
J. Cent. South Univ. (2021) 28: 1946-1954
DOI: https://doi.org/10.1007/s11771-021-4743-5
Template-free hydrothermal synthesis and gas-sensitivity of hollow-structured Cu0.3Co2.7O4 microspheres
TIAN Li(田俐)1, 2, 3, 4, 5, LIU Qiang(刘强)1, 2, 3, 4, WU Jie-ling(吴杰灵)1, 2, 3, 4, YI Yi-tao(易益涛)1, 2, 3, 4
1. School of Materials Science and Engineering, Hunan University of Science and Technology,Xiangtan 411201, China;
2. Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of
Fine Polymers, Hunan University of Science and Technology, Xiangtan 411201, China;
3. Hunan Provincial Key Lab of Advanced Materials for New Energy Storage and Conversion,
Hunan University of Science and Technology, Xiangtan 411201, China;
4. Hunan Provincial Key Defense Laboratory of High Temperature Wear-resisting Materials and
Preparation Technology, Hunan University of Science and Technology, Xiangtan 411201, China;
5. School of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: Hollow-structured Cu0.3Co2.7O4 microspheres have been synthesized by a simple one-pot template-free hydrothermal method with copper sulfate, cobalt acetate and ammonia as raw materials. The products were characterized by powder X-ray diffraction, energy dispersive X-ray analysis, selected area electron diffraction, high-resolution transmission electron microscopy, scanning electron microscopy and BET measurements. The research results show that the hollow Cu0.3Co2.7O4 microspheres consist of single-crystalline nanocubes with the diameter of about 20 nm. The formation mechanism of hollow Cu0.3Co2.7O4 microspheres is suggested as Ostwald ripening in a solid-solution-solid process, and Cu0.3Co2.7O4 microspheres are mesoporous containing two pore sizes of 3.3 and 5.9 nm. The as-prepared Cu0.3Co2.7O4 sensors have optimal gas responses to 50×10-6 mg/m3 C2H5OH at 190 oC.
Key words: Cu0.3Co2.7O4 oxides; microsphere; inorganic compounds; nanostructures; template-free hydrothermal method
Cite this article as: TIAN Li, LIU Qiang, WU Jie-ling, YI Yi-tao. Template-free hydrothermal synthesis and gas-sensitivity of hollow-structured Cu0.3Co2.7O4 microspheres [J]. Journal of Central South University, 2021, 28(7): 1946-1954. DOI: https://doi.org/10.1007/s11771-021-4743-5.
1 Introduction
As an important class of porous materials, hollow microspheres have attracted extensive attention over the past half century. These hollow materials have large surface area and low material density, which have extensive technological applications in a wide range of areas, such as catalysis, drug delivery, biosorption, interaction, filling, energy storage [1-3]. The size, morphology, and structure of hollow materials have a significant effect on their magnetic, optical, electric, catalytic properties and further their applications [4-8]. Therefore, a lot of research efforts have been made to the controlled synthesis of these hollow materials with well crystalline, favorable shape and appropriate cavity size [9, 10]. So far, a variety of preparation methods have been exploited to produce the hollow microspherical structure with adjusted inner space and shell thickness [11-13].
Spinel transition metal oxides, for example, ferrite and cobaltite, are among the most important industrial materials and have been widely used in electronic devices, information storage, magnetic resonance imaging, gas sensors, adsorbents, catalysts, pigments and biotechnology [14-18]. Recently, nanostructured CuCo2O4 have obtained much research interest as catalysts, supercapacitors, cathode materials with impressive physical and chemical properties, which inspired us to investigate the gas-sensitivity of copper cobalt oxides for some selected gases. In this work, we developed a facile and controllable strategy to solution-phase synthesis spinel Cu0.3Co2.7O4 hollow microspheres. The one-pot synthesis process is template-free, low-cost and can be readily extended to produce various hollow inorganic materials with controllable size, shape and structure. The microstructure and gas-sensitivity of the as-prepared hollow Cu0.3Co2.7O4 microspheres was studied.
2 Experimental
2.1 Materials preparation
Co(OAc)2·6H2O, CuSO4·5H2O and NH3·H2O (Guangzhou Chemical Co.), n-propanol (Tianjin Chemical Co.) were of analytical grade and used as raw materials without further purification. In a typical synthesis of hollow Cu0.3Co2.7O4 microspheres, Co(NO3)2·6H2O (3.0 mmol) and CuSO4·5H2O (1.5 mmol) was dissolved in the co-solvent (water and n-propanol is 5.0 and 10.0 mL, respectively) to form a transparent solution, and then 5.0 mL of ammonia was added into the solution to yield a brownish-black dispersion with intensive stirring. The resulting dispersion was put into a 30.0 mL autoclave with Teflon liner, and heated at 220 °C for a period of time. After the autoclave was cooled naturally to room temperature, the transparent supernatant of ca. 25 mL was removed by pipette and a black precipitate was left. The black precipitate was centrifuged, then rinsed by distilled water and absolute ethanol several times. The as-obtained products were dried in a desiccator for further characterizations.
2.2 Characterization
The phase structure of the product was identified using XRD on a Rigaku D/MAX 2200 VPC diffractometer with Cu Kα radiation (λ=0.15045 nm) and a graphite monochromator at ambient temperature from 10° to 70° at a scanning rate of 10(°)/min. X-ray tube voltage and current were set at 40 kV and 30 mA, respectively. The composition, shape, size, structure, and crystalline of the hollow nanospheres were characterized using FE-SEM (FEI Quanta 400 thermal FE environment scanning electron microscope) and TEM (JEM-2010 transmission electron microscope) with X-ray energy-dispersive spectrometry (EDS). The hollow microspheres are determined by N2 adsorption via BET measurements using an ASAP-2000 surface area analyzer.
2.3 Gas-sensitivity determination
Figure 1(a) illustrated the structure of the as-fabricated gas sensor. The samples were dry-ground, and then wet-ground with an organic binder to obtain pastes. The resulting pastes were coated on aluminium oxide tubes with a pair of gold electrodes attached with platinum wires. After being dried at room temperature, they were heated at 600 °C for 2 h. The electrodes were furnished on the circuit for measurement finally.
A resistance heater in the ceramic tube is utilized to control the temperature. The as-prepared sensor was aged at 190 °C for 24 h, firstly. For testing gas sensing properties, a stationary state gas distribution method was applied. Figure 1(b) presented the measuring electric circuit. The resistance of a sensor in air or in a sample gas was determined by monitoring Vout. The test was performed in a measuring system of HW-30A (Hanwei Electronics Co. Ltd., Henan, China). The samples were injected into a test jar and mixed with the air inside. The response/recovery time of the sensor is defined as the time needed to arrive 90% of the original resistance. The gas response (S) (p type) was defined as the ratio (Rg/ Ra) of the resistance in a sample gas (Rg) and in air (Ra). The gas response (S) (n type) was defined as the ratio (Ra/Rg).
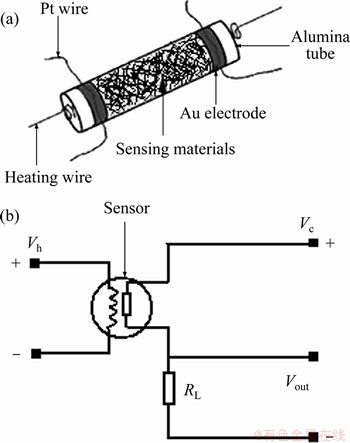
Figure 1 Schematic drawing of a sensor element (a) and electric circuit (b) (Vh is the heating voltage, RL is a constant resistance, Vc is the constant voltage (=5 V) applied on the RL and the sensor, and Vout is the output voltage of RL)
3 Results and discussion
3.1 Structure analysis
Figure 2 shows the typical scanning electron microscope (SEM) and transmission electron microscope (TEM) images of the assynthesized samples. It is clearly demonstrated that the samples have uniform microspherical morphology. The sizes of these microspheres range from 3.6 to 4.0 μm, with an average diameter of 3.8 μm. Due to the ultrasonication dispersion of the sample for the SEM determination, some microspheres were broken and a small quantity of fragments was found, as shown in Figure 2(a). Based on the observation of those broken microspheres (Figures 2(a) and (b)) and the TEM image (Figure 2(d)), it is revealed that these microspheres have hollow structure with big cavities. From the higher magnification SEM image of an individual microsphere (Figure 2(b)), it is also noticed that the surface of the hollow microsphere is quite scraggly and composed of nanoparticles (Figure 2(c)). The result of energy dispersive X-ray (EDX) analysis (Figure 2(e)) confirmed that the microsphere consists of copper, cobalt, and oxygen with the ratio of 3:27:40. The related X-ray diffraction (XRD) pattern (Figure 2(f)) exhibited the corresponding reflection characteristic of pure spinel cobalt copper oxide (JCPDS card No. 25-0270), indicating a face-center cubic phase [space group: Fd3m (227)] of Cu0.3Co0.7Co2O4 with lattice constant a=0.8074 nm. No other impurities were detected in the synthesized products.
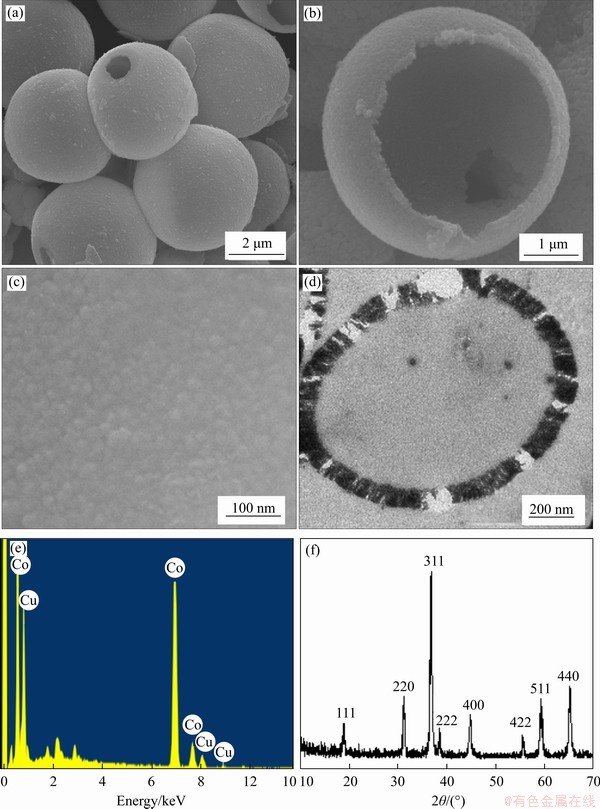
Figure 2 Low magnification SEM image of as-synthesized sample with uniform microspherical morphology (a); Higher magnification SEM image of a single hollow microsphere (b); Detailed view of the microsphere surface (c); TEM image of a hollow microtomed microsphere (d); EDS spectrum gained under SEM observation (e); XRD pattern of the sample, showing reflection characteristic of Cu0.3Co0.7Co2O4 (PDF# 25-0270) (f)
The morphology and microstructure of the as-synthesised Cu0.3Co2.7O4 were further investigated by high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED). The detailed morphological and crystalline information of the hollow Cu0.3Co2.7O4 microspheres is shown in Figure 3(d). It is very clear that all microspheres are hollow with a wall thickness less than 100 nm from the TEM image of hollow microtomed microspheres (Figure 2(d)). The as-synthesized hollow microspheres have big cavities and the dimension of the cavities is about 3.8 μm. To our knowledge, it is the first time for us to obtain hollow copper cobaltate microspheres with several micrometered cavity and nanosized wall. This hollow microspheral structure with big cavities might be more suitable to the application in minireactors, ionchanger, lightweight fillers, molecular separation, chemical storage, and so forth.
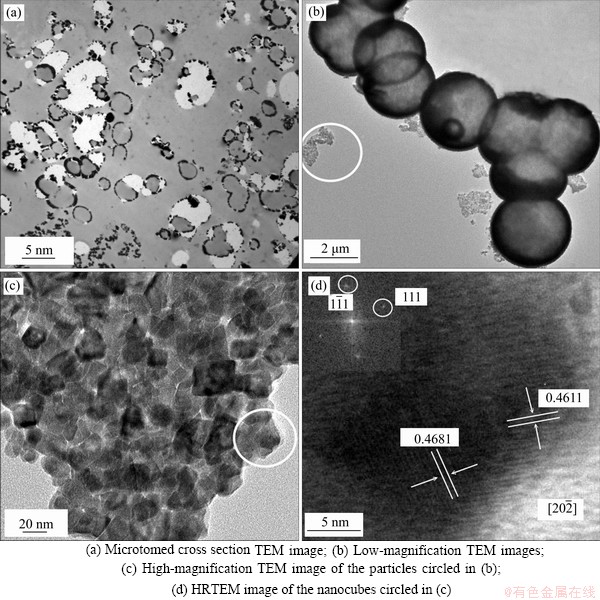
Figure 3 TEM and HRTEM images of the Cu0.3Co2.7O4 microspheres prepared at 220 °C:(inset: corresponding SAED pattern)
For better analysis of the structural characteristic of hollow microspheres, the samples for TEM observation (Figure 3) were prepared by intensely ultrasonication. The hollow Cu0.3Co2.7O4 microspherical shells were finally broken and some fragments could be seen circled in Figure 3(b). The high-magnification TEM image of the fragments shown as in Figure 3(c) confirms that the shells of hollow microspheres are made up of many cubic nanoparticles, and the diameter of those nanocubes is about 20 nm. The apparent two-dimensional lattice fringes in the HRTEM image (Figure 3(d)) testify the single-crystallinity of those nanocubes. The lattice spacings of 0.4681 and 0.4611 nm correspond to the distance of the (111) and 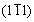 planes of cubic copper cobalt oxide, respectively. The SAED pattern of the fragment (inset in Figure 3(d)) can be indexed to the
planes of cubic copper cobalt oxide, respectively. The SAED pattern of the fragment (inset in Figure 3(d)) can be indexed to the 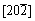 zone axis of cubic Cu0.3Co2.7O4, which further confirms that the nanocubes have well-crystalline and the shell of the hollow microspheres is single crystalline. It is believed that the single-crystalline nature of the microspheres should improve their stability and may enhance their physicochemical performance in application.
zone axis of cubic Cu0.3Co2.7O4, which further confirms that the nanocubes have well-crystalline and the shell of the hollow microspheres is single crystalline. It is believed that the single-crystalline nature of the microspheres should improve their stability and may enhance their physicochemical performance in application.
3.2 Formation mechanism
In order to illuminate the formation mechanism of hollow Cu0.3Co2.7O4 microspheres, the phases and morphology of the samples obtained at 220 °C for different hydrothermal reaction were testified by XRD and SEM characterization, respectively.Figure 4 shows the XRD patterns of the samples prepared after different reaction times, indicating that all the as-synthesized products are pure spinel Cu0.3Co0.7Co2O4 (JCPDS card No. 25-0270) with face-center cubic structure [space group: Fd3m (227)].
Figures 5 and 6 show SEM images of the corresponding products synthesized for 2, 6, 12 and 24 h, respectively. After 2.0 h reaction, the solid spinel microspheres with uniformed morphology were found and the diameter of the solid microspheres is about 2.8 μm as shown in Figure 5(a). The surface of these microspheres is smooth and glabrous. After 6 h reaction, the diameter of as-formed microspheres is about 3.2 μm. At the same time, some hollow microspheres with core-shell structure were observed. When the hydrothermal time was increased to 12 h, more and more cavities occurred inside microspheres as the arrowhead denoted in Figure 5(b). The hollow microspheres became larger and larger. When the reaction time prolonging to 24 h, Cu0.3Co0.7Co2O4 microspheres were hollowed completely (Figure 5(d)). The surface of these hollow microspheres was coarse and porous.
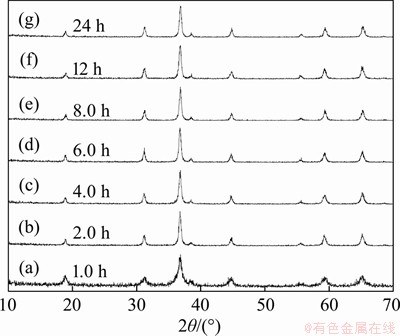
Figure 4 Powder XRD patterns of products prepared at 220 °C for different reaction times
Based on the detailed time-dependent crystallization research, it is suggested that Ostwald ripening in a solid-solution-solid process plays an important role in the formation mechanism of hollow Cu0.3Co2.7O4 microspheres. At the preliminary formation period of the Cu0.3Co2.7O4 microspheres, a number of solid crystals with poor crystallography and small dimension grow in solution due to the kinetic advantage. The dimension difference of the occurred crystallites then inevitably caused the change of concentrations of solutes across the solution. Those crystals with smaller size and nanocrystallites located in the center of the crystal particles were believed to dissolve continuously and be smaller owing to their higher thermodynamic solubility, and should be relocated and evacuated eventually, while the larger ones were essentially immobile. The uniformity of this concentration gradient will ultimately eliminate crystals of smaller sizes [13]. As a result, the interior space came into being through the dissolving and regrowing of nanocrystallites in the center of the crystal particles.

Figure 5 SEM images of products prepared at 220 °C for different reaction time:
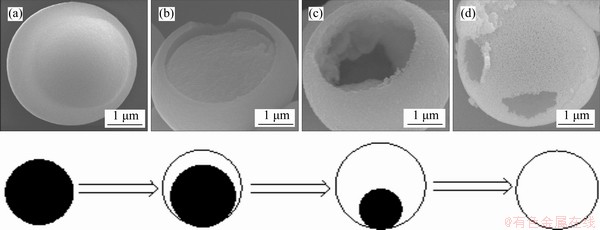
Figure 6 Schematic illustration of formation mechanism of Cu0.3Co2.7O4 microspheres and hollowing process ((a)-(d) are SEM images of products prepared at 220 °C for 2 h, 6 h, 12 h and 24 h, respectively)
Finally, hollow Cu0.3Co2.7O4 microspheres with a wall thickness of about 100 nm were obtained.
3.3 Gas-sensitivity
Figure 7 presents the nitrogen adsorption desorption isotherms and the corresponding pore size distribution curve of the as-prepared Cu0.3Co2.7O4 microspheres. The obtained isotherm of the Cu0.3Co2.7O4 microspheres shows a typical hysteresis loop, suggesting the mesoporous structure of the product. The pore size distribution shows that the as-prepared Cu0.3Co2.7O4 microspheres contain two pore sizes of 3.3 and 5.9 nm, which could also be observed in SEM image (Figure 2(c)). The BET surface area of the hollow microspheres is 23.15 m2/g.
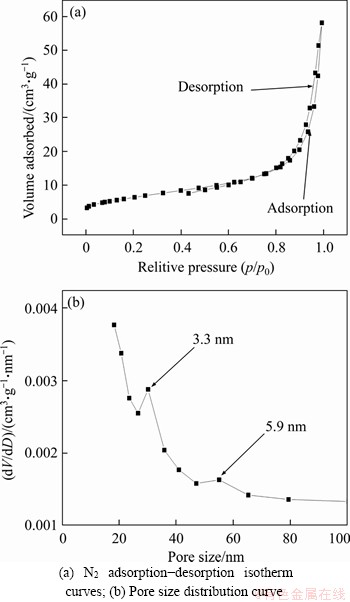
Figure 7 BET measurements of Cu0.3Co2.7O4 microspheres:
Figure 8 depicted gas responses of the as- prepared Cu0.3Co2.7O4 sensors to 50×10-6 mg/m3 of C2H5OH, HCHO, H2, O2, CO and CH4. As shown in Figure 8(a), the response of Cu0.3Co2.7O4 sensor to 50×10-6 mg/m3 of C2H5OH has a maximum value of 2.09 at 190 °C, showing the optimal working temperature of 190 °C for 50×10-6 mg/m3 C2H5OH. Keeping the same working temperature of 330 °C, the gas responses of the Cu0.3Co2.7O4 sensors to HCHO, H2, O2, CO and CH4 with the same concentration of 50×10-6 mg/m3 are represented in Figure 8(b), showing that the response of the Cu0.3Co2.7O4 sensors to 50×10-6 mg/m3 of HCHO, H2, O2, CO and CH4 decreased with the sensitivity (Rg/Ra) less than 1.21. Comparing with other as-reported spinel metal oxides such as zinc cobalt oxides, the sensitivity to 50×10-6 mg/m3 of C2H5OH below 1.0 [19], the copper cobalt oxides sensors prepared in our work give better gas sensing properties to 50×10-6 mg/m3 of C2H5OH. It is suggested that hollow nano-structures with larger specific surface area are beneficial to the improvement of gas-sensitivity of spinel metal oxides sensors. The as-prepared Cu0.3Co2.7O4 sensors could be used as a promising sensor at the detection of 10-6 mg/m3 concentrations to C2H5OH. The corresponding research is under way.
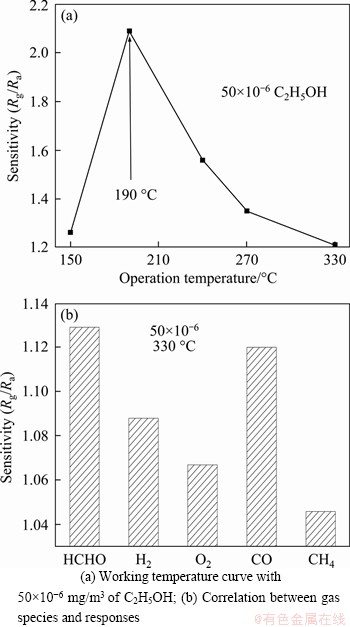
Figure 8 Responses to normal gases of as-prepared Cu0.3Co2.7O4 sensors:
4 Conclusions
In conclusion, hollow Cu0.3Co2.7O4 microspheres with spinel phase structure have been controllably synthesized via a simple and template-free hydrothermal method. The mean diameter of as-prepared hollow Cu0.3Co2.7O4 microspheres is several micrometers. And the wall thickness of hollow Cu0.3Co2.7O4 microspheres is less than 100 nm, which consists of single-crystalline nanocubes with the diameter of about 20 nm. The proposed growth mechanism of hollow Cu0.3Co2.7O4 microspheres was detailedly studied and it suggested that the Cu0.3Co2.7O4 microspheres hollowed owing to Oswald ripening. The result of BET measurements shows the mesoporous structures of the as-prepared Cu0.3Co2.7O4 microspheres with two pore sizes of 3.3 and 5.9 nm. And the BET surface area is 23.15 m2/g. The gas-sensitivity of Cu0.3Co2.7O4 sensors were determined to present a gas responses selectivity to 50×10-6 mg/m3 of C2H5OH, and the sensitivity (Rg/Ra) value is 2.09 at the optimal operation temperature of 190 °C. The research may provide a controllable and repeatable approach to obtain hollow microspheres to act as microreactors, filters, ion-exchangers, and so on.
Contributors
TIAN Li provided the concept and edited the draft of manuscript. LIU Qiang edited the draft of manuscript and conducted the literature review. WU Jie-ling and YI Yi-tao draw and modified the manuscript pictures. All authors replied to reviewers’comments and revised the final version.
Conflict of interest
TIAN Li, LIU Qiang, WU Jie-ling and YI Yi-tao declare that they have no conflict of interest.
References
[1] ZHANG Gen-qiang, LOU Xiong-wen. General synthesis of multi-shelled mixed metal oxide hollow spheres with superior lithium storage properties [J]. Angewandte Chemie International Edition, 2014, 126(34): 9087-9190. DOI: 10.1002/ anie.201404604.
[2] TIAN Li, YANG Xian-feng, LU Ping, IAN D, WANG Cai-hong, WU Ming-mei. Hollow single-crystal spinel nanocubes: The case of zinc cobalt oxide grown by a unique Kirkendall effect [J]. Inorganic Chemistry, 2008, 47(13): 5522-5524. DOI: 10.102/ic702457b.
[3] WANG Zhi-yu, ZHOU Liang, LOU Wen-xiong. Metal oxide hollow nano-structures for lithiumion batteries [J]. Advanced Materials, 2012, 24(14): 1903-1911. DOI: 10.1002/adma. 201200469.
[4] PENDASHTEH A, PALMA J, ANDERSON M. Nano-structured porous wires of iron cobaltite: Novel positive electrode for high performance hybrid energy storage devices [J]. Journal of Materials Chemistry A, 2015, 3: 16849-16859. DOI: 10.1039/C5TA02701B.
[5] SHI Yong-qian, YU Bin, ZHOU Ke-qing, RICHARD K, GUI Zhou, HU Yuan, JIANG Sai-hua. Novel CuCo2O4/graphitic carbon nitride nano-hybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic poly-urethane nanocomposites [J]. Journal of Hazardous Materials, 2015, 293: 87-96. DOI: 10.1016/j.jhazmat.2015. 03.041.
[6] LIM T, PARK S, KIM J, KIM D. Ordered mesoporous MCo2O4 (M=Cu, Zn and Ni) spinel catalysts with high catalytic performance for methane combustion [J]. Journal of Molecular Catalysis A: Chemical, 2017, 426: 68-74. DOI: 10. 1016/j.molcata.2016.11.002.
[7] ZHU Di, SUN Xun, YU Jing, LIU Qi, LIU Jing-yuan, CHEN Rong-rong, ZHANG Hong-shen, LI Ru-min, YU Jia. WANG Jun. Rationally designed CuCo2O4@Ni(OH)2 with 3D hierarchical core-shell structure for flexible energy storage [J]. Journal of Colloid and Interface Science, 2019, 557: 76-83. DOI: 10.1016/j.jcis.2019.09.010.
[8] DU Xiao-qiang, ZHANG Xiao-shuang, XU Zhou-feng, GONG Ya-qiong. CuCo2O4 microflowers catalyst with oxygen evolution activity comparable to that of noble metal [J]. International Journal of Hydrogen Energy, 2018, 43(10): 5012-5018. DOI: 10.1016/ j.ijhydene.2018.01.142.
[9] PAKNAHAD P, ASKARI M. Characterization of nano- crystalline CuCo2O4 spinel prepared by sol-gel technique applicable to the SOFC interconnect coating [J]. Ghorbanzadeh, Applied Physics A, 2015, 119: 727-734. DOI: 10. 1007/s00 339-015-9021-7.
[10] TIAN Li, ZHAO Rui-ni, WANG Jing-jing, SUN Qi-liang, CHEN Lin, XIAO Qiao-guo, LIU Sheng-li. Faclie synthesis and luminescence of hollow Eu3+-doped LaVO4 nanospheres [J]. Materials Letters, 2015, 156: 101-104. DOI: 10.10 16/ j.matlet.2015.05.002.
[11] CHEN Hong-ying, CHEN J. Preparation of p-type CuCo2O4 thin films by sol-gel processing [J]. Materials Letters, 2017, 188: 63-65. DOI: 10.1016/ j.matlet.2016.10.096.
[12] LI Gao-feng, LIU Si-qi, PAN Yang, ZHOU Tian-yue, DING Jian-dong, SUN Yue-ming, WANG Yu-qiao. Self-templated formation of CuCo2O4 triple-shelled hollow microspheres for all-solid-state asymmetric supercapacitors [J]. Journal of Alloys and Compounds, 2019, 787: 694-699. DOI: 10.10 16/j.jallcom.2019.02.134.
[13] ZENG Chun-hua. Ostwald ripening: A synthetic approach for hollow nanomaterials [J]. Current Nanoscience, 2007, 3(2): 177-181. DOI: 10.2174/ 157341307780619279.
[14] CHENG Jin-bing, YAN Hai-long, LU Yang, QIU Kang-wen, HOU Xiao-yi, XU Jing-you, LIU Xiao-ming, KIM J, LUO Yong-song. Mesoporous CuCo2O4 nanograsses as multi-functional electrodes for supercapacitors and electro-catalysts [J]. Journal of Materials Chemistry A, 2015, 3(18): 9769-9776. DOI: 10.1039/C5TA00408J.
[15] WANG Peng-xiang, SHAO Li, ZHANG Nai-qing, SUN Ke- ning. Mesoporous CuCo2O4 nanoparticles as an efficient cathode catalyst for Li-O2 batteries [J]. Journal of Power Sources, 2016, 325: 506-512. DOI: 10.1016/j.jpowsour.2016. 06.065.
[16] YANG Juan, YE Hui-li, ZHANG Zheng-qiong, ZHAO Fa-qiong, ZENG Bai-zhao. Metalorganic framework derived hollow polyhedron CuCo2O4 functionalized porous graphene for sensitive glucose sensing [J]. Sensors and Actuators B, 2017, 242: 728-735. DOI: 10.1016/j.snb.2016.11.122.
[17] ZHAO Shi-duo, LI Qi-ming, LI Fang, LIANG Zhi-hua. Synthesis of spinel CuCo2O4 nanoparticles and its application in pnitrophenol reduction [J]. Journal of Sol-Gel Science and Technology, 2017, 524: 544-555. DOI: 10.1007/s10971-016-4200-3.
[18] KUANG Min, LIU Ying-xiao, DONG Fang, ZHANG Yu-xin. Tunable design of layered CuCo2O4 nanosheets@MnO2 nanoflakes coreshell arrays on Ni foam for high-performance super-capacitors [J]. Journal of Materials Chemistry A, 2015, 3(43): 21528-21536. DOI: 10.1039/C5TA05 957G.
[19] NIU Xin-shu, DU Wei-ping, DU Wei-min. Preparation and gas sensing properties of ZnM2O4 (M=Fe, Co, Cr) [J]. Sensors and Actuators B: Chemical, 2004, 99(2): 405-409. DOI: 10.1016/j. snb.2003.12.007.
(Edited by HE Yun-bin)
中文导读
中空结构Cu0.3Co2.7O4微球的无模板水热合成和气敏性
摘要:以硫酸铜、乙酸钴和氨为原料首次通过简单的无模板水热法可控合成了空心结构的Cu0.3Co2.7O4微球。通过粉末X射线衍射、能量色散X射线分析、选区电子衍射、高分辨率透射电子显微镜、扫描电子显微镜和BET测量来表征产物。研究结果表明,空心Cu0.3Co2.7O4微球由直径约20 nm的单晶纳米立方体组成。中空Cu0.3Co2.7O4微球的形成是基于固溶-固相过程中的Ostwald熟化作用。Cu0.3Co2.7O4微球是中孔的,包含3.3和5.9 nm两种孔径。所制备的Cu0.3Co2.7O4传感器在190 ℃下对50×10-6 C2H5OH具有最佳的气体响应。
关键词:Cu0.3Co2.7O4氧化物;微球无机化合物;纳米结构;无模板水热法
Foundation item: Project(51202066) supported by the National Natural Science Foundation of China; Project(NCET-13-0784) supported by the Program for New Century Excellent Talents in University of China
Received date: 2020-03-12; Accepted date: 2020-10-25
Corresponding author: TIAN Li, PhD, Professor; Tel: +86-18627323439; E-mail: 1060072@hnust.edu.cn; ORCID: https://orcid.org/0000-0002-4035-5530