
Electric properties stability of NbO anode for new electrolytic capacitor
LI Jian(李 荐)1, 2, YI Dan-qing(易丹青)1, WEN Jun-jie(温俊杰)2, ZHONG Hui(钟 辉)2, LIU Hui-qun(刘会群)1
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 30 September 2005; accepted 8 April 2006
Abstract: By using loading test and high temperature test, the electric-property stability of NbO anode and metal Nb anode were studied in comparison. The reason for higher stability of NbO anode was analyzed by thermodynamics and kinetics. The loading test was performed in 38% H2SO4 at 85 ℃ and 12 V for 10 d. The high temperature test was carried out at 250 ℃ in air atmosphere for 30 h. It is found that the NbO anode possesses higher ability to restrain crystallization film as compared with metal Nb anode. It is considered that, oxygen in NbO anode matrix restrains the migration of oxygen in Nb2O5 anodic oxidation film to matrix, and results in that the anodic oxidation film has higher stability.
Key words: Nb; NbO; electrolytic capacitor; anode; stability
1 Introduction
Due to the large specific capacity, electrolytic capacitor has become an important part in the applications of electric capacity and has been widely applied in electronic circuit field. Electrolytic capacitors commercialized can be classified into two types for different anode materials, Ta electrolytic capacitor and Al electrolytic capacitor. Electrolytic capacitors, which are made of Ta and Al and originate from anodic oxidation film of Ta and Al with high stability, especially anodic oxidation film of Ta, show novel characteristic. The reliability and high frequency characteristic of electrolytic capacitor with Ta anode materials were superior to those of Al electrolytic capacitor, so Ta electrolytic capacitor was mostly used in military and astronavigation fields. However, the application of the Ta electrolytic capacitor was limited for its high cost.
In 1950s some researchers studied Nb electrolytic capacitor and tried to replace Ta. However, the industrialization of Nb electrolytic capacitor can not be realized for some reasons. Recently, due to scarcer resource and high price of Ta, many industries and research institutes reactivated investigation of the Nb electrolytic capacitor, and the aspects are focused as follows: 1) preparation of superfine Nb powder for high pure electrolytic capacitor Nb powder[1,2]; 2) improving electric properties by doping methods[3-5]; 3) taking NbO as anode matrix material to investigate a new type of NbO electrolytic capacitor[6-10].
The previous studies showed that NbO was a very promising electrolytic capacitor anode material. Some famous companies extensively studied NbO electrolytic capacitor and had applied for some patents[6-10]. Looking at the latest results, it is believed that NbO electrolytic capacitor will develop into a new electrolytic capacitor and will be widely applied indeed.
Anode is the most important part of an electrolytic capacitor. Properties of an electrolytic capacitor mainly depend on the anode. The properties stability is an important value for judging the credibility of electric components. The properties stability of an electrolytic capacitor is related to anode, leading-out parts of cathode and packaging structure, but mainly depends on stability of the anode. The preparation process of raw materials and fabrication of anode for NbO electrolytic capacitor have been reported in many references and patents. For example, Refs.[6,7,11] discussed the preparation technology of electrolysis capacitor with NbO powder and Refs.[8,10,12] described the preparation method of NbO anode electrolysis capacitor. However, there has not been report about anode stability of electrolysis capacitor so far. In this paper, loading test and high temperature test were performed to study electric properties stability of the NbO anode, and metal anode was also investigated in comparison. At the same time, the reason for mechanism was discussed further.
2 Experimental
2.1 Preparation of anode
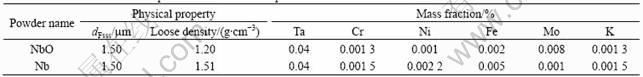
The NbO anode was prepared through process [11,12]: Nb powder (dFsss1.5 μm) of 1.1 times theoretical mass and Nb2O5 (dFsss4.5 μm) powder were mixed uniformly, and were reduced in vacuum (p<2×10-2 Pa pressure= at 1 000 ℃ for 40 min, then, niobium suboxide powder mainly composed of NbO was obtained. Physical properties and chemical composition are listed in Table 1. XRD results are given out in Fig.1. The powder was pressed into d 3.5 mm anode bulk with the mass of 0.2 g; capacitor tantalum filament was used as leading wire, and then the bulks were sintered in vacuum at 1 300 ℃ for 20 min under the pressure of less than 2×10-2 Pa. SEM images of the obtained sintering the NbO anode bulks are shown in Fig.2. From Figs.1 and 2, it can be seen that, as-received niobium suboxide powder is mainly composed of NbO, and a little Nb and Nb2O5 is also identified in the powder sample. On the surface of NbO particles lots of bulging parts and “gully” exist resulting in the large specific area and high specific capacity.
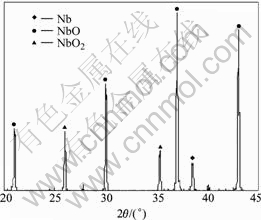
Fig.1 XRD patterns of niobium suboxide powder
The preparation method of the metal Nb anode was described as follows: metal Nb powder was selected as anode raw material (property and composition are listed in Table 1), sintered Nb anode bulk was prepared by the same methods mentioned above.
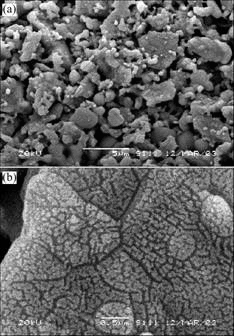
Fig.2 SEM micrographs of sintered NbO anode
The forming method of the anode was as follows: forming electrolyte was 0.01% H3PO4, temperature 90 ℃, final voltage 50 V, holding time 2 h, current density 30 mA/g, cathode was pure silver foil.
2.2 Electric property testing of anode
Specific capacitor, loss and leakage current were three main performance parameters for an electrolytic capacitor. In this paper, the properties of the NbO anode and metal the Nb anode were tested according to national standard GB/T3137—1995 “Electric properties testing method of Ta powder”.
2.3 Loading test
Loading test was performed on the metal Nb anode and the NbO anode which were treated under 50 V voltage. The test was made in the electrolyte of 38%H2SO4 at 85 ℃ with the cathode of pure silver foil. Because the anode formed at 50 V voltage can be used as 10 V product, so loading voltage was taken as 12 V considering excess 20%; the capacity, loss and leakage current of the anode were tested in every 24 h to analyze the electric properties variation of the anode.
Table 1 Characteristics and composition of NbO and Nb powder
2.4 High temperature test
The NbO anode and the metal Nb anode formed under 50 V were placed in an oven with temperature automatically controlled in air atmosphere at 250 ℃. The capacity, loss and leakage current of the anode were tested after certain interval, then the anode was rinsed by de-ionized water and heated again. The electric properties stability at high temperature was investigated due to the variation of anode electric properties of the anode. The results are helpful for the following coating processes which was performed at about 250 ℃.
3 Results and discussion
3.1 Loading test
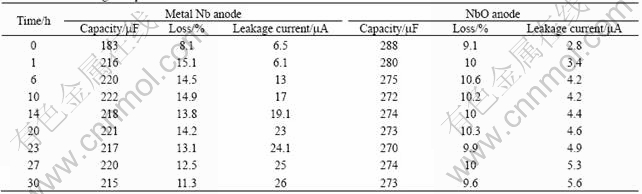
The results of loading test are listed in Table 2. The relationships between the loading time and capacity, loss and leakage current are shown in Fig.3. It can be seen that, the most obvious change is leakage current during loading test. The leakage current of the NbO anode changes a little, from 2.3 to 5.2 μA in 10 d. The leakage current of the metal Nb anode changes remarkably from 8.8 to 4 500 μA in 6 d. The variance of anode loss is smaller than that of leakage current, the maximum variance ratios of metal Nb anode and the NbO anode are 53.2% and 20.3%, respectively. The capacity variance of two anodes is unapparent, the maximum variance ratios of metal Nb anode and the NbO anode are 37.6% and 11.2%, respectively. Crystallization of anodic oxidation film in Nb anode surface occurred in the third day (grey specks were observed on the surface), but crystallization in the NbO anode surface does not occur during the test period (no grey specks on the surface of anode). It is sufficiently illustrated that the anodic oxidation film of the NbO anode had higher stability than that of metal Nb anode.
3.2 High temperature test
The results of high temperature test are listed in Table 3. The relationships between the test time and capacity, loss and leakage current are shown in Fig.4. It is found that, the leakage current also changes apparently in high temperature test. The leakage current of the NbO anode changes from 2.8 to 5.6 μA after 30 h, the leakage current of metal Nb anode changes from 6.5 to 26 μA after 30 h, the maximum variance ratio reaches 326.2%. The variance of anode loss is smaller than that of leakage current, the maximum variance ratios of the metal Nb anode and the NbO anode are 86.4% and 16.5%, respectively. Capacity variances of two anodes are not so obvious, the maximum variance ratios of the metal Nb anode and the NbO anode are 20.2% and 5.5%, respectively. So, from the results of high temperature test, it can also be seen that the NbO anode has higher stability than that of the metal Nb anode.
In the process of the production of electrolytic capacitor, MnO2 coating was made after anode forming. The anode was immerged in the solution of Mn(NO3)2, and then, thermally decomposed to MnO2 at about 250 ℃, MnO2 worked as linking cathode. The MnO2 coating was detrimental to anodic oxidation film. Unapparent variation of the properties of the NbO anode in test process which was made at 250 ℃ for 30 h (the time of coating technology was about several hours) indicated that the NbO anode was suitable for the temperature request of coating treatment.
From the analysis of loading test and high tempera-
Table 2 Results of loading test
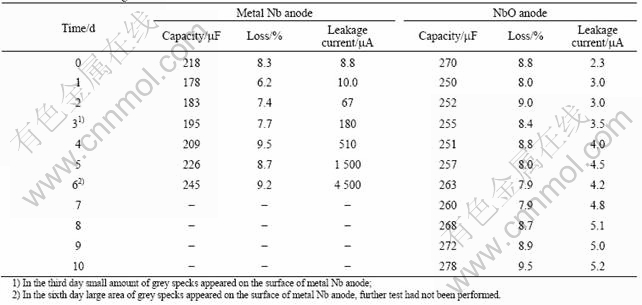
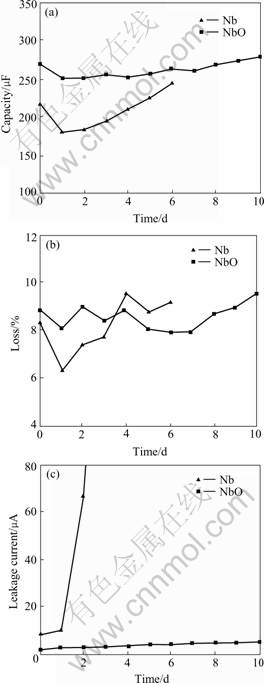
Fig.3 Relationships between loading time and electrical pro- perties of anode: (a) Capacitance; (b) Loss; (c) Leakage current
ture test mentioned above, the NbO anode shows better stability than the metal Nb anode. The properties of electrolytic capacitor mainly depended on the properties of anode, so the NbO electrolytic capacitor had better stability and credibility than metal Nb electrolytic capacitor.
Similar amorphous Nb2O5 anodic oxidation film could be formed when NbO and Nb were oxidized. The reason that the NbO anode had better stability than metal Nb anode is the existence of oxygen in the NbO anode
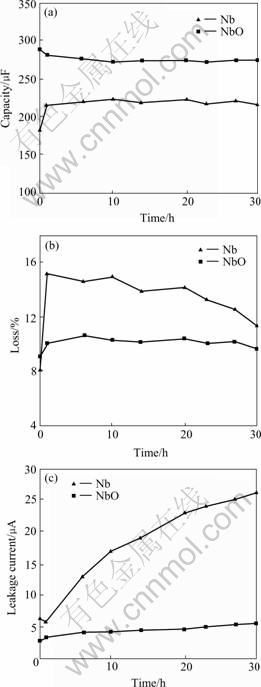
Fig.4 Anode electrical property depends on testing time at 250℃: (a) Capacitance; (b) Loss; (c) Leakage current
matrix. Many researchers indicated that[13-15], quick migration velocity of oxygen in the Nb2O5 anodic oxidation film of the Nb electrolytic capacitor resulted in the instability of properties of the Nb electrolytic capacitor. After the oxygen migrates to the matrix, partial Nb2O5 transforms into conductive NbO2 or NbO, the thickness of insulating Nb2O5 anodic oxidation film decreased, resulting in that the capacity and leakage current increased, and instability of the properties of
Table 3 Results of high temperature test at 250 ℃
electrolytic capacitor appeared. For the NbO anode, the matrix was niobium suboxide NbO, oxygen migrated from the Nb2O5 anodic oxidation film to the NbO matrix. Assuming that the oxidation reaction product is NbO2, the reaction is
Nb2O5+3NbO=5NbO2 (1)
The Gibbs free energy of this reaction is 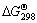 =-53.65 kJ/mol; if the matrix is Nb, oxygen migrated from the Nb2O5 anodic oxidation film to matrix. The oxide product is NbO according to the reaction as
=-53.65 kJ/mol; if the matrix is Nb, oxygen migrated from the Nb2O5 anodic oxidation film to matrix. The oxide product is NbO according to the reaction as
Nb2O5+3Nb=5NbO (2)
where 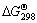 =-231.09 kJ/mol, which is smaller than that of reaction (1), indicating that thermodynamic driving force of oxygen migrating to the Nb matrix is larger. From the viewpoint of kinetics, the concentration gradient of oxygen in Nb2O5 anodic oxidation film migrating to the Nb matrix is larger than that migrating to the NbO matrix. According to the diffusion law, large concentration gradient leads to quick mass transfer, namely that the velocity of oxygen in anodic oxidation film diffusing to the Nb matrix is quicker than diffusing to the NbO matrix. From the analysis mentioned above, it is considered that if NbO is taken as the matrix, the restraining of oxygen in the Nb2O5 anodic oxidation film migrating to the matrix can make anodic oxidation film have better stability, consequently better stability of anode.
=-231.09 kJ/mol, which is smaller than that of reaction (1), indicating that thermodynamic driving force of oxygen migrating to the Nb matrix is larger. From the viewpoint of kinetics, the concentration gradient of oxygen in Nb2O5 anodic oxidation film migrating to the Nb matrix is larger than that migrating to the NbO matrix. According to the diffusion law, large concentration gradient leads to quick mass transfer, namely that the velocity of oxygen in anodic oxidation film diffusing to the Nb matrix is quicker than diffusing to the NbO matrix. From the analysis mentioned above, it is considered that if NbO is taken as the matrix, the restraining of oxygen in the Nb2O5 anodic oxidation film migrating to the matrix can make anodic oxidation film have better stability, consequently better stability of anode.
4 Conclusions
The stability of electric properties of NbO anode and metal Nb anode were investigated in comparison through loading test and high temperature test. The results show that, NbO anode has stronger restraining ability for crystallization of amorphous Nb2O5 anodic oxidation film and has better electric properties stability than metal Nb anode. It is considered that, oxygen in the NbO anode matrix restrained the migration of oxygen in Nb2O5 to the matrix, which makes anodic oxidation film and electric properties of anode have better stability.
References
[1] ODA Y (Showa Cabot Supermetals K K, Japan). Tantalum Powder, Niobium Powder their Manufacture, and Capacitor Anodes [P]. JP 2000226607, 2000.
[2] HABECKER K A, FIFE J A. (Cabot Corporation, USA). Wet Milling with Heating for Manufacture of Metal Powders, Including Niobium Powder for Electrolytic Capacitors [P]. WO 2000056486, 2000.
[3] NAITO K. Niobium Powder, Niobium Sintered Body, Capacitor Comprised of the Sintered body, and Method for Manufacturing the Capacitor [P]. WO 2000049633, 2000.
[4] NAITO K, SHIMOJIMA A. Sinter of Niobium for Capacitor, and Method of Manufacture Thereof [P]. WO 2000008662, 2000.
[5] NAITO K, SHIMOJIMA A. Niobium Capacitor and Method of Manufacture Thereof [P]. WO 2000036617, 2000.
[6] HABECKER K A, FIFE J A. High Capacitance Niobium Powders and Electrolytic Capacitor Anodes [P]. WO 2000069588, 2000.
[7] KIMMEL J L, KITCHELL R W, FIFE J A. Methods to Partially Reduce a Niobium Metal Oxide and Oxygen Reduced Niobium Oxides for Capacitor Anodes [P]. US 6373685 B1, 2002.
[8] FIFE J A. Partial Reduction of Niobium Pentoxide for its Use as a Capacitor Anode [P]. US 6416730 B1, 2002.
[9] SCHNITTER C, REICHERT K. Niobium Based Electrolytic Capacitor Anode [P]. WO 2002017338 A1, 2002.
[10] NAITO K, NAGATO N. Niobium Powder for Capacitor, Sintered Body Thereof and Capacitor Using the Sintered Body [P]. WO 2002045107 A1, 2002.
[11] KIMMEL J L, REDD R V. Refractory Metal Suboxide Powders Suitable for Sintered Capacitor Anodes [P]. WO 2002037513 A2, 2002.
[12] LI Jian, YI Dan-qing, WEN Jun-jie, ZHONG Hai-yun. Investigation of the preparation of new electrolytic capacitor anode of niobium suboxide [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(6): 893-899.(in Chinese)
[13] LI Jian, LI Qing-kui, WEN Jun-jie, ZHONG Hai-yun. Development of electrolytic capacitor anode of niobium suboxide [J]. Rare Metal Materials and Engineering, 2005, 34(7): 1144-1146.
[14] POZDEEV Y. Reliability comparison of tantalum and niobium solid electrolytic capacitors [J]. Quality and Reliability Engineering International, 1998, 14(2): 79-82.
[15] BOIKO B T, KOPACH V R, MELENTJEV S M, et al. Comparison of the degradation modes in sandwich structures including amorphous oxides of niobium and tantalum [J]. Thin Solid Films, 1993, 229(2): 207-215.
[16] FEDORENKO A I, STARIKOV V V, POZDEEV Y L, et al. Layer systems on the base of nitrogen-doped tantalum with enhanced stability [J]. Crystal Research and Technology, 1997, 32(6): 843-848.
(Edited by LONG Huai-zhong)
Corresponding author: LI Jian; Tel: +86-731-8836320; E-mail: ziliao2000@126.com