Trans. Nonferrous Met. Soc. China 23(2013) 3763-3769
Bioleaching of sphalerite sample from Kooshk lead-zinc tailing dam
J. V. MEHRABANI1, S. Z. SHAFAEI1, M. NOAPARAST1, S. M. MOUSAVI2, M. M. RAJAEI1
1. School of Mining Engineering, College of Engineering, University of Tehran, Tehran, Iran;
2. Biotechnology Group, Chemical Engineering Department, Tarbiat Modares University, Tehran, Iran
Received 21 March 2013; accepted 22 May 2013
Abstract: The zinc extraction from Kooshk lead-zinc tailing dam’s sample was investigated by bioleaching method. The Kooshk lead-zinc deposit/mine is located in Yazd province, Iran, and its tailing dam contains about 3.64% zinc, 0.97% lead and 24.18% iron. Experiments were designed and carried out by a mixed culture of mesophile bacteria as well as a mixed culture of moderate thermophile strain in the shake flasks. The results indicated that, more than 90% of sphalerite was dissolved during 14 d, while without bacteria, 44% of Zn was merely extracted. In addition, some experiments were performed in the absence of the bacterial medium (9K). The results of these experiments indicated significant difference in Zn extraction with and without 9K until the 10th day of bioleaching, but after that the Zn extraction was improved and the same extraction was achieved at the end of bioleaching tests. This improvement can be attributed to the increase of the number of bacteria or Fe3+ concentration at the last days of leaching. Zn extraction kinetics of moderate thermophile bacteria was significantly higher than that of the mesophile, therefore sphalerite was successfully dissolved in preference to the pyrite using moderate thermophile bacteria in a lower redox potential.
Key words: bioleaching; pyrite; tailing; sphalerite
1 Introduction
Kooshk lead-zinc deposit/mine is one of the sedimentary-black shale deposits in the world which is located in Yazd province, Iran. Black shale is hosted sulfide minerals of lead and zinc in this deposit. The major sulphides are pyrite, sphalerite and galena [1,2]. The average mill feed grade of Kooshk in 2011 was 1.98% lead, 8.45% zinc, 15% iron and 1% organic carbon.
The Kooshk tailing dam contains approximately 5 million tons of historic sphalerite tailings grading 3.64% zinc, 0.97% lead and 24.18% iron. Therefore, evaluation of Zn extraction from the mentioned tailings is valuable.
The tailings contain high amount of pyrite and organic carbon, and the sizes of particles are fine. Flotation results showed that lower than 50% of sphalerite can be recovered by flotation process and tailings characteristics indicated that it is difficult to recover the remained sphalerite by the flotation process [3]. Therefore, bioleaching method was implemented to study the Zn extraction from the Kooshk tailings.
In recent years, bioleaching processes have gained importance for the extraction of metals particularly from the difficult-to-treat and low grade ores/concentrates. And biomining has application as an alternative to more traditional physical-chemical methods of mineral processing [4,5].
Bioleaching is essentially a dissolution process which is based on the exploitation of acidophilic bacteria, and has the ability of deriving the energy required for their growth and other metabolic functions from the oxidation of ferrous iron (Eq. (1)), and/or elemental sulphur (Eq. (2)) or reduced sulphur compounds [6,7].
2Fe2++0.5O2+2H+→2Fe3++H2O (1)
0.125S8+1.5O2+H2O→2H++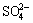 (2)
(2)
The reaction products, ferric iron and/or acid, attack the sulphide minerals, such as sphalerite (Eqs. (3) and (4)), leading to their dissolution [8].
ZnS+2Fe3+→Zn2++2Fe2++S (3)
ZnS+2H+→Zn2++H2S (4)
Bacterial tank leaching of zinc from flotation tailings was investigated by PANNI et al [9]. Zinc was leached under continuous conditions at 28-30 °C in agitated tanks with pulp densities of 16.7%, 28.6% and 40.0% of solid in mass fraction [9]. Zn(II) from Nigerian sphalerite ore was bioleached by a mixed culture of acidophilic bacteria [10]. GeoBiotics and Kumba Resources have investigated the feasibility of applying the GEOCOAT process to the leaching and recovering of zinc from a low-grade sphalerite concentrate produced from accumulated flotation tailings at Kumba’s Rosh Pinah zinc mine in Namibia [11].
The main objective of this research was to investigate the zinc extraction using bioleaching process from the Kooshk lead-zinc tailing dam’s sample. Mixture of three mesophile bacteria, and mixture of four moderate thermophile bacteria were applied to bioleaching process. In addition, because of the tailing characteristics, the effect of removing bacterial medium (9K) was studied under the optimum condition of experiments.
2 Experimental
2.1 Tailings mineralogy and characteristics
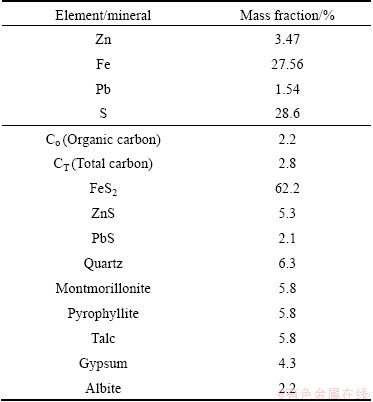
A representative sample was obtained from the Kooshk tailing dam. Although the d80 of particles size was 90 μm, particles with size of minus 53 μm was used for experiments. Particles were sized using cyclosizer. Figure 1 presents that about 50% of tailing sample is finer than 11 μm. Chemical analysis and mineralogical compositions of the sample are presented in Table 1. The tailing contains 3.42% Zn, 1.54% Pb and 27.76% Fe.
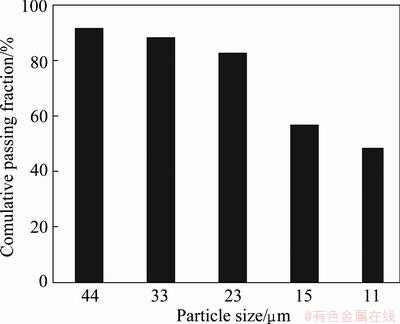
Fig. 1 Size distribution of tailings
Analysis showed that pyrite is the dominate mineral in sample with content of 62.2%. Mineralogical studies indicated that sphalerite is present in free grains, enclosed with pyrite, and in some cases pyrite is in sphalerite matrix (Fig. 2). Figure 2 also illustrates that pyrite forms include slimes, spherical (bacteriogenic and framboidal) and spherulites.
Table 1 Chemical and mineralogical composition of tailings
2.2 Carbon analysis
Carbon and sulfur were determined and analyzed by LECO. The results showed that there is about 2.2% of organic carbon and 28.6% of sulfur in the sample (Table 1). To determine the carbon which is disordered or graphitic, Raman spectroscopic analysis was used on carbonaceous matter. The Raman spectrum, in the first order region, of carbonaceous matter includes a graphite peak (G peak) at around 1580 cm-1. Disordered carbon also contains a peak at around 1360 cm-1 (D1 peak) and a peak at around 1620 cm-1 (D2). The D2 peak appears as a right shoulder on the G peak, and in more disordered carbon it merges together with the G peak, causing a broad G+D2 peak around 1600 cm-1 [12-15].
Characteristics study of tailing sample indicated that the organic matters in the sample are poorly crystalline carbon (Fig. 3); they have a D1 peak at around 1303 cm-1, and a merged G and D2 peak at around 1597 cm-1.
2.3 Medium and microorganisms
A mixed strain of mesophile bacteria of A. ferrooxidans, A. thiooxidans and L. ferrooxidans, isolated from the acidic water drainage of the Sarcheshmeh copper mine, located in Kerman province, Iran, was used in this study. The bacteria were subcultured in the laboratory, using 9K medium (3 g/L (NH4)2SO4, 0.5 g/L MgSO4 7H2O, 0.5 g/L K2HPO4, and 0.1 g/L KCl at pH=2). The bacteria were then cultured by inoculating 10 mL of a pure strain of the bacterial cells into the 90 mL of medium. Moreover, potassium nitrate was used to maintain the ionic strength. The cells were incubated at 30 °C in a rotary shaker maintained at 150 r/min.
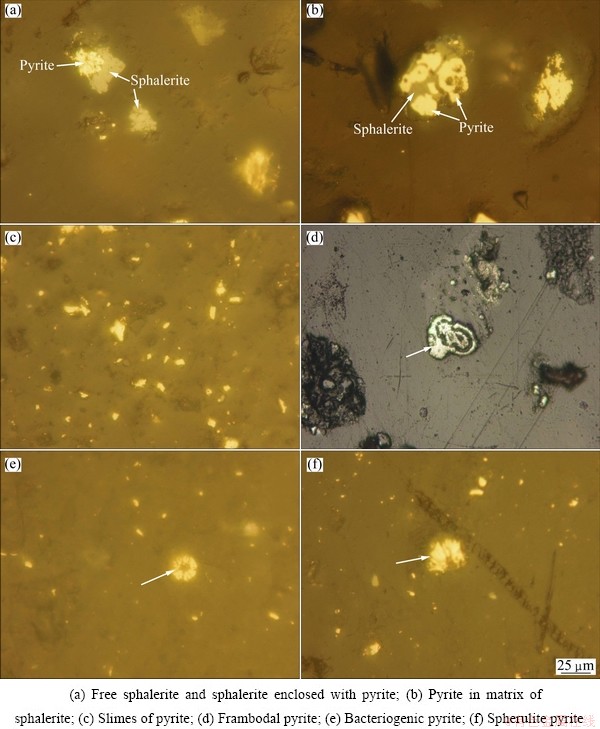
Fig. 2 Sphalerite and pyrite minerals in tailings
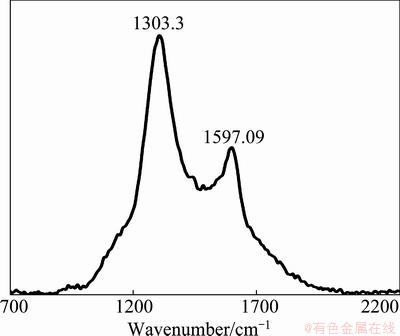
Fig. 3 Raman signature of carbon in tailings
A moderately thermophilic culture (at 45 °C) was also obtained from the Mintek Company (South Africa). This culture was mainly composed of sulfur-oxidizing bacteria with a lower proportion of iron (II) ion- oxidizing microorganisms. The moderate thermophile culture contained Acidithiobacillus caldus, Leptospirillum ferriphilum, Sulfobacillus sp. and Ferroplasma acidophilum. The bacteria were subcultured in the laboratory using 9K medium too.
2.4 Bacterial adaptation and bioleaching
The bacterial adaptation process started by adding 1 g of sample to 90 mL fresh medium and 10 mL cell culture. In each stage of adaptation, the adapted cells from the previous stage were used to the next stage, and the process continued until 20 g of tailings sample was added to 100 mL of solution.
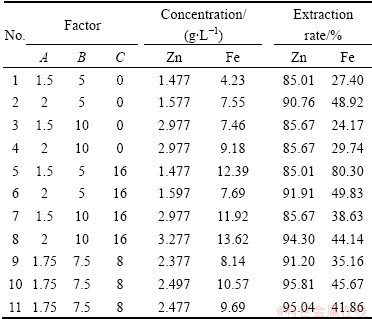
All experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL of solution. The experiments were then conducted with 10% inoculum in a rotary shaker at 150 r/min and 30 °C and 45 °C. Three control factors and their levels used in the experiments are presented in Table 2. Eleven experiments were designed and carried out in shake flasks.
Table 2 Studied factors and their levels in bioleaching experiments

3 Results and discussion
Eleven bioleaching experiments were designed and carried out. The experimental conditions and their responses are presented in Table 3. Design Expert (DX) software was used to design and analysis of the results, from which appropriate models (among several models) were chosen and fitted to the responses. Four models were fitted to Zn and Fe extraction as well as Zn and Fe grade as follows:
η(Zn)=88+2.68A
ρ(Zn)=2.3+0.75B
η(Fe)=+42.89-8.72B+10.33C-6.51AC+6.49ABC
ρ(Fe)=9.25+0.86A+4.87C+0.45AB+0.37AC
where η(Zn) and η(Fe) are the extraction rates of Zn and Fe, respectively; ρ(Zn) and ρ(Fe) are the concentrations of Zn and Fe in the solution, respectively.
In these models A is pH, B is pulp density, C is initial Fe+2 and AB, AC, ABC are the interaction of main parameters. All variables in mathematical models are in coded values.
Table 3 Bioleaching experiments plan and obtained results
3.1 Effect of designed parameters
Figure 4(a) illustrates the effect of significant parameter of pH on the Zn extraction. It shows that with changing of pH from 1.5 to 2.0, Zn extraction rate increases from about 85% to 91% significantly. Two other parameters of pulp density and initial Fe+2 are not significant factors to the Zn dissolution in the selected levels. According to the proposed and selected model, pulp density is the only parameter which could dramatically change the Zn concentration in the bioleaching solution. Figure 4(b) presents the variation of Zn concentration in different pulp densities, in which Zn concentration is considerably surged from 1.5 to 3 g/L in solution with changing of pulp density from 5% to 10%.
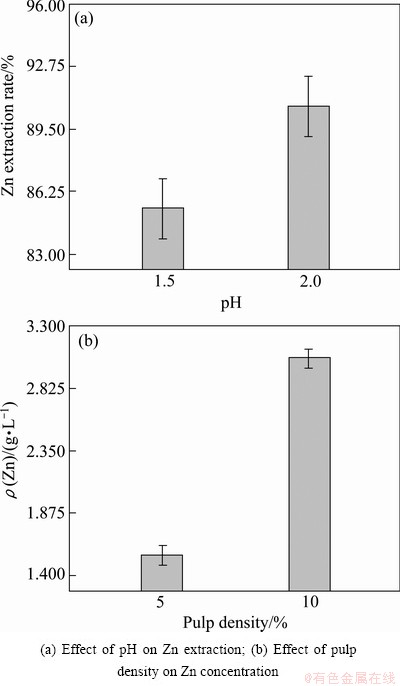
Fig. 4 Effect of designed parameters on bioleaching process of tailings
3.2 Optimization and confirmation tests
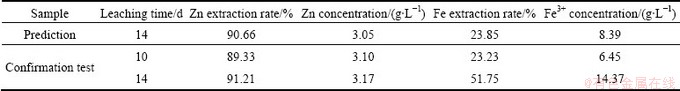
The main objective of model fitting was to find significant factors in the bioleaching process and find the optimum condition, in which the highest Zn concentration in the leachate and extraction could be achieved with the lowest Fe extraction rate in the experiments. Optimum conditions were then predicted according to the fitted models. In the proposed optimum point (pH=2, pulp density 10%, without initial Fe+2 addition), it was predicted that 90%-91% of zinc would be dissolved with concentration of 3.05 g/L in the solution. Therefore, the confirmation test was accordingly carried out in the predicted optimum conditions against the time. The predicted and actual results are shown in Table 4. The experimental values were found to be close to the predicted values of Zn concentrate and extraction rate. The results showed that after 10 d of bacterial leaching, about 89% sphalerite was leached out, and with increasing the leaching time to 14 d, Zn extraction rate was improved only 2%, while Fe extraction rate during the last 4 d of leaching was doubled. Zn concentration and extraction models were successfully validated in the confidence interval of 90%. The confirmation tests also verified the predicted results for Fe concentration and extraction rate responses for leaching time of 10 d and not for 14 d. Leaching residuals of confirmation tests were analyzed and the recoveries were calculated over feed grades and residual grades. Zn and Fe contents of residuals were 0.48% and 24%, respectively. The results indicated that 91.63% of Zn was extracted in the bioleaching of tailings. Comparing the calculated Zn extraction rate from both Zn concentrations in the leaching solution and Zn grade in the leaching residuals confirms the Zn extraction rate higher than 90% from sphalerite tailings, using bioleaching process.
Table 4 Actual and predicted values of optimum points (confirmation tests)
3.3 Effect of 9K medium in optimum condition
Figure 5 presents the variations of Zn extraction rate in the absence and presence of 9K medium for tailings against time at pH=2, pulp density 10%. It illustrated that Zn extraction rate using mixture of bacteria in the presence of 9K was 89.33% during 10 d of bioleaching. Under the same condition, bacterial medium (9K) was removed in the bioleaching test. The results showed that during 10 d of leaching in the absence of bacterial medium, Zn dissolution was about 11% lower than that with the 9K tests. Figure 5 also presents that at the end of 14 d of leaching, the same Zn extraction rate was achieved in the presence and absence of 9K medium. Figure 6 illustrates the number of bacteria under the optimum condition of leaching process in the absence and presence of 9K medium. It should be mentioned that significant difference existed in Zn extraction rate with and without 9K until the 10th day of leaching, but Zn extraction rate was improved from 78% to 89% during the last 4 d of leaching. This improvement possibly could be attributed to the increase of the number of bacteria or Fe3+ concentration (based on Fig. 7) in the last 4 d of test solution without 9K. Finally, 89.56% and 91.25% of Zn were extracted at the end of experiments with and without 9K, respectively. As is shown, their differences are not significant.
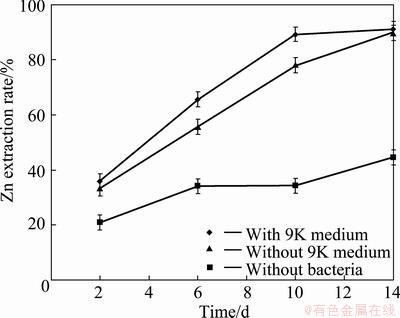
Fig. 5 Zn extraction rate against time under optimum conditions in the presence and absence of 9K medium at pH=2, pulp density 10%
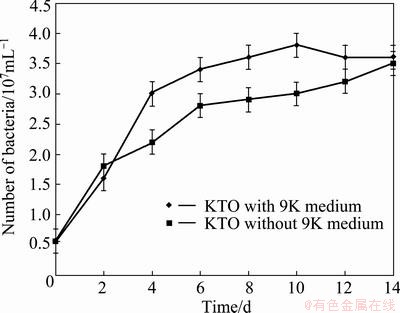
Fig. 6 Number of bacteria under optimum condition in leaching process in the presence and absence of 9K medium
Figure 5 illustrates that in the control test (in the absence of bacteria), 44% of Zn was merely extracted, which means that the addition of bacteria in the leaching process improved Zn extraction rate by up to 46%. Under the optimum condition of bioleaching process, the Fe extraction rate and concentration were monitored as well (Fig. 7). The results showed that in the bioleaching tests that 9K was applied as a bacterial culture, Fe extraction rate and Fe3+ concentration were 51.75% and 11.53 g/L respectively, while under condition in the absence of 9K, they were 27.84% and 7.06 g/L, respectively. In other words, at the end of bioleaching process, in the presence of 9K, Fe extraction rate and Fe3+ concentration were about twice of that without 9K medium. However, the same Zn extraction rate was achieved under the both mentioned conditions at the end of bioleaching tests.
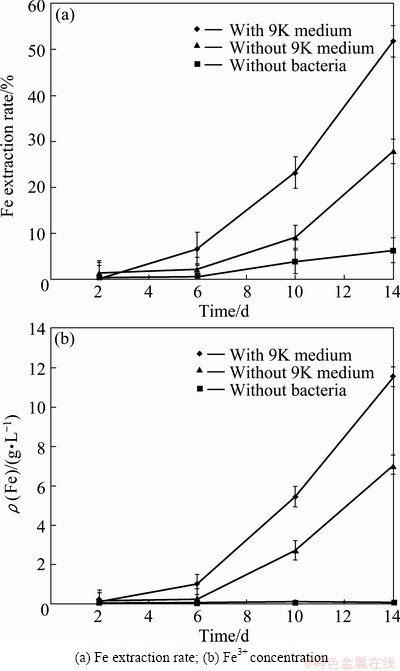
Fig. 7 Fe extraction rate and Fe3+ concentration against time at optimum conditions in the presence and absence of 9K medium
3.4 Bioleaching of tailings using moderate thermophile bacteria
After bioleaching of tailings using mixture of three mesophile bacteria in the previous sections, some experiments were carried out with the moderate thermophile bacteria in the temperature of 45 °C. Figure 8 shows Zn and Fe extraction rate of tailings over the time in the presence of mesophile and moderate thermophile bacteria under the condition of initial pH=2, pulp density of 10%(w/v). About 89% of Zn and 10.8% of Fe were extracted within 8 d of moderate thermophile bacterial leaching. Comparing Zn and Fe extraction of mesophile and moderate thermophile bacteria in Figure 8, it is indicated that Zn dissolution kinetics of moderate thermophile bacteria is significantly higher than that of mesophile. There is a remarkable difference between Fe extraction of mesophile and moderate thermophile bacteria. Under the condition of the same Zn extraction rate, about 23.2% and 10.8% of Fe were leached and, using mesophile and moderate thermophile bacteria, respectively. This difference may address the higher dissolution of sphalerite, using moderate thermophile bacteria at temperature of 45 °C, in which sphalerite was readily leached out in the lower redox potential than at 30 °C with mesophile bacteria (Fig. 9) and the pyrite was left.
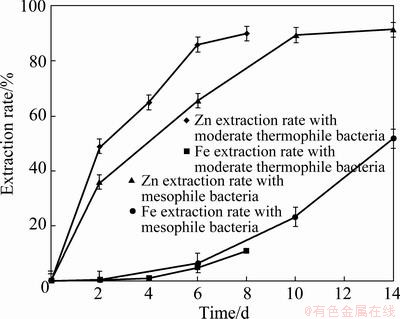
Fig. 8 Zn and Fe extraction rate using mesophile and moderate thermophile bacteria under conditions of pH=2, pulp density 10%
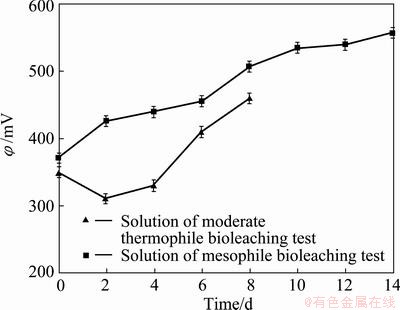
Fig. 9 Redox potential of mesophile and moderate thermophile bacteria in leaching solutions
4 Conclusions
1) Mineralogical studies showed that pyrite is the dominate mineral in forms of slimes, spherical (bacteriogenic and framboidal) and spherulites. Sphalrite is present in free grains, enclosed with pyrite and in some cases pyrite is in sphalerite matrix. Total organic carbon of sample is about 2.2%. The organic matter in tailings sample gives a signature of poor crystalline carbon. They have a D1 peak at around 1303 cm-1, and a merged G and D2 peak at around 1597 cm-1.
2) Experimental results obtained from mixture of mesophile bacteria showed that after 10 d of bacterial leaching, about 89% sphalerite was leached out, and with increasing the leaching time (14 d), Zn extraction rate was improved by 2% while Fe extraction rate during the last 4 d of leaching increased by 28%, from 23% to 51%.
3) Removing bacterial medium from bioleaching tests represented significant difference in Zn extraction rate compared with experiments with 9K medium until 10th day, but then Zn extraction was improved from 78% to 89% during the last 4 d of leaching. Therefore, the same Zn extraction was achieved at the end of bioleaching tests.
4) Comparing Zn and Fe extraction of mesophile and moderate indicates that Zn extraction kinetics of moderate thermophile bacteria is significantly higher than that of mesophile, therefore sphalerite is successfully dissolved in preference to the pyrite, using moderate thermophile bacteria in the lower redox potential. Under the condition of same Zn extraction rate, more than twice Fe was dissolved using mesholile thermophile bacteria in comparison with moderate bacteria.
Acknowledgements
The authors are grateful to Bafgh Mines Company for supporting some part of experimental costs and also Stat-Ease, Minneapolis, MN, USA, for the provision of the Design-Expert package.
References
[1] BULATOVIC S M. Handbook of flotation reagents: Chemistry, theory and practice: Volume 1 [M]. The Netherlands: Elsevier, 2007: 323-366.
[2] YAGHUBPOUR A, MEHRABI B. Kooshk lead-zinc deposit a typical black shale-hosted deposit in Yazd state [J]. Journal of Sciences, Islamic Republic of Iran, 1997, 8: 117-126.
[3] RAJAEI M M. Investigation of sphalerite recovery from the flotation tailings of Kooshk lead-zinc plant [D]. Tehran: University of Tehran, 2011.
[4] RAWLINGS D E, DEW D, DU PLESSIS C. Biomineralization of metal containing ores and concentrates [J]. Trends in Biotechnology, 2003, 21: 38-44.
[5] BRIERLEY C L. How will biomining be applied in future [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1302-1310.
[6] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review. Part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Appl Microbiol Biotechnol, 2003, 6: 239-248.
[7] SUZUKI I. Microbial leaching of metals from sulphide minerals [J]. Biotechnology Advances, 2001, 19: 119-132.
[8] SAND W, GEHRKE T, JOZSA P G, SCHIPPERS A. (Bio) chemistry of bacterial leaching—Direct vs. indirect bioleaching [J]. Hydrometallurgy, 2001, 59: 159-175.
[9] PANIN V V, ADAMOV E V, KRYLOVA L N, PIVOVAROVA T A, VORONON D Y, KARAVAIKO G I. Bacterial tank leaching of zinc from flotation tailings [C]//15th International Biohydrometallurgy Symposium. Athens: Hella, 2003: 85-90.
[10] BABA A A, ADEKOLA F A, ATATA R F, AHMED R N, PANDA S. Bioleaching of Zn(II) and Pb(II) from Nigerian sphalerite and galena ores by mixed culture of acidophilic bacteria [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2535-2541.
[11] SAMPSON M I, van der MERWE W, HARVEY T J, BATH M D. Testing the ability of a low grade sphalerite tailing to achieve autothermality during biooxidation heap leaching [J]. Miner Eng, 2005, 18: 427-437.
[12] BEYSSAC O, GOFFE B, PETITET J P, FROIGNEUX E, MOREAU M, ROUZAUD J N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy [J]. Spectrochim Acta A, 2003, 59: 2267-2276.
[13] KWIECINSKA B, SUAREZ-RUIZ I, PALUSZKIEWICZ C, RODRIQUES S. Raman spectroscopy of selected carbonaceous samples [J]. Int J Coal Geol, 2010, 84: 206-212.
[14] LINDGREN P, PARNELL J, HOLM N G, BROMAN C. A demonstration of an affinity between pyrite and organic matter in a hydrothermal setting [J]. Geochem T, 2011, 12: 3-7.
[15] WOPENKA B, PASTERIS J D. Structural characterization of kergones to granulite-facies graphite: Applicability of Raman microprobe spectroscopy [J]. Am Mineral, 1993, 78: 533-557.
伊朗Kooshk铅-锌尾矿坝闪锌矿的生物浸出
J. V. MEHRABANI1, S. Z. SHAFAEI1, M. NOAPARAST1, S. M. MOUSAVI2, M.M. RAJAEI1
1. School of Mining Engineering, College of Engineering, University of Tehran, Tehran, Iran;
2. Biotechnology Group, Chemical Engineering Department, Tarbiat Modares University, Tehran, Iran
摘 要:将取自伊朗Yazd省Kooshk铅-锌尾矿坝的闪锌矿样进行生物浸出,并对比研究不同条件下的锌浸出率。Kooshk铅-锌尾矿坝含有3.64%锌、0.97%铅和24.18%铁。先将混合嗜温菌株、混合中度嗜热菌株进行摇瓶培养,然后在9K培养基中再次培养。在浸出闪锌矿的14 d 内,在有菌株的情况下,闪锌矿的浸出率达90%,而没有菌株的情况下,只有44%的闪锌矿被浸出。实验结果表明,在有菌株浸出的前10 d,有没有9K培养液对锌的浸出率有明显影响,但随后其差别不明显,到最后趋于相同。锌浸出率的提高与细菌菌落数和Fe3+浓度的增加有关。中度嗜热菌浸取锌的动力学要求明显高于嗜温菌的,因此,在中度嗜热菌株存在的情况下,在低的氧化电位下闪锌矿优先于黄铁矿溶解而被浸出。
关键词:生物浸出;黄铁矿;尾矿;闪锌矿
(Edited by Hua YANG)
Corresponding author: J. V. MEHRABANI; Tel: +98-21-8800883; Fax: +98-21-88008838; E-mail: j_mehrabani@ut.ac.ir
DOI: 10.1016/S1003-6326(13)62927-1