Gold extraction from thiosulfate solution using trioctylmethylammonium chloride[J]. Hydrometallurgy, 2004, 73(1): 41-53.
[11] Navarro P, Vargas C, Villarroel A, ALGUACIL F J. On the use of ammoniacal/ammonium thiosulphate for gold extraction from a con- centrate[J]. Hydrometallurgy, 2002, 65(1): 37-42.
[12] CELIK H. Extraction of gold and silver from a Turkish gold ore through thiourea leaching[J]. Minerals and Metallurgical Processing, 2004, 21(3): 144-148.
[13] TANG Mo-tang, ZHAO Tian-cong. A thermodynamic study on the basic and negative potential fields of the systems of Sb-S-H2O and Sb-Na-S-H2O [J]. J Cent South Inst Min Metall, 1988, 19(1): 35-43. (in Chinese)
[14] TANG Mo-tang, ZHAO Tian-cong, LU Jun-le. Principle and application of the new chlorination-hydrolization process [J]. J Cent South Inst Min Metall, 1992, 23(4): 405-411. (in Chinese)
[15] Smith R M, Martell A E. Critical Stability Constants [M]. New York: Plenum Press, 1976(4).
[16] Yawaza A. Hitetsu Kinzoku Seiren [M]. Sendai: Japan Institute of Metal, 1980. 322-324.
Corresponding author: JU Shao-hua; Tel: +86-731-8830470; E-mail: shj_200801@sina.com
(Edited by YUAN Sai-qian)