J. Cent. South Univ. Technol. (2008) 15: 663-668
DOI: 10.1007/s11771-008-0123-7
Dispersion mechanism of nano-magnetite coated with
oleate in aqueous carrier
HU Yue-hua(胡岳华), LIU Jian-ping(刘建平), XU Jing(徐 竸), WANG Dian-zuo(王淀佐)
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: To investigate dispersion mechanism of water-based ferrofluid, the effects of electrolytes on the dispersibility of ferrofluid in the dispersing system with different pH values were discussed. The ζ-potential of magnetic nano-particles was measured to discover the adsorbent state of oleate group on the surface of magnetite particles. The mechanism that coexisting electrolyte influences the dispersibility was studied. The results show that the electrolyte affects the stability of ferrofluid through an effect on the structure of surfactant bilayer adsorption, which was proved by ζ-potential measured. The associated mechanism of steric and electrostatic is dominant in aqueous ferrofluid.
Key words: nano-magnetite; electrolyte; dispersibility; ζ-potential; dispersion mechanism
1 Introduction
Ferrofluid, a colloidal suspension containing single domain nano-sized ferromagnetic particles dispersed in a carrier liquid, attracts extensive attention due to a wide variety of potential applications[1]. To attain high magnetization ferrofluid, the volume percentage of particles in ferrofluid should be high enough to approach the upper limit about 65%[2].
Aqueous magnetite ferrofluid is the most widely applied type for the excellent performance and low cost. Stable dispersion of magnetic nano-particles in carriers to get ferrofluid implies a stabilization procedure. In highly polar carriers involving water, various stabilization mechanisms including steric stabilization and electrostatic stabilization are possible[2]. Coexisting electrolyte in aqueous ferrofluid disturbs the stability of ferrofluid seriously when the stability of ferrofluid depends on electrostatic repulsion[3]. Aqueous ferrofluid usually coexists with electrolytes, such as salt, hydrochloric acid, lactic acid and so on[4]. For example, when water-based ferrofluid is employed as drug carrier to target delivery in blood vessel flowing with blood in clinical iatrology, the electrolyte in plasma will imperil the stability of ferrofluid, and vital medical incidents could step with the dispersion invalidation of magnetic nano-particles if no measures are taken to ensure the stability of ferrofluid in electrolyte circumstance.
To ensure the dispersion stability, stabilization mechanisms should be penetrated to provide reference to prevent invalidation of ferrofluid. Electromagnetism resonance, analytical chemistry, TEM and infrared spectrum are effective methods to investigate the adsorption of surfactant on the surface of magnetite particles in ferrofluid[5]. E-DLVO calculation for interaction energy of adjacent particles is helpful and credible to predict agglomeration or dispersion of magnetic particles in ferrofluid[6-7].
In this work, a dispersion mechanism of aqueous ferrofluid was studied through analyzing effect of pH and ion intensity on the stabilities of aqueous ferrofluid, and the stabilization mechanism of sodium oleate in water was explained by ζ-potential.
2 Experimental
2.1 Materials
Ferrous chloride, ferric chloride and ammonia solution (25%) were used as reagents of synthesizing magnetite, and sodium oleate was the surfactant to present the stability of ferrofluid sample. Sodium chloride, sodium sulfate, sodium phosphate, calcium chloride and aluminum chloride were employed to review the effect from electrolytes. Double-distilled water was used as carrier and solvent.
2.2 Experimental procedure
2.2.1 Synthesis of ferrofluid
To avoid flocculation and oxidation[5], nano- magnetite particle was synthesized just before dispersion process. Superfluous ammonia solution (25%) was titrated into 200 mL mixture solution of chloride (involving 0.05 mol ferric trichloride and 0.03 mol ferrous dichloride) gradually within 30 min, intensively stirring was employed in close reactor with nitrogen as the protective atmosphere. Alkalinity of the reaction liquid was controlled to 10 to ensure the magnetic properties[8]. The reactor was then placed in supersonic cleaner to perform an ultrasonic dispersion process for  Redundant electrolyte was eliminated through washing the magnetic particles produced from the titration with distilled water in a magnetic force subsider. The deposition was dispersed in a solution involving 0.061 g (0.2 mmol) sodium oleate with the final cubage adjusted to 200 mL. The following working procedure was 1 h of ultrasonic dispersion and 6 h of intense stirring. Hydrochloric acid and sodium hydroxide were used as pH regulators to adjust the suspension to the required acidity. Finally, the suspension was processed with ultrasonic washer for 30 min.
Redundant electrolyte was eliminated through washing the magnetic particles produced from the titration with distilled water in a magnetic force subsider. The deposition was dispersed in a solution involving 0.061 g (0.2 mmol) sodium oleate with the final cubage adjusted to 200 mL. The following working procedure was 1 h of ultrasonic dispersion and 6 h of intense stirring. Hydrochloric acid and sodium hydroxide were used as pH regulators to adjust the suspension to the required acidity. Finally, the suspension was processed with ultrasonic washer for 30 min.
2.2.2 Measurement of stability
Solid phase in the suspension was settled in a centrifugal or gravitational field. According to Storks’ theory, particles settle inevitably and the velocity of settlement is in the direct ratio to its mass[9]. The suspension was done with a centrifugation of 2 000 times of gravity acceleration (about 19.6 km/s2) for 30 min to remove the flocculation. The sample was then placed in a vacuum desiccator at 80 ℃ for 12 h to remove water. The mass of remaining solid matter was weighed and recorded. Particles left in the suspension formed stable colloids. The concentration of particles in the stable colloid reflected the quality of dispersion[7].
According to the recorded mass, a mass of solid phase that could be dispersed stably in 100 g pure water could be deduced as the key parameter to evaluate the stability of ferrofluid. Here the parameter was named as dispersibility (η). Dispersibility of ferrofluid can be calculated as formula (1). Chemical titration was then carried out to get an exact quantity of ferrous ion in the remaining solid to scale the chem-stabilization compared with the weighed data[10]
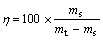 (1)
(1)
where ms is the mass of solid dispersed stably and mt is the total mass of ferrofluid sample. There is no dimension endowed to the parameter dispersibility according to formula (1).
2.2.3 Measurement of ζ-potential
Sample required in ζ-potential detection was obtained through diluting the original ferrofluid without centrifugal treatment. Solid phase concentration was controlled accurately at 1 mg/L, and electrolyte concentration in samples was 0.001 mol/L. pH was adjusted to given value using hydrochloric acid or sodium hydroxide solution. ζ-potential was measured through electrophoresis method using a Delsa 440SX zeta potential & Size Analyzer (Backmen-Caulter, U.S.A), at room temperature of 25 ℃. pH—ζ curves were drawn to provide particular information about the effect of pH on the ζ-potential of the colloid.
3 Experiment results
3.1 Dispersibility of magnetite particles in aqueous carrier without surfactant
Fig.1 shows the dispersibility of nano-magnetite in water without any surfactant or electrolyte. Good dispersibility in acidic medium, which is proved the commonness for all system involved in this work, is shown with characters including poor colloid stability in neutral and alkali circumstance. Inherent reason of this point could been indexed in Ref.[11] about surface acid-base properties of nano-magnetite particles and composing about solution chemistry of floatation[12].
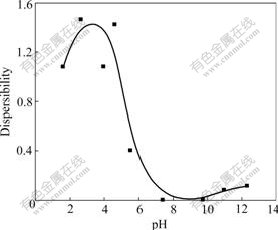
Fig.1 Dispersibility of nano-magnetite in aqueous carrier without surfactant or electrolyte
3.2 Situation with sodium oleate as dispersant
Oleic acid is one of the best surfactants in the ferrofluid industry for its excellent coating and dispersion ability[13]. Sodium oleate is a substitute for oleic acid for solubility reason in aqueous carriers. Figs.2-4 show the dispersibilities of ferrofluid in sodium chloride solutions with dispersant concentration of 0.001 mol/L according to the statement in section 2.2.1.

Fig.2 Effect of electrolyte concentration on dispersibility nano- magnetite coated with sodium oleate

Fig.3 Effect of cation on dispersibility of nano-magnetite coated with sodium oleate
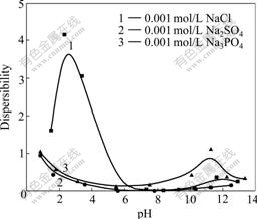
Fig.4 Effect of anion on dispersibility of nano-magnetite coated with sodium oleate
Fig.2 reveals the effect of sodium chloride concentration. The optimal dispersibility exceeds 4 when pH is about 2 and the concentration of NaCl is 0.001 mol/L, and then decreases with the dosage increase of NaCl. Additional NaCl makes colloid better dispersibility according to experimental data in Fig.2. The accessorial dispersive action of reactionless electrolyte and NaCl indicates a special electrostatic stabilization in aqueous ferrofluid when sodium oleate is served as dispersant.
Fig.3 shows the effect of cation on the dispersibility of ferrofluid at electrolyte concentration of 0.001 mol/L. Ion with higher valence leads to compression of electrical double-layer to make formation of flocculation easier although it did not destroy stability completely, which is due to the valence effect of electrolyte[14]. The degree of this effect is magnified by consumption of surfactant through reaction of metal ions and oleate group to form calcium oleate and aluminum oleate deposition[14]. It is an integrated effect from metal ion and dispersant to change dispersibility of ferrofluid (see Fig.4) when additional electrolyte with highly charged metal ion exists.
Effects of anions on dispersibility of ferrofluid are shown in Fig.4. On the surface of nano-magnetite, competition absorption of anion exists between oleate ion and electrolyte anions. An evidence of competitive absorption is the unexpected stability of sample with sodium phosphate involved. Sodium phosphate is often used as depressor in the floatation field to keep certain mineral suspending in water[14]. In the acid region from pH 2 to pH 7, H2PO4- and HPO42- are the primary forms in aqueous solution, and SO42- coexists with HSO4- in the same situation[15]. It is the same valency diversity of anion for two electrolytes that have disturbed the stability of ferrofluid samples through weakening the absorption of oleate group on the surface of particles. Cushioning action from hydrolyzation and adsorption equilibrium have made similar effect for the two sodium salts.
3.3 ζ-potential of magnetic particles
Effects of cations on ζ-potential of nano-magnetite particles are shown in Fig.5. Being a strong electrolyte, calcium chloride does not hydrolyze but exists as dissociative Ca2+ and Cl- in solution[16]. When the surface potential is positive, chloride ion acts as inert ions to affect the thickness of electrical double-layer.
Additional calcium cation made the surface potential positive throughout pH field from 2 to 12. This fact shows the specific adsorption of cations on surface of particles, by which more positive charges are taken to the surface of particles to make the surface potential higher. Aluminum ion also commits adsorption on par-

Fig.5 Surface potentials of nano-magnetite coated with sodium oleate in aqueous solution of different electrolytes: 1—0.001 mol/L NaCl; 2—0.001 mol/L CaCl2; 3—0.001 mol/L AlCl3; 4—0.001 mol/L Na2SO4; 5—0.001 mol/L Na3PO4
ticles surface and makes the surface potential more positive. The difference is the hydrolyzation of aluminum ion in alkali medium. With rising alkalinity of solution, aluminum ion combines with hydroxyl ion in the solution to reduces its positive charge until transforming into Al(OH)4- completely. Positive- charged tiny Al(OH)3 colloids particles are generated to cover on the surface of magnetite in this progress. Analogic reaction was employed to synthesize nano- particles covered by special shell layer[17-18]. The adsorption of Al3+ and stepwise hydrolyzation of aluminum ion led to a higher surface potential with an isoelectric point over 9.0.
As shown in Fig.5, sodium sulfate and sodium phosphate make surface potential of the magnetite particles more negative. The symmetry from 5 lines in Fig.5 (between 3 and 5, 2 and 4) indicates an similarity of absorption of ions with same electric quantity but contrary electrical property, and the difference is that calcium ion is captured by the film via static electric force, sulphate ion by hydrogenous bond with aqueous carrier molecular to bridge[13]. Calcium ion is captured so firmly that ζ-potential of sample keeps permanent positive in the whole measure range. Adsorption of sulfate ions is analogous but weaker than that of calcium, in the wider range of ζ-potential from -40 mV to +17 mV. The absorption mechanism of phosphate ion and sulphate ion on particles is comparable. It is easier for phosphate to commit adsorption by particles surface due to higher charge and the formation of surface precipitation[13]. As a result, surface potential of Curve 5 in Fig.5 diminishes seriously and is overthrown to negativevalue even in strong acidic medium.
According to DLVO theory, higher valence of ion enlarges the degree of the compression of electrical double layer and makes the absolute value of ζ-potential lower and stability of colloid adverse[19-20]. While the poor dispersibilities of samples (curve 3 in Fig.3 and curves 2, 3 in Fig.4) could not be explained simply through surface potential. Phosphate ion and aluminum ion are able to form different surfaces made up of spatial reticular structure on the basis of their hydrolysis[13]. It is easy for neighboring particles to form agglomeration and flocculation is avoidless because of the polycondensation of multi-hydroxyl aluminum or poly phosphate on the surface of magnetite particles[21].
It is convictive to explain the above-mentioned experimental fact on the basis of the idea about the double layer absorption of oleate ion on the surface of nano-magnetite particles[22] and evidence could be found in the contrast with Fig.6, in which the effect of electrolyte on ζ-potential of nano-magnetite without surfactant was investigated. When pH is adjusted from 2 to 12, ζ-potential of pure magnetite decreases from +10 mV to -18 mV with an isoelectric point (IEP) of pH≈6.5. Addition of NaCl brings a shift of isoelectric point to pH≈5.7, showing a slight anion adsorption of particles. Tervalent and bivalent cations transmit surface potential upwards to make surface potential of samples positive and the IEP towards high alkaline. The difference between curves in Fig.6 is also narrower than that in Fig.5. The reaction between metal ions and oleate ion captured on surface of particles is proved to be crucial in flotation engineering[23], which makes the surface potential of oleate-coated nano-magnetite more positive in the whole measure range in Fig.5. The fact that the slopes of curves in Fig.6 are generally flater contrasting with those in Fig.5, indicating a much stronger absorption of metal ion on surface of magnetite particles treated with sodium oleate.
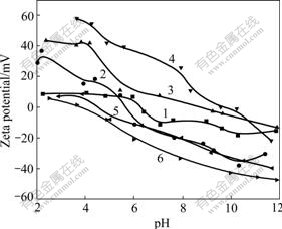
Fig.6 ζ-potential of nano-magnetite particles in electrolyte solution without dispersant: 1—Without electrolyte; 2—0.001 mol/L NaCl; 3—0.001 mol/L CaCl2; 4—0.001 mol/L AlCl3; 5—0.001 mol/L Na2SO4; 6—0.001mol/L Na3PO4
4 Discussion
Sodium oleate can ensure dispersibility of ferrofluid in acidic medium[22]. Some phenomena are explained successfully through classical DLVO theory by contrasting data from dispersibility and ζ-potential, but experiment shows that dispersibility of ferrofluid is better through addition of sodium chloride. At the same time, the surface potential of particles with calcium chloride coexisting keeps positive throughout the measurement, and is also contrary with the explanation from classical theory, the effects of high valent ions also indicate different mechanisms.
Double-deck adsorption of oleate on the surface of magnetite nano-particles in ferrofluid makes most of experimental phenomena justify themselves. With hydrophobic alkyl attracting together through hydrophobic interaction, a second oleic layer is able to commit adsorption onto particles surface of the first oleate adsorption layer, which is formatted by a layer of oleate catching onto the bare surface of magnetite particles by carboxyl group with the hydrophobic alkyl outside[24]. In this way, the outer layer of adsorption is made up of carboxyl groups of many oleate ion.
Reaction of high valent metal ion with carboxyl raises surface potential of colloid particles. With rising pH of suspension, adsorption of hydroxyl ion debases the positive surface potential lower. For calcium ion, firm adsorption but no hydrolysis is committed, effect of pH on ζ-potential is limited on the enrichment of hydroxyl ion, and the slope and the extent of surface potential reduction are small. For aluminium ion, gradual hydrolysis makes the slope and the extent larger. Anions are captured through electrostatic attraction, and polymerization tendencies of sulfate ion and phosphate ion promote the bridge flocculation of adjacent particles.
The fact that reactionless electrolyte sodium chloride improves stability of ferrofluid is also explainable on the basis of double-deck adsorption of oleate, while the evidence can only be provided through molecular simulation.
Conclusion can be drawn that double-deck absorption of oleate ions takes place in aqueous ferrofluid with sodium oleate as the surfactant. Electrolyte ions commit characteristic absorption and influence the dispersibility of ferrofluid. The experimental phenomena in this work indicate that it is the associated mechanism of steric and electrostatic, instead of single mechanism that provides stability for magnetite coated with sodium oleate in aqueous.
5 Conclusions
1) Aqueous ferrofluid with 0.001 mol/L sodium oleate as dispersant shows fine dispersibility in acidic medium. The change of pH and existence of electrolyte affect apparently on the dispersibility of ferrofluid.
2) Influence of electrolyte on ζ-potential indicates that double-deck adsorption of oleate on the surface of magnetite nano-particles takes place, and high valent inorganic ions only affect on the base of committing character adsorption on the surfactant bilayer.
3) All the facts substantiate an associated dispersion mechanism of aqueous ferrofluid assisted by sodium oleate solution: existence of oleate bilayer on surface of magnetite particles, effect means of electrolyte ion on ζ-potential and dispersibility.
References
[1] R?CUCIU M, CREANG? D E, B?DESCU V, SULITANU N. Microstructural investigation of some biocompatible ferrofluids [J]. Journal of Magnetism and Magnetic Materials, 2007, 316(2): 772-775.
[2] KHOLMETSKII A L, VOROBYOVA S A. A noval route for the preparation of ferrofluids [J]. Materials Letters, 2005, 59: 1993-1996.
[3] ALEXEY O I. Phase separation in magnetic collioids [J]. Journal of Magnetism and Magnetic Materials, 1999, 201: 234-237.
[4] L?PEZ-L?PEZ M T, DUR?N J D G, DELGADO A V, GONZALEZ-CABHLLERO F. Stability and magnetic characteriza- tion of oleate-covered magnetite ferrofluid in different nonpolar carriers [J]. Journal of Colloid and Interface Science, 2005, 291: 144-151.
[5] M?RCIO J R, SILV?RIO F. Effects of pH, temperature, and ionic strength on adsorption of sodium dodecylbenzenesulfonate into Mg-Al-CO3 layered double hydroxides [J]. Journal of Physics and Chemistry of Solids, 2004, 65: 487-492.
[6] TANG P, RAPER J A. Modelling the settling behaviour of fractal aggregates—A review [J]. Powder Technology, 2002, 123: 114-125.
[7] HARWOT P, van de VEN THEO G M. Effect of sodium oleate and calcium chloride on the deposition of latex particles on an air/water interface [J]. Colloids and Surface A: Physicochemical and Engineering Aspects, 1997, 121(2/3): 229-237.
[8] ZHANG Jin-sheng. Study of preparation, characterization and stability mechanism on superfine Fe3O4 magnetite fluids [D]. Jinan: Shandong University, 2003. (in Chinese)
[9] DUNAN J. Introduction to colloid and surface chemistry [M]. 3rd ed. ZHANG Zhong-lu, ZHANG Ren-you, trans. Beijing: Chemical Industry Press, 1983. (in Chinese)
[10] ZINS D, CABUIL V, MASSART R. New aqueous ferrofluids [J]. Journal of Molecular Liquids, 1999, 83: 217-232.
[11] SUN Zhong-xi, GUO Shu-yun. Surface acid-base properties of magnetite nanoparticles [J]. Chemical Journal of Chinese Universities, 2006, 27(7): 1351-1354. (in Chinese)
[12] WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation [M]. Changsha: Hunan Science & Technology Press, 1988. (in Chinese)
[13] BICA D, V?K?S L, RASA M. Preparation and magnetic properties of concentrated ferrofluids on alcohol and water carrier liquids [J]. Journal of Magnetism and Magnetic Materials, 2002, 252: 10-12.
[14] HU Yue-hua. Study of flotation behavior of salt-type minerals based on solution chemistry calculation and graphics [D]. Changsha: Central South University of Technology, 1989. (in Chinese)
[15] QIU Guan-zhou, HU Yue-hua. Interactions between particles and flotation of fime particles [M]. Changsha: Central South University of Technology Press, 1993. (in Chinese)
[16] YAN Xuan-shen, WANG Chang-fu. Ion balance and chemical reaction in aqueous solution [M]. Beijing: Higher Education Press, 1993. (in Chinese)
[17] PENG Hui, ZHANG Li-juan, SOELLER C, TRAVAS-SEJDIC J. Preparation of water-soluble CdTe/CdS core/shell quantum dots with enhanced photostability [J]. Journal of Luminescence,2007, 127(2): 721-726.
[18] KOBAYASHI Y, IMAI J, NAGAO D. Preparation of multilayered silica-Gd-silica core-shell particles and their magnetic resonance images [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 308(1/3): 14-19.
[19] CHENG Hai-fu, LIU Gui-zhen, LI Li-chun, GUAN Jian-guo, YUAN Run-zhang. ζ-potential and stability of Fe3O4 nano-particles [J]. Journal of Wuhan University of Technology, 2003, 25(5): 4-6, 25. (in Chinese)
[20] ZHU Yong-qiang. Special topicl lectures on paper chemicals and wet end chemistry, Chapter 4: Chemistry of aluminum ion and coordinating stablization effect [J]. Shanghai Paper Making, 2003, 34(4): 44-47. (in Chinese)
[21] TIZIANA M, ADELL A. On the applicability of DLVO theory to the prediction of clay colloids stability [J]. Journal of Colloid and Interface Science, 2000, 230: 150-156.
[22] YANG Xi-yun, GONG Zhu-qing. Effect of dispersants on surface chemical properties of magnetite [J]. Journal of Central South University: Science and Technology, 2005, 36(12): 243-247. (in Chinese)
[23] LIU San-jun. The reaction of the surfactants in system of flotation of diaspore [D]. Changsha: Central South University, 2005. (in Chinese)
[24] SONG M G, KIM J Y, KIM J D. Effect of sodium stearate and calcium ion on dispersion properties of precipitated calcium carbonate suspensions [J]. Colloids and Surfaces A: Physicochemical, 2003, 229: 75-83.
(Edited by YANG Hua)
Foundation item: Project(50374083) supported by the National Natural Science Foundation of China; Project(134375215) supported by the Research Fund for Postgraduate Innovation Project of Central South University, China
Received date: 2008-03-15; Accepted date: 2008-05-10
Corresponding author: LIU Jian-ping, PhD; Tel: +86-13017296855; E-mail: ljping6422@163.com