模拟汗液中铜币的腐蚀行为
来源期刊:中国有色金属学报(英文版)2015年第2期
论文作者:梁成浩 王树森 黄乃宝 王 鹏
文章页码:654 - 660
Key words:brass coinage; dezincification; pitting corrosion; synthetic sweat solution
摘 要:采用电化学方法以及扫描电子显微镜、X-射线电子能谱表面分析技术,研究铜币在模拟汗液中的腐蚀行为。结果表明,模拟汗液中的氯离子能加剧黄铜的阳极活性溶解反应,是发生孔蚀和脱锌腐蚀的主要原因,氨水和乳酸的存在对其阳极反应过程有一定促进作用,而尿素的存在对腐蚀过程影响不大。黄铜表面形成的腐蚀产物主要由氯化铜、氧化亚铜、尿素与铜形成的配合物以及少量的乳酸离子组成。黄铜在模拟汗液中蚀孔发展的动力学过程遵循方程J0=0.3735(t+185.93)-1/2,该过程受孔表面盐膜溶解控制。
Abstract: Corrosion behavior of brass coinage was investigated in synthetic sweat solution by electrochemical measurement and surface analysis methods including scanning electron microscope (SEM) and energy dispersive X-ray spectrometer (EDX). It is indicated that chloride ions in sweat solution accelerate the anodic active dissolution of brass, which is the main reason of pitting corrosion and dezincification corrosion. Meanwhile, lactic acid and ammonia water also promote the anode reaction. The corrosion products on the surface are mainly composed of basic copper chloride, cuprous oxide, the complex consisting of urea in association with copper, and few lactate ion. The kinetics of pitting corrosion development obeys the following equation of J0=0.3735(t+185.93)-1/2, and the process is controlled by dissolution of salt deposited on pit surface.
Trans. Nonferrous Met. Soc. China 25(2015) 654-660
Cheng-hao LIANG1, Shu-sen WANG1, Nai-bao HUANG1, Peng WANG2
1. College of Transportation Equipments and Ocean Engineering, Dalian Maritime University, Dalian 116026, China;
2. Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Received 29 April 2014; accepted 10 September 2014
Abstract: Corrosion behavior of brass coinage was investigated in synthetic sweat solution by electrochemical measurement and surface analysis methods including scanning electron microscope (SEM) and energy dispersive X-ray spectrometer (EDX). It is indicated that chloride ions in sweat solution accelerate the anodic active dissolution of brass, which is the main reason of pitting corrosion and dezincification corrosion. Meanwhile, lactic acid and ammonia water also promote the anode reaction. The corrosion products on the surface are mainly composed of basic copper chloride, cuprous oxide, the complex consisting of urea in association with copper, and few lactate ion. The kinetics of pitting corrosion development obeys the following equation of J0=0.3735(t+185.93)-1/2, and the process is controlled by dissolution of salt deposited on pit surface.
Key words: brass coinage; dezincification; pitting corrosion; synthetic sweat solution
1 Introduction
Due to its magnificent color, brass is widely used in coinage and heat exchanger [1,2]. Tarnishing of coinage is a common phenomenon during circulation process. It shortens service life of coinage and reduces the collection value of commemorative coin.
There are many reasons for tarnishing of brass coinage, such as corrosive gas in air, the water molecule adsorbed on surface. As we know, coinage often contacts with skin during its usage process, so sweat left on coinage becomes one of the main reasons for tarnishing. Until now, there have been several reports about the corrosion of copper alloys for coinage in sweat solution. For example, COLIN et al [3,4] studied the corrosion product of copper/zinc/nickel, copper/nickel/zinc and nickel/copper alloys in synthetic sweat medium by means of surface analysis. It was found that the corrosion layers are composed of copper and nickel based compounds. For the alloys with a high copper content, the corrosion layers are mainly composed of copper (I) oxide in which chloride anions are included. For the alloys with a high nickel content, copper chloride hydroxide and nickel-compounds like nickel hydroxide and nickel oxide are detected in the corrosion layer. However, most of these reports centered at the analysis of corrosion production on metal surface, there was few study on the corrosion behavior of brass in sweat solution. Furthermore, the researches on localized corrosion of brass mostly focused on dezincification corrosion, few about pitting corrosion of brass [5,6]. However, to the best of our knowledge, it has widely used in China, in sweat solution until now.
Herein, the corrosion of brass coinage was investigated with electrochemical measurement and surface analysis methods. Based on the test results, the effects of the composition in synthetic sweat solution on the corrosion of brass were discussed, and the corrosion product and type of brass in sweat solution were analyzed. Furthermore, the corrosion process of brass in sweat solution was clarified.
2 Experimental
The material used in this work was α brass with a composition of copper 70 % and zinc 30 %, which was used for manufacturing coinage.
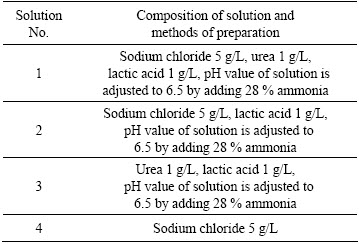
For the electrochemical test, the brass electrode was embedded into the epoxy resin with a geometrical surface area of 1 cm2 exposed to the corrosive environment. The electrode was abraded with emery paper to 2000 grade, degreased with ethanol and dried with blower before the experiment. The electrochemical tests were carried out in the four kinds of solutions shown in Table 1 at the temperature of (25±2) °C. The solution 1 in Table 1 is synthetic sweat solution [3].
Table 1 Composition of solution and methods of preparation
All electrochemical tests were carried out in a conventional three-electrode cell with Solartron 1280. Platinum plate was the counter electrode, and saturated calomel electrode (SCE) was the reference electrode.
Potentiodynamic polarization tests ranged from -0.2 V (more negative than the free corrosion potential (vs SCE)) to 1 V with the scan rate of 0.5 mV/s. The potentiodynamic cyclic anodic polarization tests commenced with the application of -0.03 V, more negative than the free corrosion potential. Subsequently, a potential scan started in the anodic direction with the scan rate of 1 mV/s until the potential reached 1 V (vs SCE), and then it was reversed in the backward direction with the same scan rate. Potentiostatic polarization test was carried out at the potential of 1 V.
Immersion test was carried out in synthetic sweat solution (solution 1 in Table 1) and the experimental temperature was (25±2) °C. In order to prevent the sweat from evaporating, the beaker filled with solution was covered with a protective plastic film. After 28 d of free corrosion, the samples were rinsed with distilled water and dried by ambient air. The corroded surface of the tested brass was inspected by scanning electron microscope (SEM) and optical microscope (OM), and the composition was revealed with X-ray diffraction (XRD), Fourier transform infrared spectrometer (IR), and energy dispersive X-ray spectrometer (EDX). In order t o confirm the corr osion type of brass in sweat solution, the composition of the solution in which brass was immersed for 24 h was analyzed by atomic absorption spectroscopy (AAS).
3 Results and discussion
3.1 Corrosion type of brass in synthetic sweat
After 28 d of immersion, brownish yellow corrosion product can be observed on brass surface. Figure 1 presents the SEM micrographs of brass after immersion. It can be observed that the corrosion product film presents map cracking shape (see Fig. 1(a)), and the corrosion product is loose and porous (see Fig. 1(b)).
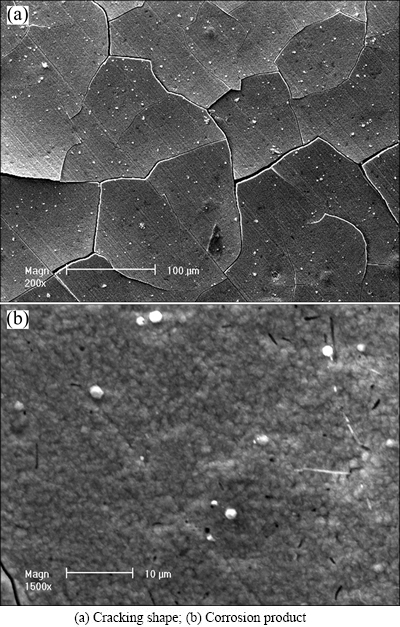
Fig. 1 SEM images of brass immersed in sweat solution for 28 d
After corrosion product on brass surface is removed, the cross section of brass is observed with OM, and its result is shown in Fig. 2. Several pits with depth of about 10 μm can be observed on brass surface. It is demonstrated that pitting corrosion occurs on brass surface in sweat solution.

Fig. 2 Side view of brass immersed in sweat solution for 28 d
After 28 d of brass immersion, the solution presents weakly alkaline with pH of 7.59. Furthermore, the color of solution changes to green, indicating that Cu (II) ions are released into solution. As we know, dezincification corrosion is a common corrosion type for brass in corrosive solution. In order to confirm its corrosion type, AAS was used to analyze the contents of copper ions and zinc ions in sweat solution. It is found that the contents of copper ions and zinc ions are 2.7569 mg/L and 2.0896 mg/L, respectively. According to the definition of dezincification coefficient:
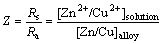 (1)
(1)
where Rs=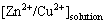 is the zinc/copper mole ratio in the solution after experiment; Ra=
is the zinc/copper mole ratio in the solution after experiment; Ra= is the zinc/copper mole ratio of brass.
is the zinc/copper mole ratio of brass.
It could be obtained that the dezincification coefficient of brass in synthetic sweat solution is 2.099, which is more than 2. Thus, it can be concluded that dezincification corrosion occurs on brass in synthetic sweat solution.
3.2 Composition of corrosion product
Figure 3 shows the EDX analysis result of the corrosion production on brass surface after being immersed in synthetic sweat solution for 28 d. Copper, chloride and oxygen are found on brass surface, and their mole ratio is 28.73 : 17.14 : 54.13, indicating that chloride and oxygen take part in the corrosion process of brass in synthetic sweat solution.

Fig. 3 EDX result of brass immersed in simulated sweat solution for 28 d
Figure 4 shows the IR result of brass after being immersed in sweat solution for 28 d. The appearance of characteristic peaks at 1640 cm-1 and 1160 cm-1 proves that urea formed complex with copper (II) in the form of oxygen—metal bond [7,8]. The peaks of carbonyl group at 1730 cm-1 and carbon—oxygen group at 1263 cm-1 suggest that the lactic acid or lactic acid radical ions (or both) exist in the corrosion product. One thing should be mentioned that carbon or nitrogen is not found on brass surface according to EDX analysis (see Fig. 3). This contradiction could be explained as follows: the contents of urea and lactic acid are so low that they could not be detected by EDX. According to the standard spectrum of copper oxides (cupric oxide and cuprous oxide), the peaks at 1380 cm-1, 1460 cm-1 and the region of 2800-3000 cm-1 all demonstrate the formation of copper oxides on brass surface, but the exact form could not be confirmed. Furthermore, the break peaks at the region of 3299-3448 cm-1 can be attributed to hydroxy with different bonding structures. They correspond to the copper—hydroxyl bond with the characteristic peaks at the region of 836-997 cm-1 and water with bending vibration bands at 1636 cm-1. These analyses indicate that water and the substance containing copper—hydroxyl bond exist on brass surface.

Fig. 4 IR result of brass after being immersed in sweat solution for 28 d
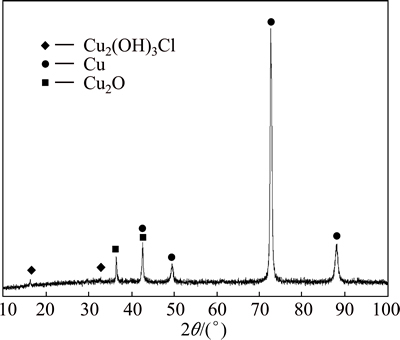
Fig. 5 XRD pattern of brass after being immersed in sweat solution for 28 d
XRD analysis result of brass is shown in Fig. 5. It is found that the corrosion product of brass immersed in synthetic sweat solution contains Cu2(OH)3Cl and cuprous oxide. Combining with IR, XRD and EDX analyses, it can be concluded that the corrosion products of brass immersed in synthetic sweat solution contain copper chloride hydroxide, cuprous oxide, urea complex formed with copper (II), and low content of lactic acid or lactic acid radical ions (or both).
3.3 Effects of composition in sweat solution on brass corrosion
Figure 6 shows the potentiodynamic polarization curves of brass in the four kinds of solutions. Compared with solution 1 (synthetic sweat solution), the free corrosion potential of brass in solution 3 is more positive (-0.08 V (vs SCE)), and the current density at the same anodic potential is lower. This means that the existence of NaCl could accelerate the anodic reaction process of brass in synthetic sweat. The polarization curves of brass in solutions 1 and 2 are almost overlapped, which proves that urea in synthetic sweat solution shows little effect on the corrosion of brass. Compared with solution 1, the anodic polarization current density of brass in solution 4 is lower in the potential range of 0.3-1 V, whereas, it is still higher than that in solution 3. These phenomena indicate that the existence of lactic acid and ammonia can also accelerate the anodic reaction process of brass, but sodium chloride is the main cause of corrosion.

Fig. 6 Polarization curves of brass in four kinds of solutions
3.4 Dynamic process of pitting corrosion
Figure 7 shows the potentiodynamic cyclic anodic polarization curves of brass in solution 1 and solution 3. The curve for brass in solution 1 (sweat solution) presents an anodic current peak at about 0.2 V, which corresponds to the formation of soluble  from copper or cuprous chloride [9]. In the potential range of 0.28-0.72 V, the anodic current density maintains at 3 mA/cm2, and cuprous chloride is formed on brass surface [10]. When polarization potential is further increased, the current density increases rapidly. Furthermore, during the reverse sweep, the curve intersects with the forward sweep curve at 0.42 V, and a hysteresis current loop appears. However, no hysteresis current loop can be found from the curve of brass in solution 3. The phenomenon indicates that chloride ion facilitates the pitting corrosion of brass in sweat solution, the critical pitting corrosion potential is 0.72 V, and the protection potential is 0.42 V.
from copper or cuprous chloride [9]. In the potential range of 0.28-0.72 V, the anodic current density maintains at 3 mA/cm2, and cuprous chloride is formed on brass surface [10]. When polarization potential is further increased, the current density increases rapidly. Furthermore, during the reverse sweep, the curve intersects with the forward sweep curve at 0.42 V, and a hysteresis current loop appears. However, no hysteresis current loop can be found from the curve of brass in solution 3. The phenomenon indicates that chloride ion facilitates the pitting corrosion of brass in sweat solution, the critical pitting corrosion potential is 0.72 V, and the protection potential is 0.42 V.
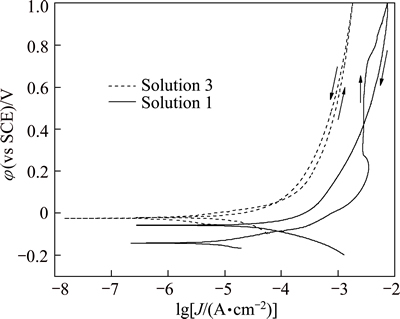
Fig. 7 Cyclic anodic polarization curves of brass in two kinds of solutions
Potentiostatic polarization test was operated to determine the mechanism of pitting corrosion of brass in sweat solution. Figure 8 shows the potentiostatic current density-time curve measured at pitting corrosion zone (1 V). The current density gradually decreases with the prolongation of time, and maintains at 8 mA/cm2 after 2000 s. White flecks are discovered on the surface of sample after test, and pits present after removing corrosion production (see Fig. 9). This proves that pitting corrosion occurs at this test condition.
Pitting corrosion is a common phenomenon for the metal in chloride-containing solution. The deduction of its dynamic process is very complex and some simplified treatments are necessary. Firstly, we regard the entire brass electrode surface as a developing pit. Secondly, the single-phase brass is considered one metal element [11].

Fig. 8 Current density-time curve of brass in sweat solution at 1 V

Fig. 9 Micrograph of brass after potentiostatic polarization at potential of 1 V
The relationship between a pit depth h and initial current density J0 is ratiocinated in advance.
In accordance to the Farady’s law,
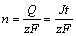 (2)
(2)
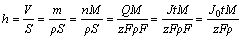 (3)
(3)
where n is the amount of metal reacted; Q is the electrical amperage consumed during the reaction; z is the transferring electrical charge number; S is the bottom area; m is the mass of metal dissolved; ρ is the metal density; M is the relative mole mass.
The differential coefficient of h in Eq. (3) to t can be obtained as follows:
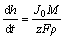 (4)
(4)
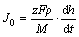 (5)
(5)
Based on the Fick’s law [12]:
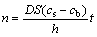 (6)
(6)
and Farady’s law:
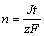 (7)
(7)
Equation (8) could be obtained:
 (8)
(8)
where D is the diffusion coefficient; cs is the concentration of metal ions at the bottom of pit; cb is the concentration of metal ions in substrate solution.
It is supposed that the development of pitting corrosion is controlled by the dissolution of salt deposit on pit surface. In this case, cs is constant because the metal ions at the bottom of pit are saturated. cb can be regarded as zero for the concentration of metal ions in substrate solution is very low. So, we could get
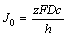 (9)
(9)
Substituting Eq. (7) into Eq. (9), integral calculus is performed,
 (10)
(10)
Substituting Eq. (10) into Eq. (9),
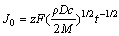 (11)
(11)
Supposing 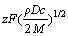 = k, it could be deduced that
= k, it could be deduced that
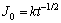 (12)
(12)
Fitting analysis is performed to the current density- time relationship curve shown in Fig. 8, and function expression of the curve is
 (13)
(13)
where k=0.3735(s0.5·A·cm-2), a=185.93 s.
Comparing Eq. (12) with Eq. (13), it is found that they had the same power index, which proves that the developing rate of pit is controlled by the dissolution of salt depositing on pits surface [11].
3.5 Corrosion process of brass in sweat solution
According to the results above, it is inferred that dezincification and pitting corrosion are the main localized corrosion types for the brass in sweat solution. These two types of corrosions occur at the same time. We discuss dezincification corrosion of brass at first.
Within the testing period (28 d), the sweat solution changes from weakly acidic (pH=6.5) to weakly alkaline (pH=7.59), so the cathodic reaction is mainly composed of reduction of oxygen:
O2+2H2O+4e→4OH- (14)
The anodic reaction is losing electrons process of metal, and zinc transfers into solution in the form of zinc ions:
Zn→Zn2++2e (15)
Different from zinc, the action related to copper is complex. During the initial stage, chloride ion reacts with copper and formed cuprous chloride porous layer [13,14]:
 (16)
(16)
Since chloride ion kept on eroding metal and reacted with cuprous chloride, copper changed to the soluble  [15]:
[15]:
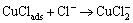 (17)
(17)
 (18)
(18)
Subsequently, the high concentration of  on brass surface leads to
on brass surface leads to  hydrolyzing to cuprous oxide (Fig. 5) [16]:
hydrolyzing to cuprous oxide (Fig. 5) [16]:
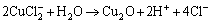 (19)
(19)
Furthermore, the cuprous oxide can be partly oxidized, and disproportionation reaction of cuprous oxide also occurs [17]:
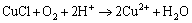 (20)
(20)
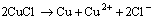 (21)
(21)
Both reactions would result in the formation of Cu (II), the fate of which contained forming stable complex with urea on brass surface (Fig. 4), forming cupric chloride hydroxide (Fig. 5) and diffusing into solution.
In addition, because the deposition potential of cupric ions is higher than the corrosion potential of brass, the Cu (II) could be reduced to Cu (0) which deposited on brass surface, and zinc ions stayed in the solution. Thus, dezincification corrosion occurred.
It had been proven that the existence of sodium chloride in sweat solution facilitates the pitting corrosion of brass (Fig. 8). Because of the oxygen in solution, zinc oxide and cuprous oxide could form on brass surface, and protect the substrate to some extent [15]. However, chloride ion could pass through the layers under the electric field action between the interfaces of layer and solution for its small radius. Thus, chloride ion, transferred to the interface between layer and substrate, could erode the substrate and resulted in pits. The related reaction equations are the same as Eqs. (15), (16) and (21).
Subsequently, the cuprous chloride and in pits hydrolyzed rapidly, which leads to the enrichment of hydrogen ion and chloride ion, and the related reaction equation is the same as Eq. (19).
in pits hydrolyzed rapidly, which leads to the enrichment of hydrogen ion and chloride ion, and the related reaction equation is the same as Eq. (19).
Meanwhile, excess cations appear in pits for the metal dissolution. In order to keep the electroneutrality, zinc ions and copper ions in pits transfer to bulk solution. On the other hand, chloride ion in the solution transfers into pits, which results in the increase of chloride ion concentration in pits.
These reasons mentioned above result in the increase of the concentratings of both chloride ion and hydrogen ion in pits, in further, the dissolution of brass is accelerated. With the prolonging of time, the corrosion product (such as cupric chloride hydroxide) formed at ostiole makes the pits occlude, and hinders the outdision of chloride ion and hydrogen ion. Thus, the erodibility of ions in pits is improved, and the pits are further expanded.
Furthermore, the existence of lactic acid and ammonia could accelerate the anodic dissolution of brass (Fig. 6). Lactic acid belongs to organic acid, and its erodibility to metal is very weak. So, it can be inferred that ammonia in sweat would react with brass, and the related reactions are as follows:
 (22)
(22)
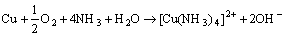 (23)
(23)
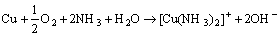 (24)
(24)
4 Conclusions
1) For the brass in sweat solution, dezincification and pitting corrosion are the main localized corrosion types. The corrosion product formed on brass surface contains cupric chloride hydroxide, cuprous oxide, urea complex formed with copper (II), and low content of lactic acid or lactic acid radical ions (or both).
2) Chloride ion in sweat solution accelerates the anodic active dissolution of brass, which is the main reason of corrosion. Lactic acid and ammonia water also promote the anode reaction. The presence of urea has little influence on the corrosion process.
3) Sodium chloride facilitates the pitting corrosion of brass in sweat solution. The development of pitting corrosion obeys the formula J0=0.3735(t+185.93)-1/2, and it is controlled by dissolution of salt deposit on pits surface.
References
[1] CHEN J L, Li Z, ZHU A Y, LUO L Y, LIANG J. Corrosion behavior of novel imitation-gold copper alloy with rare earth in 3.5% NaCl solution [J]. Materials & Design, 2012, 34(2): 618-623.
[2] ROBBIOLA L, TRAN T T M, DUBOT P, MAJERUS O, RAHMOUNI K. Characterisation of anodic layers on Cu-10Sn bronze (RDE) in aerated NaCl solution [J]. Corrosion, 2008, 50(8): 2205-2215.
[3] COLIN S, BECHE E, BERJOAN R, JOLIBOIS H, CHAMBAUDET A. An XPS and AES study of the free corrosion of Cu-, Ni- and Zn-based alloys in synthetic sweat [J]. Corrosion Science, 1999, 41(6): 1051-1065.
[4] COLIN S, KRIER G, JOLIBOIS H, HACHIMI A, MULLER J F, CHAMBAUDET A. Characterization of the corrosion layer of copper-nickel alloys in a synthetic sweat medium by FTMS and LAMMA laser microprobes [J]. Applied Surface Science, 1998, 125(1): 29-45.
[5] SONG J Y, HONG S I. Design and characterization of new Cu alloys to substitute Cu-25%Ni for coinage applications [J]. Materials & Design, 2011, 32(4): 1790-1795.
[6] ICONSTANTINIDES I, AADRIAENS A, ADAMS F. Surface characterization of artificial corrosion layers on copper alloy reference materials [J]. Applied Surface Science, 2002, 189(1-2): 90-101.
[7] DENG Fan-zheng, ZHU Ai-xia, YANG Rui. Study on prepation of CuO/Cu2(OH)3Cl powder and its spectrum behavior for photodegradation decoloration of dyes [J]. Spectro Scopy and Spectral Analysis, 2006, 26(2): 299-301. (in Chinese)
[8] AMOL P, AMRUTE, CECILIA M, MIGUEL A G H, JAVIER P R. Temporal analysis of products study of HCl oxidation on copper- and ruthenium-based [J]. The Journal of Physical Chemistry, 2011, 15(4): 1056-1063.
[9] WANG Hong-zhi, CHEN Jun, ZHOU Jian-qi, YAO Su-wei, ZHANG Wei-guo. Corrosion behavior of welded joints of copper pipe in artificial seawater [J]. Journal of Chemical Industry and Engineering, 2006, 57(11): 2677-2681. (in Chinese)
[10] RABAB M E S, KHALED M I, WAHEED A B. Effect of Zn and Pb as alloying elements on the electrochemical behavior of brass in NaCl solutions [J]. Electrochimica Acta, 2004, 49(28): 5139-5150.
[11] KABASAKALOGLU M, KIYAK T, SENDIL O, ASAN A. Electrochemical behavior of brass in 0.1 mol/L NaCl [J]. Applied Surface Science, 2002, 193(1-4): 167-174.
[12] CHEN B Y, LIANG C H, FU D J, REN D M. Corrosion behavior of Cu and the Cu-Zn-Al shape memory alloy in simulated uterine fluid [J]. Contraception, 2005, 72(3): 221-224.
[13] NAGEH K A, AHMED A N, ELSAYED A A. A review of the effects of benzotriazole on the corrosion of copper and copper alloys in clean and polluted environments [J]. Journal of Applied Electrochemistry, 2009, 39(7): 961-969.
[14] ZHANG Y N, ZI J L, ZHENG M S, ZHU J W. Corrosion behavior of copper with minor alloying addition in chloride solution [J]. Journal of Alloys and Compounds, 2008, 462(1-2): 240-243.
[15] FOLQUER M E, RIBOTTA S B, REAL S G, GASSA M. Study of copper dissolution and passivation processes by electrochemical impedance spectroscopy [J]. Corrosion, 2002, 58(3): 240-247.
[16] TUNC T, NUR K, TUGBA E N, GOKMEN S , MEHMET E. Self assembled film based on hexane-1,6-diamine and 2-mercapto-ethano on copper [J]. Applied Surface Science, 2012, 258(18): 6793-6799.
[17] RYLKINA M V, KUZNETSOV YU I, KALASHNIKOVA M V, ANDREEVA N P. Halides as activators of the local corrosion of brass [J]. Protection of Metals, 2003, 39(2): 113-119.
梁成浩1,王树森1,黄乃宝1,王 鹏2
1. 大连海事大学 交通运输装备与海洋工程学院,大连 116026;
2. 中国科学院 青岛海洋研究所,青岛 266071
摘 要:采用电化学方法以及扫描电子显微镜、X-射线电子能谱表面分析技术,研究铜币在模拟汗液中的腐蚀行为。结果表明,模拟汗液中的氯离子能加剧黄铜的阳极活性溶解反应,是发生孔蚀和脱锌腐蚀的主要原因,氨水和乳酸的存在对其阳极反应过程有一定促进作用,而尿素的存在对腐蚀过程影响不大。黄铜表面形成的腐蚀产物主要由氯化铜、氧化亚铜、尿素与铜形成的配合物以及少量的乳酸离子组成。黄铜在模拟汗液中蚀孔发展的动力学过程遵循方程J0=0.3735(t+185.93)-1/2,该过程受孔表面盐膜溶解控制。
关键词:黄铜币;脱锌;孔蚀;模拟汗液
(Edited by Xiang-qun LI)
Foundation item: Project (21276036) supported by the National Natural Science Foundation of China; Project (2009AA05Z120) supported by the National High-tech Research and Development Program of China; Project (2014025018) supported by the Liaoning Provincial Natural Science Foundation of China; Project (3132014323) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Nai-bao HUANG; Tel: +86-411-84723280; E-mail: nbhuang@dlmu.edu.cn
DOI: 10.1016/S1003-6326(15)63649-4

