DOI:10.19476/j.ysxb.1004.0609.2018.10.16
锌精矿与锌浸渣协同浸出及氧化转化行为
付中梦,邓志敢,魏 昶,李兴彬,李存兄,樊 刚
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:锌精矿与锌浸渣协同浸出过程中利用Fe3+与锌精矿发生氧化还原反应,实现锌精矿与锌浸渣的同步溶解,且缓解溶液中高浓度Fe3+对锌浸渣溶解的抑制作用。对渣矿协同浸出的矿物溶解行为进行研究,同时也以单一矿物锌精矿为研究对象,研究了其在H2SO4-Fe2(SO4)3体系中的氧化转化行为。结果表明:渣矿协同浸出能有效提高有价金属的浸出率,且浸出液中Fe3+含量较低,便于后续处理;根据XRD、SEM和XPS分析,锌精矿在氧化转化过程中不断溶解,锌精矿中的硫主要被氧化成单质硫进入渣中,且单质硫在矿物颗粒表面形成包裹,使其溶解不充分。
关键词:锌精矿;锌浸渣;协同浸出;氧化转化
文章编号:1004-0609(2018)-10-2086-08 中图分类号:TF813 文献标志码:A
从锌精矿中提取锌的传统工艺流程包括,硫化锌精矿的焙烧,焙砂的浸出,含锌溶液的净化与电解沉积(焙烧-浸出-电解)[1-6]。然而,该工艺难以处理杂质铁含量高的锌精矿,且在焙烧过程生成对环境不友好的SO2气体。硫化锌精矿的湿法浸出工艺能够消除焙烧过程中SO2污染环境的问题。为此,许多研究人员在碱性介质中或在硝酸、盐酸、硫酸等酸性介质中进行了锌精矿的浸出研究以及氧化剂的研究,例如三价铁离子(Fe3+)[7-9]。在硫酸体系中,Fe3+是重要的氧化剂,不仅能浸出硫化锌中的锌,同时硫化矿中的硫将以单质硫的形式存在于渣中[10-11]。
热酸浸出工艺处理锌浸渣时,铁酸锌的溶解使得大量的Fe3+进入溶液中,使得溶液的氧化还原电位升高,会抑制铁酸锌的溶解,使得铁酸锌不能持续地溶解[12]。对于锌浸渣中有价金属的回收国内外学者进行了一定的研究[13-16]。张帆等[17-18]利用Fe3+与硫化锌的氧化还原反应,提出了锌中性浸出渣与锌精矿协同浸出,该方法不仅能缓解溶液中高浓度Fe3+对铁酸锌溶解的抑制作用,同时能溶解锌精矿,实现了锌浸渣和锌精矿的同步浸出的效果[19]。通过实验分析,锌精矿在协同浸出过程中溶解率只有60%左右。本文作者研究了锌精矿与锌浸渣协同浸出,同时又以锌精矿单一物相为研究对象,在H2SO4-Fe2(SO4)3体系下,研究协同浸出过程中锌精矿氧化转化过程中的溶解行为。
1 实验
1.1 实验原料
实验所用的锌浸渣、锌精矿与闪锌矿均来自云南某炼锌厂,主要化学成分如表1所列,XRD分析结果如图1所示。
由图1可知,锌浸渣的主要物相为铁酸锌,以及少量硅酸锌,锌精矿的主要物相为闪锌矿和铁闪锌 矿,闪锌矿的主要物相为ZnS。实验所用的矿物粒度均为<74 mm。
1.2 实验方法
本实验在一个1 L的防爆双层玻璃反应釜(EXs212-1L)中进行,设置反应温度及搅拌转速。将浸出剂加入到反应釜中,当到达反应温度时,将矿物加入玻璃反应釜中并开始搅拌,反应过程需要冷却装置进行冷却。反应过程中进行过程取样。所有矿浆经过液固分离后获得滤液和滤渣。对浸出液和浸出渣进行成分分析和物相分析。
表1 实验原料的主要化学成分
Table 1 Main composition of experimental raw materials
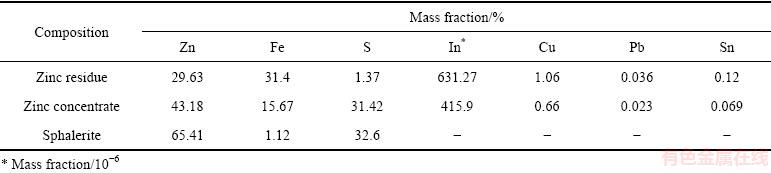
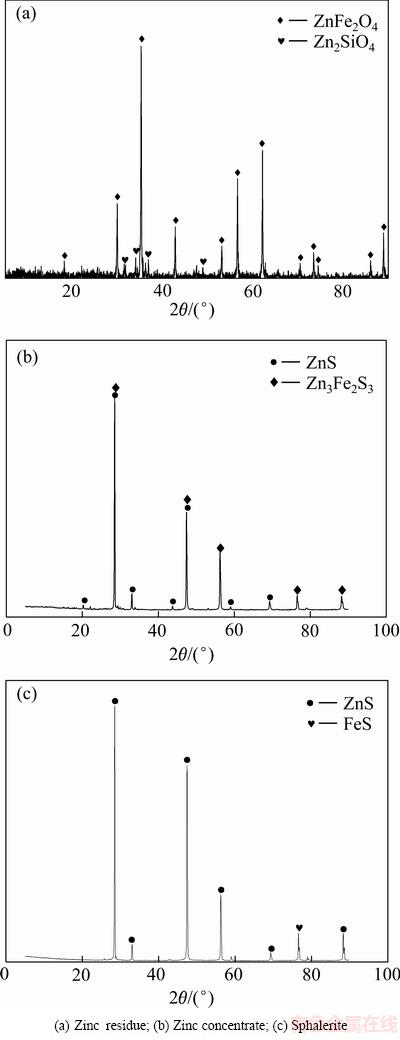
图1 实验原料的XRD谱
Fig. 1 XRD patterns of experimental raw materials
2 结果与分析
2.1 锌精矿与锌浸渣协同浸出
在搅拌转速400 r/min、反应温度90 ℃、初始硫酸浓度160 g/L及锌精矿与锌浸渣质量比值为1的条件下,浸出时间对浸出率的影响如图2所示。
由图2可知,随着反应时间的延长,锌、铁和铟的浸出率升高,其浸出率分别达到90.8%、89.4%和89.1%。协同浸出终渣含锌9.14%、铁10.62%、铜0.328%、铟176.6×10-6,由此可知,浸出终渣中也有部分的有价金属未溶解。对浸出终渣进行XRD分析,结果如图3所示。浸出渣中的主要物相为单质硫、闪锌矿和铁闪锌矿;XRD图中并未发现铁酸锌的特征衍射峰。结合图1 中锌浸渣和锌精矿的XRD检测可知,在浸出过程中,锌浸渣中难溶的铁酸锌物相基本全部溶解;锌精矿有部分未溶解而残留在协同浸出渣中;锌精矿中的硫主要被氧化成单质硫而进入浸出渣中。由表2可知,渣中的锌主要以硫化锌为主,为未反应的锌精矿,锌中浸渣几乎完全溶解。
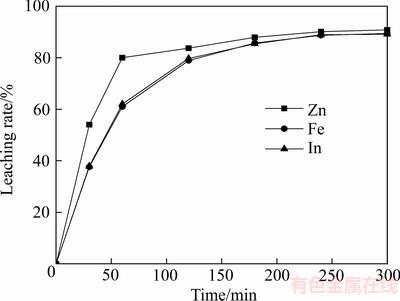
图2 锌浸渣-锌精矿协同浸出结果
Fig. 2 Simultaneous leaching results of neutral-leach residue and zinc concentrate
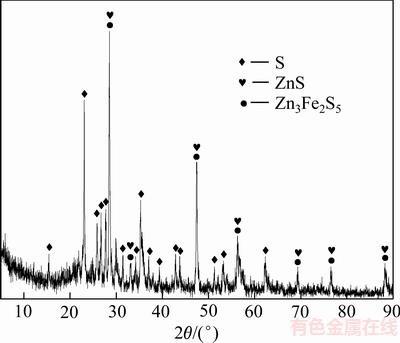
图3 协同浸出渣的XRD谱
Fig.3 XRD pattern of simultaneous leaching residue
表2 协同浸出渣中锌物相分析
Table 2 Phase composition of zinc in simultaneous leaching residue
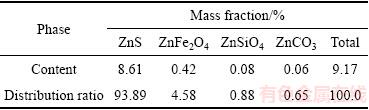
协同浸出过程中,浸出液中Fe3+、Fe2+浓度随时间的变化情况如图4所示。由图4可知,随着反应时间的延长,浸出液中Fe2+浓度逐渐升高,而溶液中Fe3+浓度则呈现先升高后降低的趋势;协同浸出液中的铁离子主要以Fe2+形式存在,Fe3+含量较低。
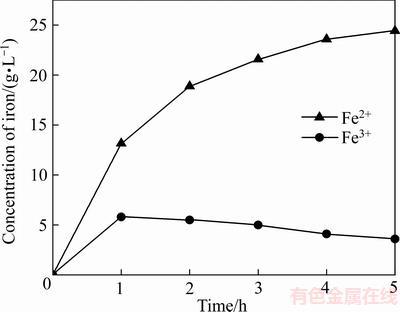
图4 浸出时间对溶液中Fe3+、Fe2+浓度的影响
Fig. 4 Effect of leaching time on concentration of iron
随着锌浸渣的溶解,大量的Fe3+进入溶液。在硫酸溶液中,Fe3+与锌精矿发生反应:
Fe2(SO4)3(aq)+ZnS(s)=2FeSO4(aq)+ZnSO4(aq)+S(s) (1)
Fe2(SO4)3(aq)+FeS(s)=3FeSO4(aq)+S(s) (2)
由反应式可知,锌精矿与Fe3+发生氧化还原反应,将溶液中的Fe3+还原成Fe2+,同时生成单质硫。在反应初期,锌浸渣中铁酸锌的溶解速率要大于Fe3+与锌精矿的反应速率;在反应后期,随着锌中浸渣的铁酸锌逐渐溶解完全,溶液中Fe3+与锌精矿的氧化还原反应成为该体系中的主导反应[14]。
2.2 锌精矿的氧化转化
由锌精矿和锌浸渣协同浸出可知,锌浸渣中的铁酸锌几乎全部溶解,而锌精矿则有部分未能溶解,进入渣中。为了探究锌精矿在协同浸出的氧化转化行为,在H2SO4-Fe2(SO4)3体系中,针对锌精矿的氧化转化行为展开实验研究。
在搅拌转速400 r/min、反应温度90 ℃、初始硫酸浓度160 g/L、初始Fe3+浓度70 g/L、液固比(mL/g) 20:1的条件下,浸出时间对锌、铁、铟的浸出率的影响结果如图5所示。
由图5锌精矿的氧化浸出结果,可知,锌、铁、铟的浸出率随时间的变化规律可以分为两部分。第一部分,即反应时间为0~60 min这一段,在此时间范围内,金属浸出率上升速度很快。而在第二阶段,金属浸出速率上升速度较慢。
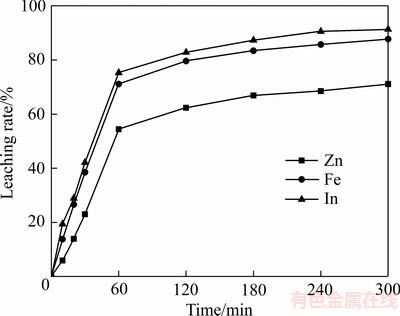
图5 锌精矿氧化浸出结果
Fig. 5 Result of oxidation leaching of zinc concentrate
反应过程中,溶液中Fe3+、Fe2+浓度随反应时间变化的结果如图6所示。由图6可知,在反应初期,溶液中Fe3+浓度随反应时间的延长而减少,Fe2+浓度而逐渐增加,在反应后期,溶液中Fe3+、Fe2+浓度的变化会逐渐变缓,在反应后期,Fe3+、Fe2+浓度的变化基本不明显。在硫酸溶液中,Fe3+具有氧化性,能够与锌精矿中的硫化锌发生氧化还原反应:
2Fe3++ZnS=Zn2++2Fe2++S0 (3)
使锌精矿中的锌进入溶液,而S2-被氧化成单质硫进入渣中,同时将溶液中的Fe3+还原成Fe2+。因此随着反应时间的延长,溶液中Fe3+逐渐被还原成Fe2+,Fe3+浓度逐渐减少,而Fe2+浓度逐渐增加。
图6 反应时间对溶液中Fe3+、Fe2+浓度的影响
Fig. 6 Effect of leaching time on concentrations of Fe3+ and Fe2+ in solution
对反应过程中不同时间的渣样进行XRD分析,结果如图7所示。由图7可知,随着反应时间的延长,浸出渣中闪锌矿物相的特征衍射峰强度逐渐减弱,而新生物相单质硫的特征衍射峰强度逐渐增强。这说明随着反应过程的进行,锌精矿不断被分解,锌精矿中的硫被氧化成单质硫,单质硫是锌精矿最主要的氧化产物。
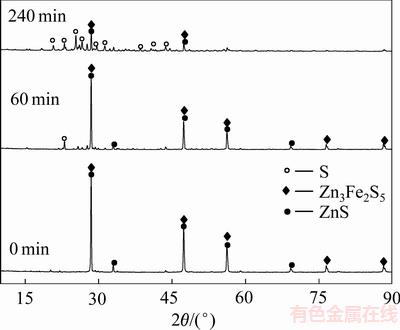
图7 锌精矿浸出不同时间后的XRD谱
Fig. 7 XRD patterns of leached zinc concentrate at different time
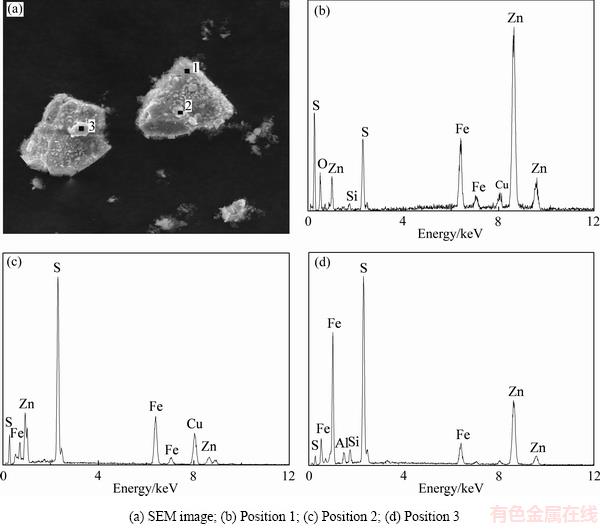
图8 浸出渣的SEM像及EDS谱
Fig. 8 SEM image of leaching residue and EDS spectra of positions
对浸出终渣进行扫描电镜能谱分析,结果如图8所示。由图8(a)可见,矿物表面附着许多细小颗粒,矿物边缘有一些细小的絮状颗粒,也有许多絮状颗粒所形成的大颗粒。通过EDS分析,得知该矿物为闪锌矿(见图8(b)),那些细小颗粒的主要成分为硫(见图8(c)和(d))。结合图5锌精矿的浸出结果,可知在反应后期,锌精矿溶解速率趋于平缓,有价金属的浸出率也未明显增加。根据分析结果可知,氧化产物单质硫颗粒附着在矿物表面,使得矿物颗粒与浸出剂不能充分接触,导致反应后期矿物溶解缓慢。
2.3 闪锌矿的氧化转化
由图5所示锌精矿氧化浸出结果可知,在锌精矿氧化转化过程中,锌精矿中的铁浸出率达到90%以上,铁基本全部溶出,可知在锌精矿氧化浸出过程中,对其溶解过程造成影响的主要还是锌的浸出。因此,以Fe含量只有1%左右的闪锌矿为研究对象,研究其氧化转化过程的行为。
在搅拌转速400 r/min、反应温度90 ℃、初始硫酸浓度160 g/L、初始Fe3+浓度70 g/L、液固比(mL/g) 20:1的条件下,闪锌矿的溶解行为如图9所示。在反应开始1 h,锌的浸出率上升速度很快。随着反应时间的延长,锌浸出速率上升速度较慢。在反应开始1 h,大约50%的金属被浸出。该浸出规律与锌精矿的氧化转化规律相一致。
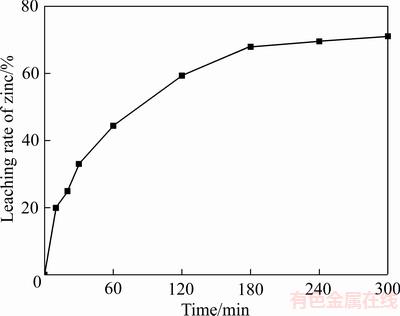
图9 闪锌矿氧化浸出结果
Fig. 9 Result of oxidation leaching of sphalerite
对不同反应时间的浸出渣进行了XRD检测,其结果如图10所示。
由XRD谱(见图10)可知,闪锌矿存在的主要物相为硫化锌以及少量的磁黄铁矿。随着反应时间的延长,硫化锌的特征衍射峰的强度则在逐渐减弱,而新生物相单质硫的特征衍射峰则不断增强。随着反应时间的延长,硫化锌物相则不断地与溶液中的Fe3+发生氧化还原反应,硫化锌物相不断溶解,而氧化产物单质硫则不断生成。结合锌浸出率曲线图(见图9)可知,在反应4 h后,延长反应时间至5 h,金属锌的浸出率并未能发生大的改变,浸出率曲线趋于平缓。随着氧化产物单质硫的不断生成,单质硫颗粒附着在闪锌矿颗粒表面,阻碍了矿物颗粒与浸出剂的进一步接触反应,使得在反应后期,闪锌矿的溶解速率变缓,锌的溶解率趋于不变。
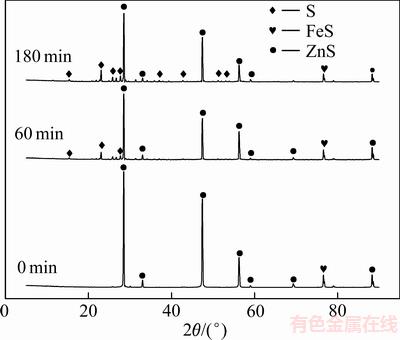
图10 闪锌矿浸出不同时间后的XRD谱
Fig. 10 XRD patterns of leached sphalerite at different time
2.4 渣矿协同浸出与锌精矿氧化转化
在锌精矿与锌中浸渣协同浸出过程中,溶液氧化还原电位随反应时间的变化如图11所示。在协同浸出过程中,溶液氧化还原电位随反应时间的延长而降低,溶液中氧化还原电位主要受溶液中[Fe3+/Fe2+]比值高低的影响[20]。
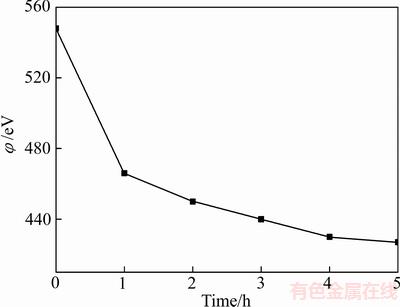
图11 协同浸出过程中溶液氧化还原电位随反应时间的变化
Fig. 11 Variation of oxidation-reduction potential(φ) of solution with time during simultaneous leaching process
结合图4可知,在渣矿协同浸出过程中,浸出液中Fe2+浓度随反应时间的延长而逐步升高,而溶液中Fe3+浓度在反应1 h后,随反应时间的延长而逐步降低,故溶液中氧化还原电位随反应时间的延长而降低。通过查阅锌浸渣浸出的热力学数据[20]可知,溶液的氧化还原电位越低,越有利于铁酸锌的溶解。在协同浸出前期,锌浸渣中的铁酸锌不断溶解,大量的Fe3+进入溶液中,使溶液的氧化还原电位升高,阻碍铁酸锌进一步溶解;添加的锌精矿作为还原剂,可还原溶液中的Fe3+,使得溶液中Fe2+浓度升高,降低了溶液中的氧化还原电位,同时能够使得铁酸锌持续溶解,从而实现锌浸渣和锌精矿的协同浸出。
以锌精矿单一矿物为研究对象,对其在H2SO4- Fe2(SO4)3体系中的氧化转化行为展开实验研究。对浸出前及浸出30 min的锌精矿和浸出终渣进行XPS检测,对获得的结果与电子结合能数据[21]进行对比分析,结果如图12所示。
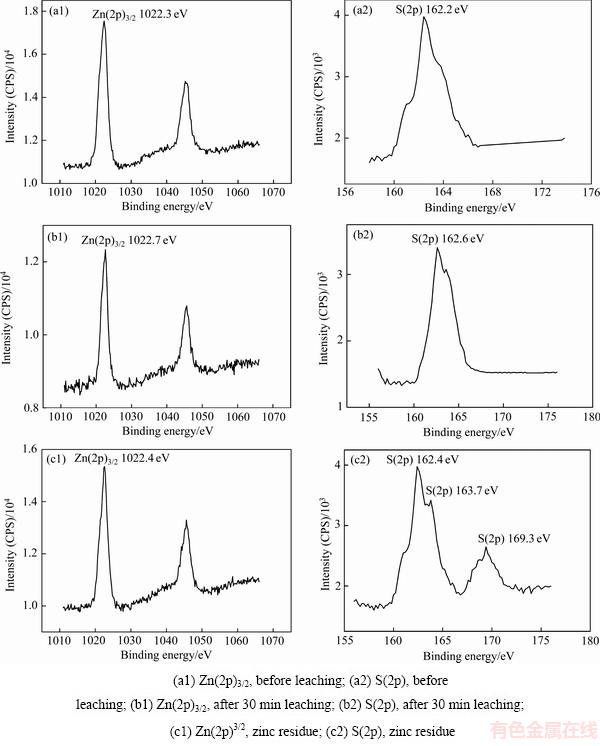
图12 锌精矿颗粒表面锌和硫的XPS分析
Fig. 12 XPS patterns of Zn(2p) and S(2p) on surface of zinc concentrate particle
浸出前及浸出30 min和浸出终渣,锌精矿矿物颗粒表面Zn(2p)3/2结合能分别为1022.3、1022.7和1022.4 eV。由此可知,锌是以Zn—S键存在的,即是未溶解的硫化物。说明在锌精矿氧化转化过程中,元素锌仅发生溶解反应,溶解锌能稳定的存在于溶液中。
浸出前及浸出30 min后,S(2p)结合能为162.2 eV和162.6 eV。经查阅的相关数据[21]进行对比可知,矿物颗粒表面的硫主要是以Me—S键的形式存在的。反应30 min 后,其表面未见明显的有S—S键形式的硫存在,由此可得出结论:在锌精矿氧化转化的初期,锌精矿中的硫直接转化成单质硫的反应较为滞后,单质硫直接转化生成的量较少,而锌精矿的直接酸溶反应是反应初期的主要反应。结合能161.8 eV峰值的出现表明了,这部分的硫为未溶解的硫化物;峰值163.7 eV的出现则表明了,这部分硫为单质硫。故在锌精矿溶解反应的后期,锌精矿中的硫被氧化生成了单质硫,这与图7中浸出终渣XRD的检测结果相一致,确实有大量的单质硫生成。
随着反应时间的延长,锌精矿的硫不断被氧化成单质硫,结合XPS分析,氧化产物单质硫在浸出渣颗粒表面形成了包裹,阻碍了锌精矿与浸出剂充分接触,使得反应后期金属浸出率增加缓慢,锌精矿也未能够充分溶解。
3 结论
1) 在渣矿协同浸出过程中,铁酸锌溶解产生Fe3+,利用Fe3+的氧化性,与锌精矿发生氧化还原反应,不仅能够缓解高浓度Fe3+对锌浸渣溶解的抑制作用,而且实现了锌精矿中有价金属的同步溶解。
2) 渣矿协同浸出时,锌精矿的利用率较低。针对锌精矿单一矿物的氧化转化行为进行研究。锌精矿在氧化转化过程中,锌精矿不断溶解,大量有价金属溶出,氧化产物单质硫不断生成,同时在矿物颗粒表面形成包裹,阻碍了锌精矿与浸出剂的充分接触,这是造成锌精矿在反应过程中未能溶解完全的主要因素。
REFERENCES
[1] 魏 昶, 李存兄. 锌提取冶金学[M]. 北京: 冶金工业出版社, 2013: 15.
WEI Chang, LI Cun-xiong. Zinc extraction metallurgy[M]. Beijing: Metallurgical Industry Press, 2013: 15.
[2] 田 磊, 张廷安, 吕国志, 刘 燕, 周 双, 张伟光, 张国权. 机械活化对闪锌矿物化性质及焙烧动力学的影响[J]. 中国有色金属学报, 2015, 25(12): 3535-3542.
TIAN Lei, ZHANG Ting-an, Lü Guo-zhi, LIU Yan, ZHOU Shuang, ZHANG Wei-guang, ZHANG Guo-quan. Effect of mechanical activation on physical and chemical properties and roasting kinetics of sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(12): 3535-3542.
[3] 张 纯, 闵小波, 张建强, 王 密, 李辕成. 锌冶炼中浸渣锌还原浸出机制与动力学[J]. 中国有色金属学报, 2016, 26(1): 197-203.
ZHANG Chun, MIN Xiao-bo, ZHANG Jian-qiang, WANG Mi, LI Yuan-cheng. Mechanisms and kinetics on reductive leaching of zinc from neutral leaching residue[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(1): 197-203.
[4] 巨 佳, 王吉坤, 张向阳. 锌焙砂中浸渣氧压酸浸新工艺探讨[J]. 有色金属, 2011, 63(02): 159-162.
JU Jia, WANG Ji-kun, ZHANG Xiang-yang. Discussion on oxidizing pressure leaching of residues from zinc neutral leaching process[J]. Nonferrous Metals, 2011, 63(02): 159-162.
[5] 杨显万, 邱定蕃. 湿法冶金[M]. 北京: 冶金工业出版社, 1998: 154-215, 255-263.
YANG Xian-wan, QIU Ding-fan. Hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1998: 154-215, 255-263.
[6] 刘 洋, 谭 军, 尹周澜, 刘常青, 陈启元, 张平民, 廖 舟, 王心皞. 湿法炼锌沉铁渣和浸锌渣的焙烧预处理[J]. 中国有色金属学报, 2016, 26(1): 212-222.
LIU Yang, TAN Jun, YIN Zhou-lan, LIU Chang-qing, CHEN Qi-yuan, ZHANG Ping-min, LIAO Zhou, WANG Xin-hao. Roasting pretreatment of iron-sinking slag and zinc leaching residue in zinc hydrometallurgy[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(1): 212-222.
[7] SANTOS S M C, MACHADO R M, CORREIA M J N, REIS T A, ISMAEL M R C, CARVALHO J M R. Ferric sulphate/chloride leaching of zinc and minor elements from a sphalerite concentrate[J]. Minerals Engineering, 2010, 23: 606-615.
[8] SOUZA A D. PINA P S, LEAO V A, SILVA C A, SIQUEIRA P F. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate[J]. Hydrometallurgy, 2007, 89: 72-81.
[9] BALDWIN S A, DEMOPOULOS G P. Assessment of alternative iron sources in the pressure leaching of zinc concentrates using a reactor model[J]. Hydrometallurgy, 1995, 39(1/3): 147-162.
[10] LU Z Y, MUIR D M. Dissolution of metal ferrites and iron oxides by HCl under oxidising and reducing conditions[J]. Hydrometallurgy, 1988, 21(1): 9-21.
[11] MARKUS H, FUGLEBERG S, VALTAKARI D. Kinetic modelling of a solid-liquid reaction: Reduction of ferric iron to ferrous iron with zinc sulphide[J]. Chemical Engineering Science, 2004, 59: 919-930.
[12] DUTRIZAC J E, MACDONALD R J C. The dissolution of sphalerite in ferric chloride solutions[J]. Metallurgical Transactions B, 1978, 9(4): 543-551.
[13] ELGERSMA F, WITKAMP G J, ROSMALEN V G M. Kinetics and mechanism of reductive dissolution of zinc ferrite in H2O and D2O[J]. Hydrometallurgy, 1993, 33(1/2): 165-176.
[14] 王玉芳, 蒋开喜, 王海北. 高锌闪锌矿低温低压浸出新工艺研究[J]. 有色金属(冶炼部分), 2004(4): 4-6.
WANG Yu-fang, JIANG Kai-xi, WANG Hai-bei. Study on low temperature and pressure leaching of marmatite[J], Nonferrous Metals, 2004(4): 4-6.
[15] 张燕娟, 黎铉海, 潘柳萍, 韦岩松. 机械活化对铟铁酸锌溶解动力学及物化性质的影响[J]. 中国有色金属学报, 2012, 22(1): 315-323.
ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, WEI Yan-song. Influence of mechanical activation on dissolution kinetics and physicochemical properties of indium-bearing zinc ferrite[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 315-323.
[16] 韩俊伟, 刘 维, 覃文庆, 蔡 鑫, 罗虹霖, 王大伟. CO还原焙烧铁酸锌的选择性分解行为[J]. 中国有色金属学报, 2016, 26(6): 1324-1331.
HAN Jun-wei, LIU Wei, QIN Wen-qing, CAI Xin, LUO Hong-lin, WANG Da-wei. Selective decomposition behavior of zinc ferrite by reduction roasting with CO[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(6): 1324-1331.
[17] 张 帆, 魏 昶, 邓志敢, 李兴彬, 李存兄, 李旻廷. 锌中浸渣-硫化锌精矿协同浸出锌和铟[J]. 有色金属工程, 2016, 6(3): 40-44.
ZHANG Fan, WEI Chang, DENG Zhi-gan, LI Xing-bin, LI Cun-xiong, LI Min-ting. Simultaneous leaching of zinc and indium from zinc neutral-leaching residue and zinc concentrate[J]. Nonferrous Metals Engineering, 2016, 6(3): 40-44.
[18] ZHANG Fan, WEI Chang, DENG Zhi-gan. Reductive leaching of zinc and indium from industrial zinc ferrite particulates in sulphuric acid media[J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 2495-2501.
[19] 张帆. 湿法炼锌浸出渣与高铁锌精矿协同浸出机理与工艺研究[D]. 昆明: 昆明理工大学, 2017: 1-2.
ZHANG Fan. Study on mechanism and process of synergistic leaching of leaching residue from zinc hydrometallurgy with high-iron zinc concentrate[D]. Kunming: Kunming University of Science and Technology, 2017: 1-2.
[20] 李洪桂. 湿法冶金学[M]. 长沙: 中南大学出版社, 2002: 101-123.
LI Hong-gui. Hydrometallurgy[M]. Changsha: Central South University Press, 2002: 101-123.
[21] 王建祺. 电子能谱学(XPS/XAES/UPS)引论[M]. 北京: 国防工业出版社, 1992: 506-519.
WANG Jian-qi. Electron spectroscopy(XPS/XAES/UPS)[M]. Beijing: National Defense Industry Press. 1992: 506-519.
Simultaneous leaching of zinc residue and zinc concentrate and oxidative conversion behavior
FU Zhong-meng, DENG Zhi-gan, WEI Chang, LI Xing-bin, LI Cun-xiong, FAN Gang
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: Based on the redox reaction of Fe3+ and zinc concentrate, the method of the simultaneous leaching of zinc concentrate and zinc residue was proposed. The method achieved the dissolution of the zinc concentrate and the zinc residue, and also relieved the inhibitory effect of high concentration Fe3+ on the dissolution of zinc residue. The dissolution behavior of simultaneous leaching was studied, at the same time, taking zinc concentrate as the research object, the oxidative conversion behavior in H2SO4-Fe2(SO4)3 system was also studied. The results show that the simultaneous leaching process can effectively increase the leaching rate of valuable metals, and the content of Fe3+ in the leachate is low, which is convenient for subsequent treatment. According to the XRD, SEM and XPS analyses, zinc concentrate is continuously dissolved during the process of oxidative conversion. The sulphur of zinc concentrate is mainly oxidated into the elemental sulfur entered the residue. The elemental sulfur forms a wrap on the surface of the mineral particles, which results the zinc concentrate cannot fully dissolve.
Key words: zinc concentrate; zinc residue; simultaneous leaching; oxidation conversion
Foundation item: Projects(51564030, 51664030, 51664029) supported by the National Natural Science Foundation of China
Received date: 2018-02-08; Accepted date: 2018-07-21
Corresponding author: DENG Zhi-gan; Tel: +86-871-6518889; E-mail: dengzhigan83@163.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51564030,51664030,51664029)
收稿日期:2018-02-08;修订日期:2018-07-21
通信作者:邓志敢,讲师,博士;电话:0871-6518889;E-mail: dengzhigan83@163.com