J. Cent. South Univ. Technol. (2011) 18: 42-47
DOI: 10.1007/s11771-011-0656-z
Influence of dopant content on morphology and optical constants of ZnO:Na films by sol-gel method
L? Jian-guo(吕建国)1, 2, CAO Chun-bin(曹春斌)1, 3, SONG Xue-ping(宋学萍)1, SUN Zhao-qi(孙兆奇)1
1. School of Physics and Material Science, Anhui University, Hefei 230039, China;
2. Department of Physics and Electronic Engineering, Hefei Normal University, Hefei 230061, China;
3. School of Sciences, Anhui Agricultural University, Hefei 230036, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Undoped and Na-doped ZnO films were deposited by sol-gel method. The effects of sodium incorporation on structure, surface morphology and optical constants of the films were investigated. X-ray diffraction patterns show the hexagonal wurtzite polycrystalline structure and that the sodium incorporation leads to the change in the structural characteristics of ZnO films. The SEM observations show that the surface morphology of the films is affected by the sodium incorporation. The transmission spectra show that the average transmittance of the films is above 85% in the visible range. The absorption edge initially blue-shifts and then red-shifts with the increase of Na doping content. The optical constants of these films were calculated using transmission spectra. Refractive indices of the films in the visible range decrease at first and then increase with increasing Na doping content.
Key words: Na-doped ZnO; sol-gel method; transmission spectra; optical constants
1 Introduction
Zinc oxide (ZnO) is a direct wide-band-gap semi- conductor material (Eg=3.37 eV at room temperature). It has a large excitation binding energy of 60 meV, which makes the exciton hard to be thermally ionized. Therefore, ZnO is a very promising material for optoelectronic devices, such as surface acoustic wave devices [1-2], ultraviolet light-emitting diodes [3-5], photodetectors [6-7] and laser diodes [8].
Refractive index is one of the fundamental properties for an optical material, because it is closely related to the electronic polarizability of ions and the local field inside materials. The evaluation of refractive indices of optical materials is considerably important for the applications in integrated optic devices, such as switches, filters and modulators, where refractive indices are the key constants for device design [9]. The optical constants of undoped [10-12], In-doped [13], Al-doped [14-15] and Li-doped [16] ZnO films were investigated. LIN et al [3] reported that Na could be a good dopant to create stable p-type ZnO. WANG and GAO [4] studied the effects of annealing condition on the structural and optical properties of Na-doped ZnO thin films. It is possible to determine optical constants, such as refractive index, absorption coefficient, and dielectric constant by analyzing transmittance spectrum [17-19]. Few studies have been reported to extract the optical constants of Na-doped films from transmittance spectrum.
In this work, undoped and Na-doped ZnO thin films were deposited by sol-gel method. The optical constants of the thin films with different Na doping contents were calculated by transmittance spectrum. The effects of Na doping content on the structure, surface morphology and optical constants, such as refractive index, extinction coefficient and dielectric constant, were investigated.
2 Experimental
Zinc acetate dehydrate (Zn(CH3COO)2?2H2O) and sodium chloride (NaCl) were dissolved in ethylene glycol monomethyl ether. Then, monoethanolamine (MEA) and methanamide were added under stirring. The molar ratio of MEA to zinc acetate was 1.0 and the concentration of zinc acetate was 0.5 mol/L. The molar ratio of dopant (sodium chloride) in the solution was varied between 0% and 16%. The resultant solution was stirred at 60 °C for 2 h to yield a clear and homogeneous solution. The sol-gel coating was made usually one day after the solution was prepared. Na-doped ZnO films were prepared on glass substrate by repeated coating. Spin coating was performed at room temperature, with a rate of 3 000 r/min for 30 s. After the deposition, the films were preheated in air at 250 °C for 10 min. The coating process was repeated after cooling down to room temperature in order to increase the thickness of the coatings. After repeating the coating procedure 15 times for the final film thickness of approximately 458 nm, the films were finally heated at 550 °C for 60 min in air.
Crystal structure of the Na-doped ZnO film was investigated by XRD using Cu Kα radiation with wavelength of 0.154 06 nm. Morphology of the thin films was observed by SEM. Transmission spectra of the thin films were obtained with the normal incident transmittance with a UV-Vis spectrophotometer in the wavelength of 370-900 nm.
Spectral ellipsometer is generally acknowledged to be a measuring method of high precision. It is based on the measurement of two elliptical polarization parameters, ψ and Δ. The two elliptical polarization parameters have the defining relationships with the refractive index n0 of the ambient, the film thickness d and its refractive index nF, the refractive index nS of substrate, the incident angle φ0 and the wavelength λ of incident light, and can be presented as
F(ψ, Δ)=F(n0, nF, nS, φ0, λ, d) (1)
If there is absorption in the film, complex refractive index nF can be defined as nF=n+ik. The n and k are optical refractive index and extinction coefficient, respectively. By using regression calculation process, the values of film thickness d and its refractive index nF can be obtained. The ellipsomitric data were analyzed by using the Film WizardTM software package (Scientific Computing International). A set of ψ and Δ data measured by ellipsometer are the target file in the analysis process. In this work, the transmission spectrum was converted to a target file, which was analyzed by the Film WizardTM software package. The analysis software identified the target file and executed the calculation relationship between transmission spectrum and elliptical parameters. If the dispersion relation was employed properly and the initial parameters were set suitably, the simulation results can be obtained finally.
Accuracy of the simulation results can be judged by two aspects as follows: 1) the deviation between the calculated data and the target (measured) data and 2) the root mean squared error (RMSE, E) that is defined as
E= (2)
(2)
where n is the number of selected targets, Ytar is the target (experimental) value, Ycal is the calculated value and w is the weight at each target point.
3 Results and discussion
3.1 Structure and morphology
Fig.1 shows the XRD patterns of undoped and Na-doped ZnO films annealed at 550 °C for 60 min. The diffractive peaks, which correspond to (100), (002), (101), (102), (110) and (103) of ZnO, indicate that the sample is composed of polycrystallines. All diffractive peaks match the hexagonal wurtzite ZnO structure with lattice constants of a=3.250 ? and c=5.195 ?. No peaks corresponding to either Na metal or any of its oxides are observed on the patterns, which indicates that there is no additional phase present in the Na-doped films. In addition, the undoped ZnO thin film cannot form preferential c-axis ((002) crystal plane) orientation. As Na doping content increases, preferential c-axis orientation becomes more and more obvious, and the intensity of the diffraction peaks from (103) increases. It can be seen from Fig.1 that the intensity of the diffraction peaks from (103) is much weaker than that from (002) of 8% Na-doped ZnO film. However, the intensity from (103) is similar to that from (002) as Na doping content rises to 16%. The grain size from ZnO (002) peaks calculated by Scherrer relation, ranges from 6.2 to 16.6 nm with Na doping content increasing. Variations of the full width at half maximum (FWHM) and grain size from ZnO (002) peaks with different Na doping contents are shown in Fig.2.
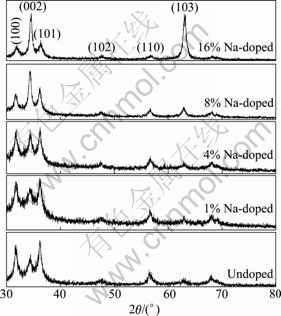
Fig.1 XRD patterns of undoped and Na-doped ZnO films
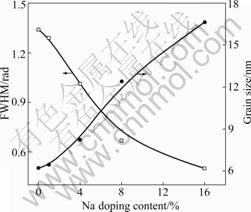
Fig.2 Variations of FWHM and grain size with Na doping content
Fig.3 shows the SEM images of ZnO thin films with 0, 1%, 4%, 8% and 16% Na doping content. It can be seen that the surface morphology of the films is strongly dependent on the content of sodium. Rough surface and sheet shape microstructure are observed in Fig.3 for the undoped ZnO film. The surface morphology of 1% Na-doped film exhibits a porous microstructure and the grain size is approximately 30-50 nm. When the doping content is 4% or 8%, the surface of the film exhibits nano-rod shape particles [13]. The density of nano-rod shape particles decreases as the doping content increases to 16%. This coincides with the results of XRD experiment. The c-axis orientation may be explained with the change of internal stress and surface free energy [20] caused by Na doping content.
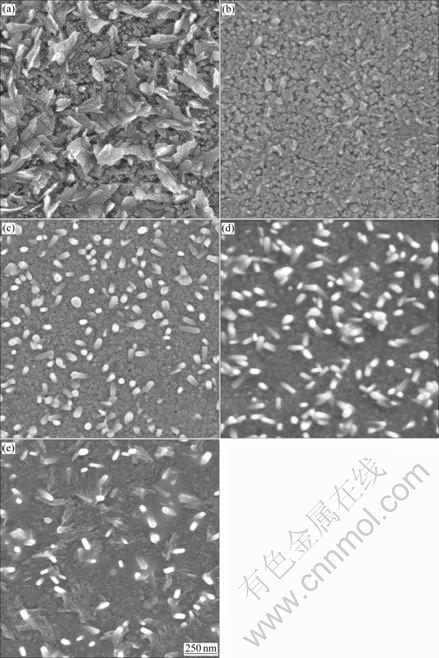
Fig.3 SEM images of ZnO films with different Na doping contents: (a) Undoped; (b)1%; (c) 4%; (d) 8%; (e) 16%
3.2 Optical constants
Fig.4 shows the transmission spectra of the undoped and Na-doped ZnO films. All films have an average optical transparency over 85% in the visible range. It can be seen from the inset of Fig.4 that the UV absorption edge blue-shifts at first and then red-shifts with increasing the Na doping content.
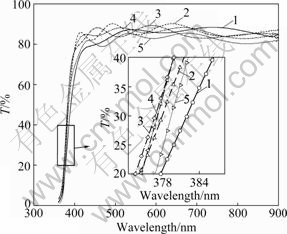
Fig.4 Optical transmittance spectra of ZnO films: 1—Undoped; 2—1% Na-doped; 3—6% Na-doped; 4—8% Na-doped; 5—16% Na-doped
Light absorption of the undoped and Na-doped ZnO films is attributed to the free-carrier and lattice scattering. The Lorentz Oscillator and Drude dispersion model is adopted to fit the transmission spectrum. The formula of the Lorentz oscillator and Drude dispersion model [21] can be described as
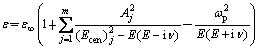 (3)
(3)
where ε∞ is the high-frequency lattice dielectric constant. j is the number of oscillators, which should be between one and five. One should attempt first to use the minimum number of oscillators possibly to avoid correlation effects. Ecen is the center energy of each oscillator. Aj is the amplitude of oscillator. 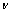 is the electron collision frequency.
is the electron collision frequency.
The plasma frequency (ωp) is determined as
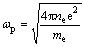 (4)
(4)
where ne, e and me are the electron (conduction electron) density, charge and mass, respectively.
Fig.5(a) shows the refractive index of the films with different Na doping contents in the wavelength range of 370-900 nm. It can be seen from Fig.5(a) that the refractive index of the films in the visible range decreases at first and then increases with increasing Na doping content. The decrease of refractive index with the increase of the Na doping content may be mainly attributed to an increase of the carrier concentration (donor electrons) [14], which indicates that most of the Na ions must be incorporated as interstitial donors into the structure rather than substitutional acceptors in the 1% and 4% Na-doped ZnO films. The increase of refractive index of the films can be explained as follows. As the Na content increases from 4% to 16%, there are more chances for the Na atoms to occupy substitutional sites and to generate Na acceptor. Substitutional incorporation of Na atoms neutralizes the donor electrons in ZnO. Therefore, the refractive index of the Na-doped ZnO films can be controlled by varying the Na doping content, which is important for the applications in designing the integrated optic devices. The extinction coefficient k dependent of the wavelength is shown in Fig.5(b). The k values of the Na-doped ZnO films decrease with increasing the wave length to a certain value and then increase.
The fundamental electron excitation spectrum of the film is described by a frequency dependent of the complex
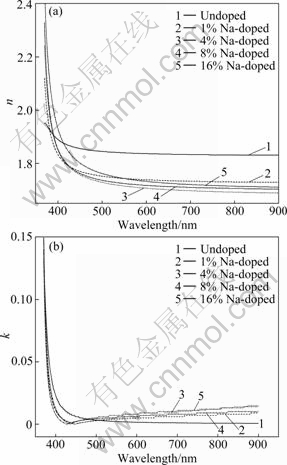
Fig.5 Variations of refractive index (a) and extinction coefficient (b) of undoped and Na-doped ZnO films
electronic dielectric constant. Real and imaginary parts of the dielectric constant are related to the n and k values. The real and imaginary parts are calculated using the formulas [22]:
 (5)
(5)
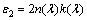 (6)
(6)
The real and imaginary parts dependent of wavelength are shown in Fig.6(a) and 6(b), respectively. The values of real part are higher than those of imaginary part. It can be seen that the real part of the dielectric constant decreases while the imaginary part initially decreases and then increases with increasing the wavelength.
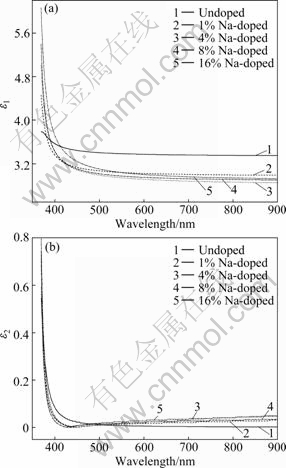
Fig.6 Variations of real (a) and imaginary (b) parts of dielectric constants of undoped and Na-doped ZnO films
4 Conclusions
1) The structure, surface morphology and optical transmittance spectra of undoped and Na-doped ZnO films, prepared by sol-gel method, were investigated by XRD, SEM and UV-Vis spectrophotometry.
2) The XRD analysis indicates that the films are of polycrystalline structure. The SEM results show that surface morphology is strongly dependent on the content of sodium.
3) The optical constants (refractive index, extinction coefficient, dielectric constant) were determined by analyzing the transmittance spectrum. The refractive indices of the films in the visible range decrease at first and then increase with increasing the Na doping content.
References
[1] SHIH W C, SU H Y, WU M S. Deposition of ZnO thin films on SiO2/Si substrate with Al2O3 buffer layer by radio frequency magnetron sputtering for high frequency surface acoustic wave devices [J]. Thin Solid Films, 2009, 517(11): 3378-3381.
[2] KRISHNAMOORTHY S, ILIADIS A A. Properties of high sensitivity ZnO surface acoustic wave sensors on SiO2/(100) Si substrates [J]. Solid State Electronics, 2008, 52(11): 1710-1716.
[3] LIN S S, LU J G, YE Z Z, HE H P, GU X Q, CHEN L X , HUANG J Y, ZHAO B H. P-type behavior in Na-doped ZnO films and ZnO homojunction light-emitting diodes [J]. Solid State Communications, 2008, 148(1/2): 25-28.
[4] WANG D Y, GAO S X. Influence of annealing condition on the structure and optical properties of Na-doped ZnO thin films prepared by sol-gel method [J]. Journal of Alloys and Compounds, 2009, 476(1/2): 925-928.
[5] ZHANG Z Z, WEI Z P, LU Y M, SHEN D Z, YAO B, LI B H, ZHAO D X, ZHANG J Y, FAN X W, TANG Z K. P-type ZnO on sapphire by using O2-N2 co-activating and fabrication of ZnO LED [J]. Journal of Crystal Growth, 2007, 301/302: 362-365.
[6] CHEN K J, HUNG F Y, CHANG S J, YOUNG S J. Optoelectronic characteristics of UV photodetector based on ZnO nanowire thin films [J]. Journal of Alloys and Compounds, 2009, 479(1/2): 674-677.
[7] WANG K, VYGRANENKO Y, NATHAN A. Optically transparent ZnO-based n-i-p ultraviolet photodetectors [J]. Thin Solid Films, 2007, 515(17): 6981-6985.
[8] LOOK D C, CLAFLIN B. P-type doping and devices based on ZnO [J]. Physics Status Solid B, 2004, 241(3): 624-630.
[9] NEUMANN H, HORIG W, RECCIUS E, SOBOTTA H, SCHUMANN B, KUHN G. Growth and optical properties of CuGaTe2 thin films [J]. Thin Solid Films, 1979, 61(1): 13-22.
[10] LIU Y C, HSIEH J H, TUNG S K. Extraction of optical constants of zinc oxide thin films by ellipsometry with various models [J]. Thin Solid Films, 2006, 510(1/2): 32-38.
[11] XUE S W, ZU X T, ZHOU W L, DENG H X, XIANG X, ZHANG L, DENG H. Effects of post-thermal annealing on the optical constants of ZnO thin film [J]. Journal of Alloys and Compounds, 2008, 448(1/2): 21-26.
[12] HUANG B, LI J, WU Y B. Optical constants of transparent ZnO films by RF magnetron sputtering [J]. Materials Letters, 2008, 62(8/9): 1316-1318.
[13] CAGLAR M, ILICAN S, CAGLAR Y. Influence of dopant concentration on the optical properties of ZnO:In films by sol-gel method [J]. Thin Solid Films, 2009, 517(17): 5023-5028.
[14] LI Q H, ZHU D L, LIU W J, LIU Y. Optical properties of Al-doped ZnO thin films by ellipsometry [J]. Applied Surface Science, 2008, 254(10): 2922-2926.
[15] CAGLAR Y, ILICAN S, CAGLAR M, YAKUPHANOGLU F. Effects of In, Al and Sn dopants on the structural and optical properties of ZnO thin films [J]. Spectrochimica Acta: Part A, 2007, 67(3/4): 1113-1119.
[16] EL-FADL A A, MOHAMAD G A, EL-MOIZ A B A, RASHAD M. Optical constants of Zn1-xLixO films prepared by chemical bath deposition technique [J]. Physica B, 2005, 366(1/2/3/4): 44-54.
[17] GUNGOR T, SAKA B. Calculation of the optical constants of a thin layer upon a transparent substrate from the reflection spectrum using a genetic algorithm [J]. Thin Solid Films, 2004, 467(1/2): 319-325.
[18] MINKOV D A. Calculation of the optical constants of a thin layer upon a transparent substrate from the reflection spectrum [J]. Journal Physics D: Applied Physics, 1989, 22(8): 1157-1161.
[19] XUE S W, ZU X T, ZHENG W G, XIANG X. Effects of Al doping concentration on optical parameters of ZnO:Al thin films by sol-gel technique [J]. Physica B, 2006, 381(1/2): 209-213.
[20] BAO D, GU H, KUANG A. Sol-gel-derived c-axis oriented ZnO thin films [J]. Thin Solid Films, 1998, 312(1/2): 37-39.
[21] LOSURDO M, BARRECA D, CAPEZZUTO P, BRUNO G, TONDELLO E. Interrelation between nanostructure and optical properties of oxide thin films by spectroscopic ellipsometry [J]. Surface and Coatings Technology, 2002, 151/152: 2-8.
[22] KIM K H, PARK K C, MA D Y. Structural, electrical and optical properties of aluminum doped zinc oxide films prepared by radio frequency magnetron sputtering [J]. Journal of Applied Physics, 1997, 81(12): 7764-7772.
(Edited by YANG Bing)
Foundation item: Project(50872001) supported by the National Natural Science Foundation of China; Project(20060357003) supported by Research Fund for the Doctoral Program of Higher Education of China; Project(KJ2010A284) supported by the Natural Science Foundation of Anhui Higher Education Institution of China
Received date: 2009-12-31; Accepted date: 2010-03-23
Corresponding author: SUN Zhao-qi, Professor, PhD; Tel: +86-551-5107237; E-mail: szq@ahu.edu.cn