文章编号:1004-0609(2012)09-2656-06
CaCl2-Ca(OH)2-H2O体系中氢氧化钙的平衡浓度
刘卫平,徐 徽,石西昌,杨喜云,陈 亚,程俊峰,李 贵
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:绘制了25℃时CaCl2-Ca(OH)2-H2O体系的c(CaCl2)—c(Ca(OH)2)图和c(CaCl2)—pH值图。结果表明:体系中c(Ca(OH)2)和pH值随着c(CaCl2)的增加而不断减小, c(Ca(OH)2)变化趋势与Debye-Huckel计算值相符,当c(CaCl2)<3.78 mol/L时,体系pH值>10.4。当c(CaCl2)>2.57mol/L时,有沉淀物CaClOH生成。经计算,理论上CaCl2母液可以循环配制石灰水132次,研究结果为石灰水法制备氢氧化镁工艺中CaCl2母液循环配制石灰水提供了一定的理论依据。
关键词:CaCl2-Ca(OH)2-H2O;石灰水;CaCl2母液;pH值;循环
中图分类号:TF111.3 文献标志码:A
Equilibrium concentration of calcium hydroxide in
CaCl2-Ca(OH)2-H2O system
LIU Wei-ping, XU Hui, SHI Xi-chang, YANG Xi-yun, CHEN Ya, CHENG Jun-feng, LI Gui
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The c(CaCl2)—c(Ca(OH)2) chart and c(CaCl2)—pH value chart in CaCl2-Ca(OH)2-H2O system under 25 ℃ were plotted. The results represent that c(Ca(OH)2) and pH value decrease with the increase of c(CaCl2), the trend of c(Ca(OH)2) is supported by the calculation results of Debye-Huckel. When c(CaCl2)<3.78 mol/L, the pH value of the system is bigger than 10.4, and CaClOH is preciptated while c(CaCl2)>2.57 mol/L. Through calculation, the CaCl2 mother liquor can reuse 132 times for collocating lime water in theory. The research results provide a theoretical basis for the treatment and reuse of calcium chloride mother liquor for collocating lime water which is the precipitant in the process of synthesizing magnesium hydroxide.
Key words: CaCl2-Ca(OH)2-H2O; lime water; CaCl2 mother liquor; pH value; recycling
基金项目:国家科技支撑计划“十一五”重大项目(2008BAB35B04);中央专项中南大学前沿研究计划重点项目(2010QZZD003)
收稿日期:2011-09-07;修订日期:2012-01-16
通信作者:徐 徽,教授,博士;电话:0731-88877352;E-mail: xuhui_0318@hotmail.com
氢氧化镁作为一种重要的无机化合物,可广泛应用于阻燃剂[1-3]、废水处理[4]、烟气脱硫及医药等领 域[5-7]。由于氢氧化镁用途广泛,故对其制备方法的研究备受关注。
目前,工业上生产氢氧化镁的方法有氨法、烧碱法和石灰乳法等,且以氨法为主。氨法产生的氨氮废水对环境污染大,同时氨易于挥发、有刺激性气味,造成工人劳动环境差,此法扩产受到限制。烧碱法生产成本较高。石灰乳法成本较低,若能攻克产品纯度较低的瓶颈,该方法有较好的市场应用前景,且卤水生产氢氧化镁比矿石生产氢氧化镁具有明显优势[8],因此,研究以石灰水和卤水为原料制备氢氧化镁新工艺具有重要意义。石灰水法制备氢氧化镁时副产大量氯化钙母液,利用氯化钙母液循环配制石灰水是降低石灰水法成本的有效途径,但随着氯化钙母液配制石灰水的不断循环,氯化钙会在石灰水中不断富集,循环到一定次数后,氯化钙母液将不宜继续配制石灰水。目前对氯化钙母液循环配制石灰水的工艺研究较少,并且其理论研究亦未见报道。
本文作者利用德拜休克尔极限定律探讨CaCl2- Ca(OH)2-H2O体系中氯化钙浓度对氢氧化钙溶解度的影响,同时测定该体系pH值的变化规律,并计算氯化钙母液配制石灰水可以循环的次数,为氯化钙母液循环配制石灰水提供理论指导。
1 实验
1.1 试剂与仪器
主要试剂:Ca(OH)2(分析纯,广东汕头市西陇化工厂生产);CaCl2(分析纯,广东汕头市西陇化工厂生产);乙二胺四乙酸二钠(分析纯,国药集团化学试剂有限公司生产);NaOH(优级纯,天津市华真特种化学试剂厂生产);钙-羧酸(分析纯,天津市化学试剂研究所生产);超纯水等。
主要仪器:PHS-3C精密pH计;AUY220型电子分析天平;JBV-Ⅲ变频调速搅拌器;HH-601恒温数显水浴;SHZ-CD型循环水式多用真空泵等。
1.2 实验方法
配制一定浓度的氯化钙溶液,经过滤除杂后装入三口烧瓶中,置于25 ℃的恒温数显水浴锅中,固定于铁架台,插入搅拌桨,调节转速为300 r/min左右。用电子分析天平称取一定量的分析纯氢氧化钙,加入三口烧瓶中,反应达到平衡后取样过滤后分析。钙离子浓度采用EDTA二钠滴定法测定。氢氧根离子的测定采用滴定法测定。将氯化钙溶液称为Ai溶液,向Ai溶液中加入过量氢氧化钙,达到溶解平衡后过滤的溶液称为Bi溶液。
2 实验原理
2.1 固液溶解平衡的溶度积和活度积
在一定温度下,各种盐在水中溶解达到饱和时,便达到了溶解平衡[9]。对于一种给定的盐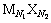 ,其溶解平衡可以表示为
,其溶解平衡可以表示为
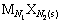

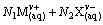 (1)
(1)
当达到平衡时: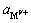 和
和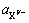 分别为阳离子和阴离子在溶解平衡时的活度;
分别为阳离子和阴离子在溶解平衡时的活度;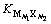 为盐在水中的溶解平衡常数。它与温度和离子强度有关,故称为电解质活度积。若忽略固有溶解度及各阴阳离子的水解,而只考虑溶液中离子强度的影响,即可进行活度积计算。假设此结论适用于CaCl2-Ca(OH)2-H2O三元体系。
为盐在水中的溶解平衡常数。它与温度和离子强度有关,故称为电解质活度积。若忽略固有溶解度及各阴阳离子的水解,而只考虑溶液中离子强度的影响,即可进行活度积计算。假设此结论适用于CaCl2-Ca(OH)2-H2O三元体系。
3.2 25 ℃时CaCl2-Ca(OH)2-H2O系Ca(OH)2平衡浓度的计算CaCl2-Ca(OH)2-H2O系是一个由含有共同阳离子Ca2+的盐、碱和水组成的三元体系。若Ca(OH)2是饱和的,其浓度为c(Ca(OH)2);若CaCl2没有饱和,其浓度为c(CaCl2),则Ca(OH)2 的溶解平衡常数为根据式(12)即可得到溶液中氯化钙浓度与氢氧化钙浓度的关系。
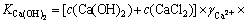
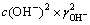 (2)
(2)
若c(CaCl2)是已知的,而c(Ca(OH)2)未知,在CaCl2的浓度为c(CaCl2)时可利用式(2)求得c(Ca(OH)2):

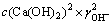 (3)
(3)
25 ℃时,KSP=[Ca2+][OH-]2=5.5×10-6[10]采用Debye-Huckel公式[11]估算Ca2+和OH-的活度系数:
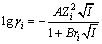 (4)
(4)
式中:ri为离子半径;A、B为系数,与溶剂的温度和介电常数有关,查表[11]知25 ℃时,A=0.511 5,B= 0.329 1,r(Ca2+)=0.6 nm,r(OH-)=0.35 nm。
溶液离子强度I:
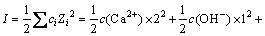
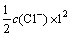 (5)
(5)
式中:ci为i离子的物质的量浓度,Zi为i离子的电荷数,其中将式(9)代入式(4)中可得式(10)和(11)。
c(Ca2+)=c(Ca(OH)2)+c(CaCl2) (6)
c(OH-)=2c(Ca(OH)2) (7)
c(Cl-)=2c(CaCl2) (8)
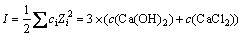 (9)
(9)
钙离子活度因子:
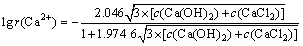 (10)
(10)
氢氧根离子的活度因子:
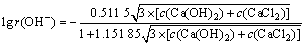 (11)
(11)
将式(10)和(11)代入式(3)得式(12):
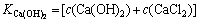 ×
×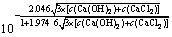 ×4×c(Ca(OH)2)2×
×4×c(Ca(OH)2)2×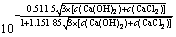 (12)
(12)
3 结果与讨论
3.1 平衡时间的确定
在0.48 mol/L的氯化钙溶液中加入过量氢氧化钙,溶液体积为800 mL,温度为25 ℃,搅拌速度为300 r/min。为尽量避免溶液与CO2反应及水分的蒸发影响实验结果,烧杯用胶带和保鲜膜密封。隔一定时间后取样分析,考察OH-浓度和pH值的变化情况,实验结果如图l所示。由图1可知,反应2 h后,OH-浓度和pH值基本保持不变,表明溶解达到平衡。
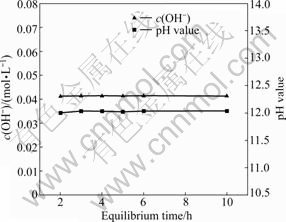
图l c(OH-)和pH值与平衡时间的关系
Fig. 1 Relationships between c(OH-), pH value and equilibrium time
3.2 25 ℃时CaCl2-Ca(OH)2-H O体系中Ca(OH)2平衡浓度
OH-平衡浓度的变化情况结果如图2所示。由图2可知,随着氯化钙浓度的增加,氢氧化钙的浓度不断下降。
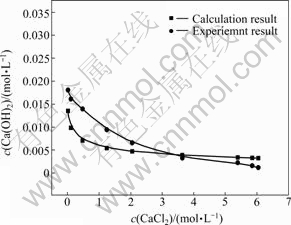
图2 计算值与实验值的关系
Fig. 2 Relationship between calculation result and experiment result
利用德拜休克尔极限定律计算不同氯化钙浓度下氢氧化钙浓度,计算结果由在图2中绘出,由图2可知,计算获得的氢氧化钙浓度变化趋势和实际结果一致,但由于理论计算时忽略了固有溶解度及各阴阳离子的水解,导致数值上有一定的偏差。同时,由于c(CaCl2)>2.57 mol/L时,CaCl2和Ca(OH)2反应生成了CaClOH。因此,如图2所示,当c(CaCl2)>2.57mol/L后,随着氯化钙浓度的增加,氢氧化钙浓度因为参与反应而不断降低,当c(CaCl2)>3.3 mol/L后氢氧化钙的实验值比理论值低。
3.3 CaCl2-Ca(OH)2-H2O体系中pH值的变化规律
石灰水法制备氢氧化镁过程中,通过加入氢氧化钙溶液调节pH值,控制反应过程的进行,pH值对氢氧化镁的成核和结晶生长过程的影响很大[12-14]。因此,研究B溶液中氯化钙浓度与溶液pH值之间的关系非常重要。
如图3所示,随着氯化钙浓度的增加,B溶液的pH值是呈下降趋势。这是由于随着氯化钙浓度增加,导致溶液中钙离子增加,根据同离子效应,氢氧化钙浓度必然下降。制备氢氧化镁过程中反应终点pH值大于10.4[14],故当B溶液pH值大于10.4时,即可用于制备氢氧化镁的反应。图3中a点具有代表性,a点氯化钙浓度为3.78 mol/L,pH值为10.45,即氯化
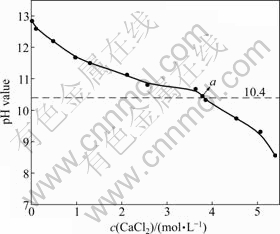
图3 B溶液c(CaCl2)与pH值的关系
Fig. 3 Relationship between c(CaCl2) and pH value of B solution
钙母液循环配制石灰水的最高氯化钙浓度为3.78 mol/L。实际反应过程中,可以通过测定反应母液氯化钙浓度和pH值来判定氯化钙母液是否可以进入下一步循环配制石灰水。
3.4 CaCl2-Ca(OH)2-H2O体系结晶物的生成
如图4所示, B1~B7溶液钙离子浓度始终大于对应的A1~A7溶液钙离子浓度,但是A8溶液和B8溶液钙离子浓度都为2.57 mol/L。此后,B9~B11溶液钙离子浓度始终小于其对应的A9~A11溶液钙离子浓度。而根据图5所示,B1~B11溶液的pH值始终是高于A1~A11溶液的pH值,所以B1~B11溶液中都有氢氧化钙溶解。

图4 c(Ca2+)Ai和c(Ca2+)Bi的关系
Fig. 4 Relationship between c(Ca2+)Ai and c(Ca2+)Bi
因此,当c(Ca2+)A>2.57 mol/L时,c(Ca2+)B<c(Ca2+)A,这因为CaCl2和Ca(OH)2反应生成了CaClOH。将B9溶液过滤真空干燥后即得结晶物,XRD分析如图6所示。
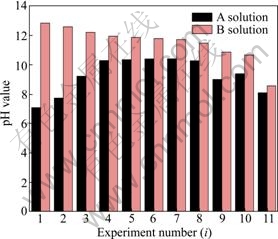
图5 pH(Ai)和pH(Bi)的关系
Fig. 5 Relationship between pH(Ai) and pH(Bi)
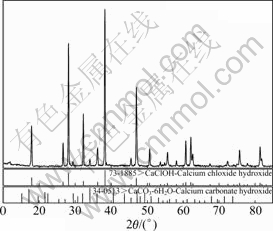
图6 结晶物的XRD谱
Fig. 6 XRD pattern of crystal
如图6所示,结晶物中主要含CaClOH和CaCO3? 6H2O两种固相。CaCO3?6H2O是由于实验中石灰水与空气接触,原理如式(13)所示。据文献报道[15-17],CaClOH的生成原理如式(14)所示:
Ca(OH)2+5H2O+CO2→CaCO3?6H2O↓ (13)
CaCl2+Ca(OH)2→2CaClOH↓ (14)
因此,当氯化钙母液浓度大于2.57 mol/L时,该母液不适合配制石灰水,因为会生成碱式氯化钙(CaClOH),造成氢氧化钙的损失和成本的提高,同时也会降低产物氢氧化镁的纯度。此时,由图3可知,当c(Ca2+)=2.57时,B溶液pH值为10.81。
3.5 氯化钙母液循环次数的计算
从制备氢氧化镁反应所需pH值考虑,氯化钙母液浓度低于3.78 mol/L即可配制石灰水。但由于氯化钙母液浓度过高会与氢氧化钙生成碱式氯酸钙,造成氢氧化钙的损失,所以从成本上考虑,当氯化钙母液浓度大于2.57 mol/L时,即不能配制石灰水。
MgCl2+Ca(OH)2→Mg(OH)2↓+CaCl2 (15)
石灰法制备氢氧化镁原理如式(15)所示。假设第一次反应时石灰水浓度为0.019 5 mol/L,氢氧化钙与氯化镁添加量按照1:1进行。原料为5 mL 的95 g/L MgCl2溶液,1 L的0.019 5 mol/L石灰水。反应完成后母液氯化钙浓度为0.019 4 mol/L。假设反应母液无损失,每次得到1.005 L反应母液,配制石灰水后为1.005 L,取1 L参与下一次循环。通过计算,可知反应母液可以循环132次。
3 结论
1) 25 ℃时,CaCl2-Ca(OH)2-H2O体系需经2 h达到平衡。
2) 2 5℃时,CaCl2-Ca(OH)2-H2O体系中氢氧化钙浓度随着氯化钙浓度的升高而降低,利用德拜休克尔极限定律计算氯化钙浓度与氢氧化钙浓度的关系,发现实验值和计算值符合。
3) 25 ℃时,CaCl2-Ca(OH)2-H2O体系中氯化钙浓度小于3.78 mol/L时,溶液pH值>10.4。
4) 25 ℃时,CaCl2-Ca(OH)2-H2O体系中氯化钙母液浓度小于2.57 mol/L时,有CaClOH相生成。
5) 从成本综合考虑,确定氯化钙母液浓度小于2.57 mol/L,pH值大于10.81时,才能用于配制石灰水;根据反应简化模型,理论上氯化钙母液可以循环配制石灰水132次。
REFERENCES
[1] 徐旺生, 张 翼. 新型无机阻燃剂的研究进展[J]. 江苏化工, 2002, 30(4): 20-22.
XU Wang-sheng, ZHANG Yi. Research progress of novel inorganic five retarding agent [J]. Jiangsu Chemical Industry, 2002, 30(4): 20-22.
[2] KIM S. Flame retardancy and smoke suppression of magnesium hydroxide filled polyethylene [J]. Journal of Polymer Science Part B: Polymer Physics, 2003, 41(9): 936-944.
[3] HORNSBY P, WANG J, ROTHON R, JACKSON G, WILKINSON G, COSSICK K. Thermal decomposition behaviour of polyamide fire-retardant compositions containing magnesium hydroxide filler [J]. Polymer Degradation and Stability, 1996, 51(3): 235-249.
[4] 郭如新. 氢氧化镁在工业废水处理中的应用[J]. 工业水处理, 2000, 20(2): 1-4.
GUO Ru-xin. The applications of magnesium hydroxide to industrial wastewater treatment [J]. Industrial Water Treatment, 2000, 20(2): 1-4.
[5] 郭如新. 镁剂在烟气脱硫领域中的应用[J]. 海湖盐与化工, 2003, 32(3): 8-11.
GUO Ru-xin. Magnesium-agents and its application to flue gas desulfurization [J]. Sea-Lake Salt & Chemical Industry, 2003, 32(3): 8-11.
[6] 魏炳举, 张万峰. 一种极具发展前景的镁系产品―氢氧化镁[J]. 海湖盐与化工, 2003, 32(1): 26-29.
WEI Bing-ju, ZHANG Wan-feng. A king of development prospect magnesium product-magnesium hydroxide [J]. Sea-Lake Salt & Chemical Industry, 2003, 32(1): 26-29.
[7] 宋彦梅, 衣守志. 氢氧化镁的生产及应用技术进展[J]. 海湖盐与化工, 2006, 35(2): 15-20.
SONG Yan-mei, YI Shou-zhi. Progress on the production and application of Mg(OH)2 [J]. Sea-Lake Salt & Chemical Industry, 2006, 35(2): 15-20.
[8] 王兰君. 石灰法生产氢氧化镁工艺研究[J]. 盐业与化工, 2010, 39(6): 19-23.
WANG Lan-jun. Study on the craft of magnesium hydroxide by lime method [J]. Journal of Salt and Chemical Industry, 2010, 39(6): 19-23.
[9] 李亚红, 高世扬, 宋彭生, 夏树屏. Pitzer混合参数对HCl-NaCl-H2O体系溶解度预测的影响[J]. 物理化学学报, 2001, 17(1): 91-94.
LI Ya-hong, GAO Shi-yang, SONG Peng-sheng, XIA Shu-ping. Effects of pitzer mixing parameters on the solubility prediction of the phase system HCl-NaCl-H2O [J]. Acta Phys Chim Sin, 2001, 17(1): 91-94.
[10] 刘光启, 马连湘, 刘 杰. 化学化工物性手册(无机卷)[M]. 北京: 化学工业出版社, 2002.
LIU Guang-qi, MA Lian-xiang, LIU Jie. Physical property of chemistry & chemical engineering handbook (Inorganic volume) [M]. Beijing: Chemical Industry Press, 2002.
[11] 姚允斌, 解 涛, 高英敏. 物理化学手册[M]. 上海: 上海科学技术出版社, 1985.
YAO Yun-bin, XIE Tao, GAO Ying-min. Handbook of physical chemistry [M]. Shanghai: Science & Technology Press of Shanghai, 1985.
[12] 李秋菊, 刘华彦, 卢晗锋, 郑敏珠, 谢 晶, 陈银飞. pH 值对氢氧化镁晶体生长的影响[J]. 过程工程学报, 2007, 25(4): 609-611.
LI Qiu-ju, LIU Hua-yan, LU Han-feng, ZHANG Min-zhu, XIE Jing, CHEN Yin-fei. Effects of pH on the growth of Mg(OH)2 crystals [J]. Journal of Materials Science & Engineering, 2007, 25(4): 609-611.
[13] PHILLIPS V A, KOLBE J L, OPPERHAUSER H. Effect of pH on the growth of Mg(OH)2 crystals in an aqueous environment at 60 ℃ [J]. Journal of Crystal Growth, 1977, 41(2): 228-234.
[14] 高春娟, 张雨山, 黄西平, 蔡荣华. 浓海水-钙法制取氢氧化镁工艺研究[J]. 盐业与化工, 2011, 40(1): 5-7.
GAO Chun-juan, ZHANG Yu-shan, HUANG Xi-ping, CAI Rong-hua. Study on producing magnesium hydroxide by concentrated seawater-calcium method [J]. Journal of Salt and Chemical Industry, 2011, 40(1): 5-7.
[15] ALLAL K M, DOLIGNIER J C, MARTIN G. Determination of thermodynamical data of calcium hydroxichloride [J]. Revue de l'Institute Francais du Petrole, 1997, 52(Compendex): 361-368.
[16] ALLAL K M, DOLIGNIER J C, MARTIN G. Determination of the residence time distribution of solid particles by a photometric method [J]. Chemical Engineering Research and Design, 1998, 76(5): 643-648.
[17] ALLAL K M, DOLIGNIER J C, MARTIN G. Reaction mechanism of calcium hydroxide with gaseous hydrogen chloride [J]. Revue del' Institute Francais du Petrole, 1998, 53(Compendex): 871-880.
(编辑 何学锋)