DOI:10.19476/j.ysxb.1004.0609.2017.03.013
纳米H2TiO3锂吸附剂的水热合成及其吸附性能
陈自正,沈卫华,陈立芳,成洪业,漆志文
(华东理工大学 化工学院 化学工程联合国家重点实验室,上海 200237)
摘 要:以TiO2和LiOH·H2O为原料,经水热反应、煅烧后得到纳米级别锂吸附剂前驱体Li2TiO3。用盐酸将Li+洗脱后得到纳米级别锂吸附剂H2TiO3。通过XRD、SEM、动力学测试等手段考察煅烧温度、煅烧时间对吸附剂的结构和洗脱吸附性能的影响,用拟一级和拟二级动力学方程对吸附过程进行拟合,并用Langmuir和Freundlich等温线方程拟合吸附平衡数据。结果表明:在773 K下煅烧2 h制备的吸附剂对锂离子吸附容量最高达到36.16 mg/g,并且具有极快的洗脱和吸附速率;洗脱5 h时,锂洗脱率为98.8%,吸附速率常数达到0.0339 g/(mg·h);吸附动力学符合拟二级动力学方程,吸附平衡数据符合Freundlich方程,锂离子对镁离子的分离因子达到154.17。
关键词:纳米H2TiO3;水热法;吸附动力学;吸附速率常数;Freundlich方程
文章编号:1004-0609(2017)-03-0547-08 中图分类号:TQ424.2;TQ028 文献标志码:A
锂已成为国内外重要的战略资源,被人们称为“21世纪的能源元素”。从矿石中提取锂步骤繁琐,且能耗较大,因此,从溶液中提取锂将成为今后提锂的发展方向[1]。中国的液体锂资源量极为丰富,主要集中在盐湖卤水、地热水以及海水中。但是大部分盐湖卤水中的Li+浓度很低[2-3],从这些稀锂溶液中提锂成为当下的热门课题[4-5]。
离子筛型吸附剂是目前最常用的一类从低品位锂资源中提锂的吸附剂。对于锂离子筛的研究目前主要集中在锰系锂离子筛[6-7],其中锰系锂离子筛存在锰溶损大、循环次数少等缺点,而钛系锂离子筛则具有结构稳定这一突出优点。颜辉等[5]用固相烧结法合成了锂离子筛前驱体Li4Ti5O12,对锂饱和吸附容量达到32.29 mg/g。石西昌等[8-9]用固相烧结法合成了偏钛酸型前躯体Li2TiO3,对锂的吸附量高达39.8 mg/g。ZHANG等[10]用软化学方法合成TiO2纳米管,再用固相烧结法制备Li2TiO3,对锂的吸附量达到了25.46 mg/g。李骁龙等[11]采用了溶胶-凝胶法,制备出尖晶石前驱体Li4Ti5O12,对锂的吸附量为17.46 mg/g。上述采用不同制备方法获得的钛系离子筛前驱体,具有不同的结构,能达到较高的吸附容量,但尺寸都处于微米级别,均存在离子交换速率较慢这一问题,例如吸附速率常数较低[8-9],酸洗脱锂时间达到72 h[10],达到吸附平衡时间长达120 h[11]。如何加快离子交换速率成为本文在制备吸附剂时首要考虑的问题。纳米级别离子筛具有吸附速率快、对锂离子的选择性高等优势[12]。水热法合成的Li2TiO3具有粒径超细、尺寸均一、纯度极高等特点[13-16]。但采用这一方法合成锂吸附剂H2TiO3的研究却鲜有报道。本文作者通过水热法合成纳米级别锂吸附剂前驱体Li2TiO3,酸洗脱锂后得到纳米级别锂吸附剂H2TiO3,相比于微米级别钛系离子筛,极大加快了洗脱、吸附速率;并对Li2TiO3和H2TiO3进行结构与形貌的表征,优化了制备过程中的主要参数,建立了动力学模型和吸附等温线模型。
1 实验
1.1 锂吸附剂的制备
将LiOH·H2O(AR,国药集团化学试剂有限公司生产)和TiO2 (P25,Degussa公司生产)按Li与Ti摩尔比为2:1,在去离子水中混合均匀,然后置于水热釜中在433 K下反应24 h。将水热产物过滤,在323 K下干燥,在不同温度(573~973 K)下煅烧不同时间(1~10 h)后得到锂吸附剂前驱体Li2TiO3。
对锂的洗脱即将锂吸附剂前驱体Li2TiO3放入盐酸溶液中,置于恒温水浴摇床(TS-110X30型)中振荡,直至达到平衡,洗涤、离心分离、干燥后得到锂吸附剂H2TiO3。
1.2 洗脱与吸附性能
将3 g的锂吸附剂前驱体Li2TiO3放入150 mL 0.6 mol/L盐酸溶液中,置于恒温水浴摇床中在308 K下振荡,在不同的时间取上层清液,采用电感耦合等离子体发射光谱仪(ICP,ARCOS FHS12型)分析清液中锂离子和少量钛离子含量,直至锂离子含量达到稳定,研究洗脱动力学。分别采用式(1)和(2)计算酸洗脱过程中锂的洗脱量(A)和洗脱率(R):
A=ρV/m (1)
R=ρVM1/(zmM2) (2)
式中:ρ为不同时间清液中锂的浓度;V为溶液体积;m为吸附剂质量;z为2,即Li2TiO3中Li的化学计量数;M1为Li2TiO3的相对分子质量;M2为锂的相对原子质量。当用式(2)计算钛的溶损率时,ρ为不同时间清液中钛的浓度;z为1,M2为钛的相对原子质量。
将2 g锂吸附剂H2TiO3放入150 mL不同浓度LiCl溶液中,pH=10.1(由体积比为1:4的0.1 mol/L NH4Cl和0.1 mol/L NH3·H2O的混合溶液调节pH),置于恒温水浴摇床中在308 K下振荡,在不同的时间取上层清液,采用ICP分析清液中锂离子含量,直至锂离子含量达到稳定,研究吸附动力学。采用文献[6]中的方法计算吸附剂对锂的吸附量。
1.3 结构与形貌的表征
采用X射线衍射仪(BRUKER D8 Focus型)分析锂吸附剂前驱体Li2TiO3、锂吸附剂H2TiO3和吸附后H2TiO3的形貌,工作参数如下:Cu Kα靶,扫描电压40 kV,电流40 mA,步长0.02°,扫描速度4 (°)/min,扫描范围10°~70°。采用扫描电子显微镜(HITACHI S-4800型)观察锂吸附剂前驱体Li2TiO3、锂吸附剂H2TiO3和吸附后H2TiO3的形貌。
2 结果与讨论
2.1 锂吸附剂前驱体Li2TiO3
2.1.1 煅烧温度
为了探究煅烧温度对结构和吸附容量的影响,保持煅烧时间2 h条件不变,对在不同煅烧温度下制备的吸附剂进行结构表征以及平衡吸附容量测试。
一般来说,Li2TiO3随着温度升高依次呈现3种稳定晶型:低温下为稳定的立方晶α相,573 K以上逐渐转变为单斜晶β相,1428 K以上转变为稳定的立方晶γ相[14]。图1所示为煅烧前(即水热产物)和不同温度下煅烧的Li2TiO3的XRD谱。由图1可以看出,煅烧温度对Li2TiO3晶型和纯度有很大影响。Li2TiO3的结构可以被描述成Liic(Li1/3Ti2/3)mcO3[15](其中,Ti原子占据主晶版2/3的4e晶位;Li原子占据主晶片其余1/3的4e晶位。ic代替间晶版;mc代替主晶片),所有样品的(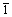 33)衍射峰峰形尖锐,强度较高,表明了主晶片(LiTi2层)中锂离子和钛离子的有序排列。煅烧前和573 K下煅烧时,(002)衍射峰没有形成,表明间晶片中Li离子排列的无序,此时产物为立方晶α相Li2TiO3。煅烧温度升高至673 K时,样品的(002)衍射峰开始形成,表明了间晶片中Li离子趋于有序排列,Li2TiO3开始由立方晶α相向单斜晶β相转变。随着煅烧温度继续升高(773 ~973 K),样品的(002)衍射峰强度持续升高,峰形更为尖锐,表明间晶片中Li离子的有序排列。从而可知,通过水热反应得到立方晶Li2TiO3,通过温度在773 K以上的煅烧可获得纯度较高的单斜晶β相Li2TiO3。
33)衍射峰峰形尖锐,强度较高,表明了主晶片(LiTi2层)中锂离子和钛离子的有序排列。煅烧前和573 K下煅烧时,(002)衍射峰没有形成,表明间晶片中Li离子排列的无序,此时产物为立方晶α相Li2TiO3。煅烧温度升高至673 K时,样品的(002)衍射峰开始形成,表明了间晶片中Li离子趋于有序排列,Li2TiO3开始由立方晶α相向单斜晶β相转变。随着煅烧温度继续升高(773 ~973 K),样品的(002)衍射峰强度持续升高,峰形更为尖锐,表明间晶片中Li离子的有序排列。从而可知,通过水热反应得到立方晶Li2TiO3,通过温度在773 K以上的煅烧可获得纯度较高的单斜晶β相Li2TiO3。
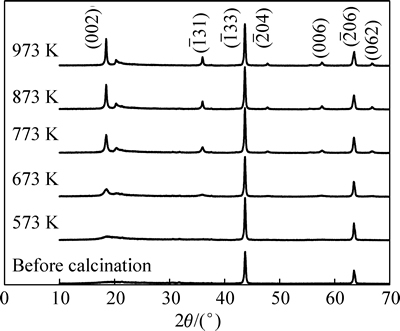
图1 不同煅烧温度Li2TiO3的XRD谱
Fig. 1 XRD patterns of Li2TiO3 under different calcination temperatures
根据晶型变化趋势,选择673 K、773 K和973 K的煅烧温度,考察不同煅烧温度吸附剂的平衡吸附容量。它们在0.68 g/L Li+溶液中吸附24 h达到吸附平衡,平衡吸附容量分别为16.91 mg/g (673 K)、28.98 mg/g (773 K)、27.67 mg/g (973 K)。根据吸附剂的最高平衡吸附容量,确定煅烧温度773 K。
2.1.2 煅烧时间
CHITRAKAR等[17]报道,减少煅烧时间能够提高酸洗脱锂过程中离子交换速率。为了考察煅烧时间对吸附剂结构和性能的影响,保持煅烧温度773 K条件不变,对在不同煅烧时间下制备的吸附剂进行结构表征和动力学测试。
图2所示为不同煅烧时间下制备的锂吸附剂前驱体Li2TiO3的XRD谱。由图2可以看出,煅烧时间仅为1 h时,已经获得纯度较高的单斜晶Li2TiO3,煅烧时间的延长对产品的晶型变化影响较小。据此,选择1 h、2 h、10 h煅烧时间,进一步考察煅烧时间对吸附剂洗脱和吸附性能的影响。

图2 不同煅烧时间Li2TiO3的XRD谱
Fig. 2 XRD patterns of Li2TiO3 for different calcination time
图3所示为煅烧时间分别为1 h、2 h和10 h下制备的Li2TiO3在0.6 mol/L盐酸溶液中的洗脱速率曲线。从图3中可以看出,煅烧2 h和10 h时的速率较快,均在洗脱5 h左右达到了125 mg/g的锂洗脱量,即洗脱率98.8%;煅烧1 h时的速率最慢,在洗脱24 h时才达到123 mg/g的锂洗脱量,即洗脱率97.6%。
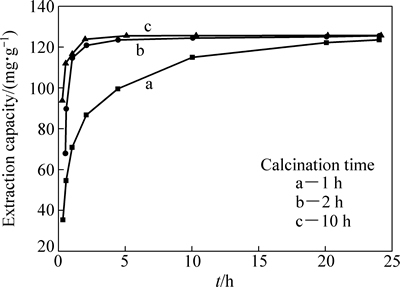
图3 洗脱不同煅烧时间Li2TiO3中Li+的速率曲线
Fig. 3 Li+ extraction rate of Li2TiO3 for different calcination time
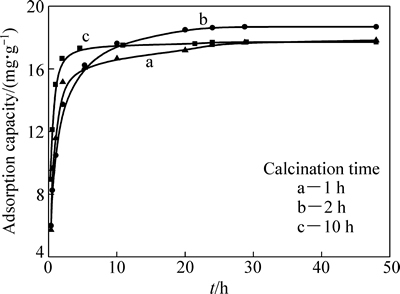
图4 不同煅烧时间H2TiO3吸附Li+的速率曲线
Fig. 4 Li+ adsorption rate by H2TiO3 for different calcination time
图4所示为煅烧时间分别为1 h、2 h、10 h下制备的H2TiO3在0.34 g/L Li+溶液中的吸附速率曲线。由图4可看出,煅烧24 h时,达到了吸附平衡。其中,煅烧2 h时平衡吸附量最高,达到18.74 mg/g;煅烧1 h和10 h的平衡吸附量较低,为17.70 mg/g。综合洗脱和吸附性能的考察结果,确定煅烧时间为2 h。
2.2 锂吸附剂H2TiO3
酸洗脱锂过程中,吸附剂前驱体Li2TiO3中的少量钛会溶解到盐酸溶液中。将Li2TiO3在0.6 mol/L盐酸溶液中洗脱5 h,得到吸附剂H2TiO3,锂洗脱率为98.8%,钛溶损率为2.10%。在一定范围内随盐酸浓度降低,锂洗脱率维持较高水平,而钛溶损率显著降低。当盐酸浓度为0.3 mol/L时,锂洗脱率98.1%,钛溶损率0.04%。图5所示为锂吸附剂前驱体Li2TiO3,及吸附前和吸附后的H2TiO3吸附剂的XRD谱。
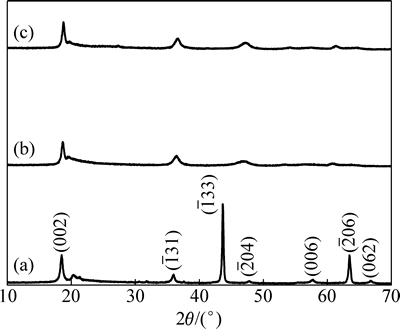
图5 Li2TiO3、吸附前和吸附后的H2TiO3离子筛的XRD谱
Fig. 5 XRD patterns of Li2TiO3 (a), H2TiO3 before (b) and after (c) adsorbing Li+
图5(a)所示为锂吸附剂前驱体Li2TiO3的XRD谱图,特征峰均与标准Li2TiO3 (JCPDS 33-0831)相吻合,属单斜晶相,空间点群结构为C2/c。图5(b)所示为Li2TiO3经过盐酸洗脱锂后得到的H2TiO3的XRD谱,属单斜晶相,空间点群C2/c。对比图5(a)和(b)可以看出,酸洗脱锂后(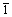 33)峰基本消失,这是由于锂几乎完全被氢置换出来,晶体结构发生变化,导致对应的峰消失,部分峰略微向右偏移,出现衍射峰宽化,表明氢置换锂后样品的晶胞一定程度上发生了收缩[9]。图5(c)所示为吸附锂后的H2TiO3的XRD谱,可以看出吸附后的H2TiO3与吸附前相比峰形极为接近,表明吸附前后吸附剂结构基本维持稳定。吸附过程中只有部分氢被锂置换出来,没有造成晶体结构上的较大变化。(002)、(
33)峰基本消失,这是由于锂几乎完全被氢置换出来,晶体结构发生变化,导致对应的峰消失,部分峰略微向右偏移,出现衍射峰宽化,表明氢置换锂后样品的晶胞一定程度上发生了收缩[9]。图5(c)所示为吸附锂后的H2TiO3的XRD谱,可以看出吸附后的H2TiO3与吸附前相比峰形极为接近,表明吸附前后吸附剂结构基本维持稳定。吸附过程中只有部分氢被锂置换出来,没有造成晶体结构上的较大变化。(002)、(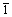 31)等衍射峰在酸洗、吸附的过程中变化不大,说明晶胞在这些方向上能保持原有结构,这些不易形变的部位保证了离子交换反应过程主体结构的稳定,这一结论也与文献[9,18]的结果一致。
31)等衍射峰在酸洗、吸附的过程中变化不大,说明晶胞在这些方向上能保持原有结构,这些不易形变的部位保证了离子交换反应过程主体结构的稳定,这一结论也与文献[9,18]的结果一致。
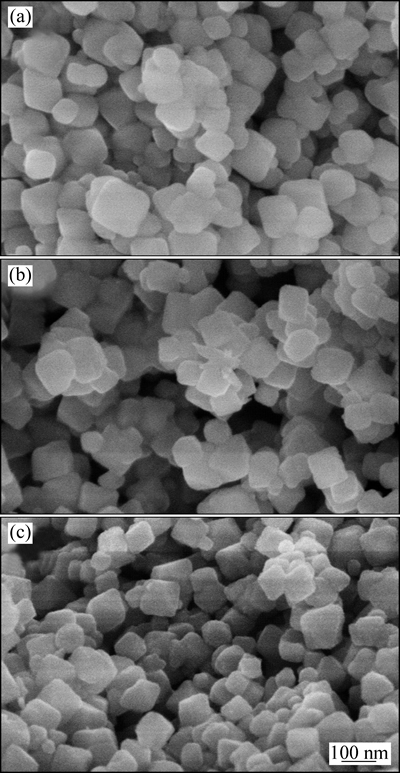
图6 Li2TiO3、吸附前和吸附后的H2TiO3离子筛的SEM像
Fig. 6 SEM images of Li2TiO3 (a), H2TiO3 before (b) and after (c) adsorption
图6所示为Li2TiO3和H2TiO3吸附前、吸附后的SEM像。由图6可以看出,Li2TiO3和H2TiO3均呈现规则的立方体形貌,洗脱和吸附过程中形貌未发生明显变化,说明结构较为稳定,有利于吸附剂的循环利用。Li2TiO3微粒的粒径在80~130 nm范围之内,吸附前H2TiO3微粒的粒径在65~120 nm,尺寸的微小变化来源于氢和锂的交换,另外,图6(b)与图6(a)和(c)相比结构略为疏松,应当是尺寸变小后微粒间空隙变大导致。相比固相法合成的H2TiO3[9-10, 17]的SEM像比较可以看出,水热法合成的离子筛尺寸更小,结构更为规整,形状更为均一,使得吸附过程中结构更为稳定。
2.3 吸附性能
2.3.1 吸附动力学
对吸附动力学的研究有助于了解吸附过程的机理,目前,关于金属离子吸附剂的动力学研究已有很多报道。其中,拟一级动力学方程(见式(3))和拟二级动力学方程(见式(4))被广泛用来研究吸附机理[8-9, 19],确定吸附过程的速率常数。
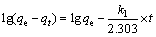 (3)
(3)
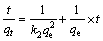 (4)
(4)
式中:qe为达到吸附平衡时吸附剂对锂的吸附容量;qt为吸附时间为t时的吸附容量;k1为拟一级动力学模型的吸附速率常数;k2为拟二级动力学模型的吸附速率常数。
纳米H2TiO3吸附剂在0.68 g/L Li+溶液中吸附24 h达到吸附平衡,平衡吸附容量为28.98 mg/g,图7和表1为拟一级动力学方程和拟二级动力学方程对该吸附过程的线性拟合结果。结果表明,用拟二级动力学方程拟合的线性相关系数达到了0.9956,而拟一级仅为0.6169,可以推断吸附过程符合拟二级动力学模型,说明该吸附过程为化学吸附。经计算得速率常数k2为0.0339 g/(mg·h),对比微米级H2TiO3吸附剂[8-9]的速率常数0.0051 g/(mg·h)、0.00574 g/(mg·h),吸附速率常数有了数倍提高。速率的加快很大程度上是由于粒径减小后,吸附剂颗粒表面上的原子数与总原子数之比急剧增大,表面上的离子交换速率迅速增加。
2.3.2 吸附等温方程
吸附平衡数据可以用Langmuir(见式(5))和Freundlich(见式(6))等温方程来拟合:
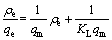 (5)
(5)
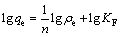 (6)
(6)
式中:ρe为达到吸附平衡时的吸附液浓度;qe为达到吸附平衡时的吸附量;qm为最大吸附量;KL为Langmuir吸附平衡常数;n为与吸附剂和锂相互作用有关的Freundlich常数;KF为与吸附速率有关的Freundlich吸附平衡常数。
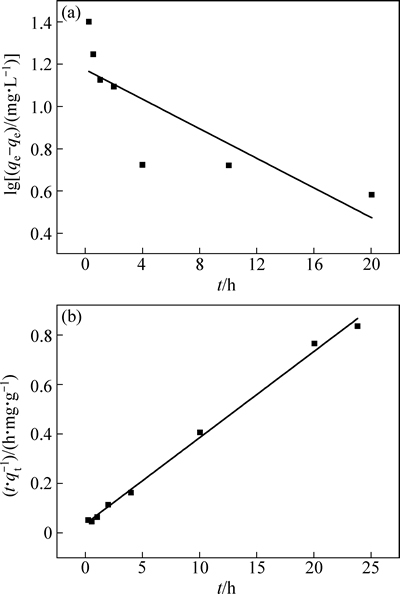
图7 H2TiO3在0.68 g/L Li+溶液中吸附的拟一级和拟二级动力学方程拟合图
Fig. 7 Fitting models of H2TiO3 adsorbing in 0.68 g/L Li+ solution with pseudo-first-order (a) and pseudo-second-order kinetics equations (b)
表1 H2TiO3吸附Li+过程的动力学方程参数
Table 1 Kinetics equations parameters of H2TiO3 adsorbing Li+ process
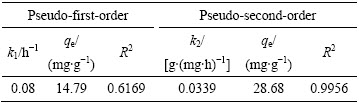
图8和表2所示为Langmuir方程和Freundlich方程对吸附平衡数据拟合的结果。拟合结果表明:采用Langmuir方程拟合的线性相关系数仅为0.3910,而用Freundlich方程拟合的线性相关系数达到0.9533。据此可以推断,在一定的浓度范围内,吸附平衡数据服从Freundlich方程,说明吸附剂各吸附位点与锂离子结合能不相同。纳米晶体通常在边/角有很高的表面浓度和各类缺陷点,导致纳米级别吸附剂各吸附位点与锂离子的结合能不同。
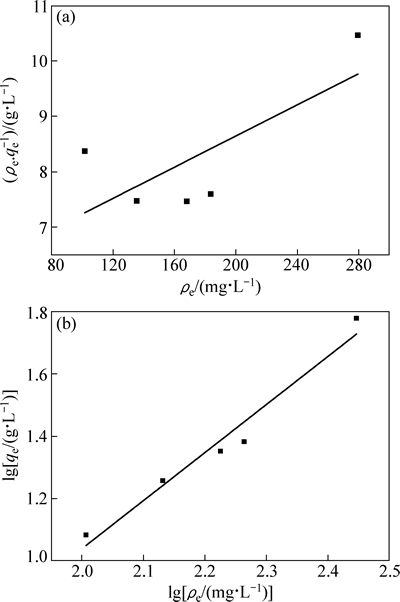
图8 H2TiO3在不同Li+浓度溶液中吸附的Langmuir和Freundlich等温方程拟合图
Fig. 8 Fitting models of H2TiO3 adsorbing in different Li+ concentrations solution with Langmuir (a) and Freundlich (b) isothermal equations
表2 H2TiO3吸附Li+的等温方程参数
Table 2 Isothermal equations parameters of H2TiO3 adsorbing Li+

HE等[20]报道,吸附液Li+初始浓度是影响吸附容量的关键因素。本文作者通过水热法合成的纳米H2TiO3吸附剂在0.68 g/L和0.34 g/L Li+溶液中的吸附量分别为28.98 mg/g和18.74 mg/g,表明Li+初始浓度对吸附容量影响较大。考察其在1 g/L Li+溶液中的平衡吸附量,发现24 h达到吸附平衡,平衡吸附容量为36.16 mg/g,表明所制备的纳米H2TiO3吸附剂对Li+具有较强的吸附能力。
2.3.3 吸附选择性
在模拟卤水中进行吸附实验,以探究吸附剂对锂的吸附选择性。模拟卤水含有一定量的Li+、Na+、K+、Ca2+、Mg2+,pH=9.19。分配系数(Kd)和分离因子(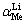 )根据式(7)和式(8)计算:
)根据式(7)和式(8)计算:
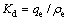 (7)
(7)
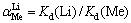 (8)
(8)
式中:qe为达到吸附平衡时的吸附量,ρe为达到吸附平衡时的吸附液浓度;Me为Li、Na、K、Ca和Mg。
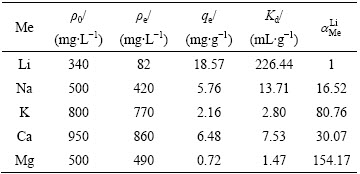
表3所列为对吸附剂选择性的考察结果。由表3可知,吸附剂在模拟卤水中对锂的吸附量为18.57 mg/g,与在LiCl溶液中吸附量(18.74 mg/g)非常接近,说明模拟卤水中的共存离子对吸附锂影响不大。另外,锂离子的分配系数达到了226.44 mL/g,远高于其他金属离子的分配系数。尤其Mg2+的分配系数低至1.47,锂离子对镁离子的分离因子达到154.17,说明H2TiO3吸附剂对Li+的选择性明显优于对Mg2+的。
表3 H2TiO3对模拟卤水中各金属离子的吸附选择性
Table 3 Selectivity of adsorbent for main ions in simulated salt-lake brine solution
3 结论
1) 采用水热法可以制备立方晶型吸附剂前驱体Li2TiO3。随着煅烧温度的升高,Li2TiO3由立方晶向单斜晶转变。而煅烧时间对Li2TiO3的相纯度影响较小,煅烧时间的延长对晶型变化影响不大。在煅烧温度773 K、煅烧时间2 h下制备的吸附剂平衡吸附容量最高。
2) 酸洗脱Li2TiO3中锂的速率快,在0.6 mol/L盐酸溶液中洗脱锂,5 h后锂洗脱率达到98.8%。
3) 通过酸洗脱锂得到的纳米级吸附剂H2TiO3,在1 g/L Li+溶液中吸附,24 h时达到吸附平衡,吸附容量为36.16 mg/g。
4) 纳米H2TiO3吸附剂在LiCl溶液中吸附Li+的过程符合拟二级动力学模型,在0.34 g/L Li+溶液中吸附速率常数达到0.0339 g/(mg·h)。吸附平衡数据符合Freundlich等温方程,说明纳米H2TiO3吸附剂各吸附位点与Li+结合能不相同。
5) 模拟卤水中共存离子对吸附锂影响不大,锂离子对镁离子的分离因子达到154.17。
REFERENCES
[1] 徐占武, 岳德宇, 张 蕾, 程鹏高, 高天成, 唐 娜. 锂离子筛吸附剂及成型的研究进展[J]. 无机盐工业, 2014, 46(6): 12-16.
XU Zhan-wu, YUE De-yu, ZHANG Lei, CHENG Peng-gao, GAO Tian-cheng, TANG Na. Research progress of preparation and formation of lithium ion sieve[J]. Inorganic Chemicals Industry, 2014, 46(6): 12-16.
[2] 陈 念, 钟 辉, 颜 辉. 国内外卤水提锂工艺技术现状[J]. 盐业与化工, 2014, 43(3): 1-4.
CHEN Nian, ZHONG Hui, YAN Hui. Present situation of the process and technique of lithium recovery from brine around the world[J]. Journal of Salt and Chemical Industry, 2014, 43(3): 1-4.
[3] 高 峰, 郑绵平, 乜 贞, 刘建华, 宋彭生. 盐湖卤水锂资源及其开发进展[J]. 地球学报, 2011, 32(4): 483-492.
GAO Feng, ZHENG Mian-ping, NIE Zhen, LIU Jian-hua, SONG Peng-sheng. Brine lithium resource in the salt lake and advances in its exploitation[J]. Acta Geoscientia Sinica, 2011, 32(4): 483-492.
[4] VIKSTROM H, DAVIDSSON S, HOOK M. Lithium availability and future production outlooks[J]. Applied Energy, 2013, 110: 252-266.
[5] 颜 辉, 钟 辉, 陈 念. 新型锂吸附剂的制备研究[J]. 无机盐工业, 2014, 46(2): 38-40.
YAN Hui, ZHONG Hui, CHEN Nian. Preparation of new lithium adsorbents[J]. Inorganic Chemical Industry, 2014, 46(2): 38-40.
[6] SUN Shu-ying, XIAO Jia-li, WANG Jin, SONG Xing-fu, YU Jian-guo. Synthesis and adsorption properties of Li1.6Mn1.6O4 by a combination of redox precipitation and solid-phase reaction[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15517-15521.
[7] 石西昌, 张志兵, 周喜诚, 周定方, 肖 湘, 陈白珍. MnO2·0.5H2O的固相法制备及其对Li+的吸附动力学[J]. 中国有色金属学报, 2012, 22(11): 3135-3143.
SHI Xi-chang, ZHANG Zhi-bing, ZHOU Xi-cheng, ZHOU Ding-fang, XIAO Xiang, CHEN Bai-zhen. Synthesis of MnO2·0.5H2O by solid method and its adsorption dynamics for Li+[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(11): 3135-3143.
[8] SHI Xi-chang, ZHANG Zhi-bing, ZHOU Ding-fang, ZHANG Li-fen, CHEN Bai-zhen, YU Liang-liang. Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 253-259.
[9] 张丽芬, 陈白珍, 石西昌, 马立文, 陈 亚. 偏钛酸型锂吸附剂的合成及吸附性能[J]. 中国有色金属学报, 2010, 20(9): 1849-1854.
ZHANG Li-fen, CHEN Bai-zhen, SHI Xi-chang, MA Li-wen, CHEN Ya. Synthesis and adsorption property of H2TiO3 type adsorbent[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1849-1854.
[10] ZHANG Qin-hui, LI Shao-peng, SUN Shu-ying, YIN Xian-sheng, YU Jian-guo. Lithium selective adsorption on low-dimensional titania nanoribbons[J]. Chemical Engineering Science, 2010, 65(0): 165-168.
[11] 李骁龙, 李少鹏, 张钦辉, 于建国. TiO2离子筛的合成及锂吸附性能研究[J]. 功能材料, 2009, 40(8): 1338-1341.
LI Xiao-long, LI Shao-Peng, ZHANG Qin-Hui, YU Jian-guo. Preparation and lithium adsorption of titania ion-sieves[J]. Journal of Functional Materials, 2009, 40(8): 1338-1341.
[12] 钟 辉, 殷辉安. 偏钛酸型锂离子交换剂表面性质与选择吸附性研究[J]. 离子交换与吸附, 2003, 19(1): 55-60.
ZHONG Hui, YIN Hui-an. Study on the properties of the surface and absorb of Li+ ion-exchange of H2TiO3 type[J]. Ion Exchange and Adsorption, 2003, 19(1): 55-60.
[13] WU Xiang-wei, WEN Zhao-yin, LIN Bin, WU Xiao-gang. Sol-gel synthesis and sintering of nano-size Li2TiO3 powder[J]. Materials Letters, 2008, 62(6/7): 837-839.
[14] LAUMANN A, FEHR K, WACHSMANN M, HOLZAPFEL M, IVERSEN B. Metastable formation of low temperature cubic Li2TiO3 under hydrothermal conditions—Its stability and structural properties[J]. Solid State Ionics, 2010, 181(33/34): 1525-1529.
[15] YU Cheng-long, YANAGISAWA K, KAMIYA S, KOZAWA T, UEDA T. Monoclinic Li2TiO3 nano-particles via hydrothermal reaction: Processing and structure[J]. Ceramics International, 2014, 40(1): 1901-1908.
[16] KATAOKA K, TAKAHASHI Y, KIJIMA N, NAGGI H, AKIMOTO J, IDEMOTO Y, OHSHIMA K. Crystal growth and structure refinement of monoclinic Li2TiO3[J]. Materials Research Bulletin, 2009, 44(1): 168-172.
[17] CHITRAKAR R, MAKITA Y, OOI K, SONODA A. Lithium recovery from salt lake brine by H2TiO3[J]. Dalton Transactions, 2014, 43(23): 8933-8939.
[18] 刘峙嵘, 王长福, 赖 毅, 张 寰, 王 云, 周利民. 偏钛酸型离子交换剂的制备及其对锂的富集性能[J]. 过程工程学报, 2015, 15(1): 147-152.
LIU Zhi-rong, WANG Chang-fu, LAI Yi, ZHANG Huan, WANG Yun, ZHOU Li-min. Synthesis of H2TiO3 type adsorbent and its enrichment performance for Li+[J]. The Chinese Journal of Process Engineering, 2015, 15(1): 147-152.
[19] 曲肖彦, 刘广义, 刘 胜, 钟 宏, 肖静晶. 3-己基-4-氨基- 1,2,4-三唑-5-硫酮在黄铜矿表面的吸附动力学与热力学[J]. 中国有色金属学报, 2015, 25(7): 2006-2014.
QU Xiao-yan, LIU Guang-yi, LIU Sheng, ZHONG Hong, XIAO Jing-jing. Adsorption kinetics and thermodynamics of 3-hexyl-4-amino-1,2,4-triazole-5-thione on surface of chalcopyrite[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(7): 2006-2014.
[20] HE Gang, ZHANG Li-yuan, ZHOU Da-li, ZOU Yuan-wen, WANG Fan-hou. The optimal condition for H2TiO3–lithium adsorbent preparation and Li+ adsorption confirmed by an orthogonal test design[J]. Ionics, 2015, 21(8): 2219-2226.
Hydrothermal synthesis and adsorption properties of nano scale H2TiO3 adsorbent
CHEN Zi-zheng, SHEN Wei-hua, CHEN Li-fang, CHENG Hong-ye, QI Zhi-wen
(State Key Laboratory of Chemical Engineering, School of Chemical Engineering,
East China University of Science and Technology, Shanghai 200237, China)
Abstracts: Nano scale H2TiO3 adsorbent was obtained by acid-modifying adsorbent precursor Li2TiO3, which was synthesized by calcining products of hydrothermal reaction between TiO2 and LiOH·H2O. The effects of calcination time and calcination temperature on structure and ion-exchange properties were investigated via XRD, SEM and kinetic experiment. The pseudo- first and second order rate equations were used to investigate the adsorption process, and the Langmuir and Freundlich adsorption isotherm equations were fitted by the equilibrium data. The results show that the verified optimal calcination temperature and time are 773 K and 2 h, respectively. For such prepared adsorbent, the maximum adsorption capacity reaches 36.16 mg/g, the adsorption kinetic constants is 0.0339 g/(mg·h) and the extraction rate of lithium is 98.8% after 5 h. Moreover, the adsorption process obeys a pseudo-second order equation, and the equilibrium data fits well to the Freundlich model. The separation coefficient of Li+ to Mg2+ reaches 154.17.
Key words: nano scale H2TiO3; hydrothermal method; adsorption kinetics; adsorption rate constants; Freundlich equation
Foundation item: Project (2012AA061601) supported by National High Technology Research and Development Program of China
Received date: 2015-12-15; Accepted date: 2016-06-13
Corresponding author: QI Zhi-wen; Tel: +86-21-64250935; E-mail: zwqi@ecust.edu.cn
(编辑 李艳红)
基金项目:国家高技术研究发展计划资助项目(2012AA061601)
收稿日期:2015-12-15;修订日期:2016-06-13
通信作者:漆志文,教授,博士;电话:021-64250935;E-mail:zwqi@ecust.edu.cn