CaO-SiO2熔渣精炼去除冶金级硅中杂质硼
来源期刊:中国有色金属学报(英文版)2014年第4期
论文作者:伍继君 李彦龙 马文会 魏奎先 杨 斌 戴永年
文章页码:1231 - 1236
关键词:冶金级硅;除硼;热力学;分配系数;熔渣精炼
Key words:metallurgical grade silicon; boron removal; thermodynamics; distribution coefficient; slag refining
摘 要:利用CaO-SiO2熔渣去除冶金级硅(MG-Si)中的杂质硼。热力学分析和实验结果表明:纯SiO2基本上不能去除冶金级硅中的杂质硼。通过建立硼的分配系数与熔渣中SiO2和CaO活度之间的关系,从热力学上对CaO-SiO2熔渣的除硼能力进行表征。结果表明:随着渣中CaO配比的升高,硼的分配系数和去除效率大大提高。当熔渣组成为60%CaO-40%SiO2(质量分数)时,硼的分配系数达到最大值1.57。当渣硅比为2.5,精炼温度为1600 °C以及精炼时间为3 h时,利用60%CaO-40%SiO2熔渣可以将冶金级硅中的硼含量从18×10-6降低至1.8×10-6,去除效率达到90%。
Abstract: Boron removal from metallurgical grade silicon (MG-Si) using a calcium silicate slag was studied. The results show that it is impossible basically to remove boron using a pure SiO2 refining. The oxidizing ability of CaO-SiO2 slag for boron removal was characterized by establishing the thermodynamic relationship between the distribution coefficient of boron (LB) and the activities of SiO2 and CaO. The experimental results show that the distribution coefficient and the removal efficiency of boron are greatly improved with the increase of CaO proportion in the slag. The maximal value of LB reaches 1.57 with a slag composition of 60%CaO-40%SiO2 (mass fraction). The boron content in the refined silicon is reduced from 18×10-6 to 1.8×10-6 using slag refining at 1600 °C for 3 h with a CaO-SiO2/MG-Si ratio of 2.5, and the removal efficiency of boron reaches 90%.
Trans. Nonferrous Met. Soc. China 24(2014) 1231-1236
Ji-jun WU1,2,3, Yan-long LI1,3, Wen-hui MA1,2,3, Kui-xian WEI1,2,3, Bin YANG1,2, Yong-nian DAI1,2,3
1. State Key Laboratory of Complex Nonferrous Metal Resources Cleaning Utilization in Yunnan Province/National Engineering Laboratory for Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
2. Yunnan Provincial Key Laboratory of Nonferrous Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
3. Engineering Research Center for Silicon Metallurgy and Silicon Materials of Yunnan Provincial Universities, Kunming University of Science and Technology, Kunming 650093, China
Received 8 April 2013; accepted 26 August 2013
Abstract: Boron removal from metallurgical grade silicon (MG-Si) using a calcium silicate slag was studied. The results show that it is impossible basically to remove boron using a pure SiO2 refining. The oxidizing ability of CaO-SiO2 slag for boron removal was characterized by establishing the thermodynamic relationship between the distribution coefficient of boron (LB) and the activities of SiO2 and CaO. The experimental results show that the distribution coefficient and the removal efficiency of boron are greatly improved with the increase of CaO proportion in the slag. The maximal value of LB reaches 1.57 with a slag composition of 60%CaO-40%SiO2 (mass fraction). The boron content in the refined silicon is reduced from 18×10-6 to 1.8×10-6 using slag refining at 1600 °C for 3 h with a CaO-SiO2/MG-Si ratio of 2.5, and the removal efficiency of boron reaches 90%.
Key words: metallurgical grade silicon; boron removal; thermodynamics; distribution coefficient; slag refining
1 Introduction
Renewable energy is the only way to substitute fossil fuel [1,2]. In recent years, solar cell has been paid close attention for its potential advantages. Traditionally, the off-spec silicon feeds from semiconductor industry produced by the Simens process have been supplied to the photovoltaic industry [3,4]. The metallurgical route is an increasingly popular method in the range from purifying metallurgical grade silicon (MG-Si) to solar grade silicon (SoG-Si). However, boron removal from MG-Si is still a puzzle as a result of the low vapour pressure for boron [5] and the large segregation coefficient for boron to silicon [6]. Boron, which will accelerate the auger recombination and reduce the mobility ratio of minority carriers in silicon wafer [7,8] on account of the existence of the recombination centers such as Fe-B pairs, B-O metastable complexes and Cu-related extended defects [9], is a trouble maker to the photoelectric conversion.
As a rule, the boron content in SoG-Si is required to be lower than 0.5×10-6. To meet this requirement, a purification process for boron removal must be operated. These methods include gas blowing, thermal plasma and slag refining. Through blowing the reactive gases such as O2, H2 and H2O into MG-Si melt, the gaseous boric species BxOy can be generated and volatilized. It is similar to the thermal plasma refining, which usually has preferable boron removal efficiency [10,11]. Slag refining employed in the steelmaking industry is also an efficient way to remove boron from MG-Si and these slags include CaO-SiO2, CaO-SiO2-CaF2, CaO-SiO2- Al2O3, CaO-SiO2-MgO and CaO-SiO2-Na2O [12,13]. KHATTAK and SCHMID [14] studied the boron removal using a CaO-SiO2 slag refining and the boron content in MG-Si was reduced from 18×10-6 to 1.0×10-6. JOHNSTON and BARATI [15] and LUO et al [16] found that the slag baisicity interpreted as the concentration of free oxygen ions in slag had an important effect on boron removal.
In the present work, the boron removal from MG-Si using CaO-SiO2 slag was studied, and the role of CaO in slag was illustrated. The distribution of boron between slag and silicon (LB) was determined using different slag composition. Simultaneously, the oxidizing ability of CaO-SiO2 slag to impurity boron was calculated and characterized. The boron removal was finally carried out with different ratios of slag to silicon and refining time.
2 Experimental
The MG-Si with a boron content of 18×10-6 was used in this work. It was smashed and ground to powders with sizes of 50-200 μm. The MG-Si powder and the reagent grade chemicals of CaO and SiO2 were mixed and loaded in a graphite crucible. The crucible was put into a quartz tube of intermediate frequency inductive furnace. Argon with 99.9% purity was blown into quartz tube for protection, and the temperature was determined by an infrared thermometer. The schematic diagram of experimental apparatus is shown in Fig. 1.

Fig. 1 Schematic diagram of apparatus for molten slag refining
Boron removal using the pure SiO2 and CaO-SiO2 binary slag refining was carried out under different conditions of slag composition, mass ratios of slag to silicon and refining time, respectively. The boron contents in the refined slag and silicon samples were determined by inductively coupled plasma mass spectrometry (ICP-MS, Elan-5000A, USA).
3 Results and discussion
3.1 Boron removal using pure oxide SiO2
Boron removal from MG-Si using pure SiO2 refining was carried out with different refining temperatures and mass ratios of SiO2 versus MG-Si. TEIXEIRA and MORITA [17], JOHNSTON and BARATI [18] described that boron in silicon (expressed as [B]) could be oxidized into boric oxide by free oxygen ion (O2-) and oxygen (O2). Then, the generated boric oxide would enter a basic slag phase based on calcium oxide in the form of negative ion 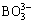 , and a calcium borate phase was finally generated via the ionic equation as follows:
, and a calcium borate phase was finally generated via the ionic equation as follows:
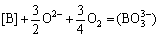 (1)
(1)
The studies focused on the free oxygen ion released by the basic oxides CaO and oxygen decomposed by SiO2. In thermodynamics, the activity of free oxygen ion and the partial pressure of oxygen were crucial for boron removal. However, the oxygen partial pressure would be restricted by the decomposition of SiO2.
In addition, TEIXEIRA et al [19] detailed that boron in silicon was directly oxidized into BO1.5 by SiO2 and it was expressed as:
 (2)
(2)
It is found that this reaction would only take place at a higher temperature than 1961.8 °C according to the standard Gibbs free energy of formation, and it could be thought that the oxidation of boron by the pure SiO2 was especially difficult. Experimentally, boron removal was done in the intermediate frequency furnace at the refining temperatures of 1500 and 1750 °C, respectively. The mass ratios of SiO2 to MG-Si were set as 1:10, 2:10, 3:10 and 4:10. Figure 2 shows the refined sample using pure SiO2 at 1500 °C.
It is seen from the longitudinal section of refined sample in Fig. 2(a) that SiO2 distributes on the top of refined silicon and takes on the state of granule, which implies that SiO2 is unmelted during the refining process. The cross section image of sample in Fig. 2(b) shows a same distribution for SiO2 as the longitudinal section. The boron removal using SiO2 with a refining time of 2 h and different mass ratios of SiO2 to MG-Si at 1500 and 1750 °C, respectively, are shown in Fig. 3.
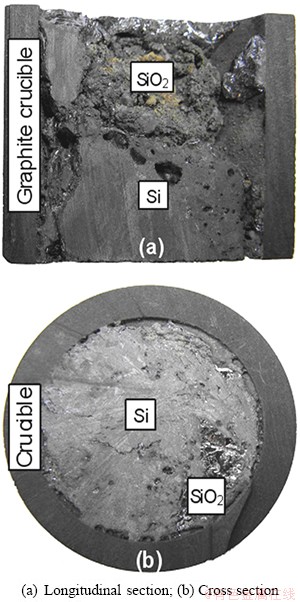
Fig. 2 Silicon sample refined by pure SiO2
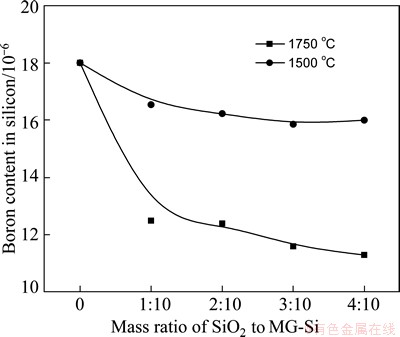
Fig. 3 Effects of mass ratio of SiO2 to MG-Si and refining temperature on boron removal
It is found that the boron content is reduced from 18×10-6 to 16×10-6 at 1500 °C and the removal efficiency of boron is only 11%. However, it is reduced to 11.3×10-6 at a refining temperature of 1750 °C and the removal efficiency reaches 37%. Though it is hardly effective for boron removal using pure SiO2 refining, it is more helpful for a higher refining temperature. The result is also in accordance with the thermodynamic calculation. It is thought that the liquid SiO2 may make the oxidation of boron in molt silicon more easily compared with the solid state one. As shown in the SiO2-B2O3 binary phase diagram [20], the acidic B2O3 generated in the interface of SiO2 and MG-Si can not be easily dissolved into a same acidic slag. Therefore, the diffusion of B2O3 towards slag phase is obstructed on account of the accumulation of B2O3 in the interface of slag and silicon.
3.2 Boron removal using CaO-SiO2 binary slag
The basic oxide CaO was added to SiO2, however, the oxidation of boron became possible. At the moment, Eq. (2) might be written as:
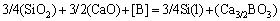 (3)
(3)
It is found that the theoretical initial temperature of Eq. (3) is greatly reduced compared with that of Eq. (2) according to the standard Gibbs free energy of formation. The molten silicon and slag are considered as a dilute solution. On condition that the standard state for the Henry’s law is chosen, the equilibrium constant (Kp) of Eq. (3) might be written as:
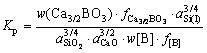 (4)
(4)
where w(Ca3/2BO3) and w[B] are the mass fraction of calcium borate in slag and the mass fraction of boron in silicon, respectively; a and f are the activity and the activity coefficient, respectively. For the Henry’s law, it is considered that 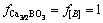 and aSi(l)=1.
and aSi(l)=1. 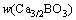 can be substituted with the concentration of boron in slag (w(B)), and it can be expressed as:
can be substituted with the concentration of boron in slag (w(B)), and it can be expressed as:
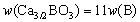 (5)
(5)
The distribution coefficient of boron between slag and silicon (LB) is expressed as:
 (6)
(6)
Lastly, the distribution coefficient of boron might be written as:
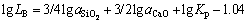 (7)
(7)
The activities of SiO2 and CaO in CaO-SiO2 binary system (0.4< <0.7) were determined by ZOU et al [21], as shown in Fig. 4.
<0.7) were determined by ZOU et al [21], as shown in Fig. 4.
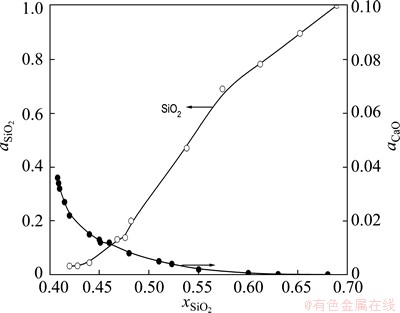
Fig. 4 Activities of CaO and SiO2 in CaO-SiO2 binary system at 1600 °C [21]
It is found that the activity of SiO2 increases with the rise of its proportion in CaO-SiO2 slag. It is completely opposite to CaO for this case. As shown in Fig. 5, the thermodynamic relationship displays that the value of item  in Eq. (7) decreases gradually when the mole fraction of SiO2 in slag is larger than 0.4.
in Eq. (7) decreases gradually when the mole fraction of SiO2 in slag is larger than 0.4.
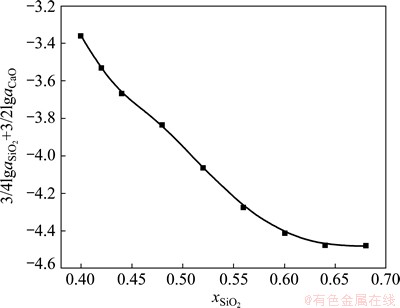
Fig. 5 Relationship between item 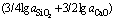 and mole fraction of SiO2 in slag
and mole fraction of SiO2 in slag
Figure 6 shows the refined silicon sample using CaO-SiO2 binary slag at 1600 °C. It is seen that the CaO-SiO2 slag is melted completely and separated out from silicon melt. After refining, the refining slags separated are distributed on the top of silicon (Fig. 6(a)) and between silicon and graphite crucible (Fig. 6(b)).
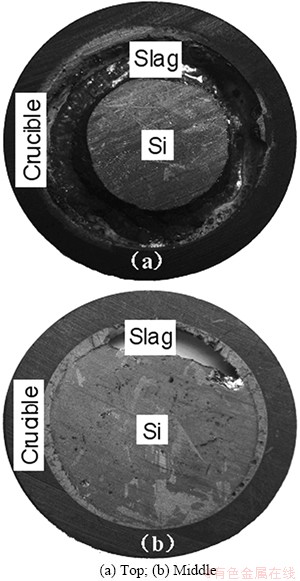
Fig. 6 Refined silicon sample in crucible using CaO-SiO2 slag
The SEM images of silicon sample before and after refining are shown in Fig. 7. It is found that the amounts of impurities in MG-Si are greatly reduced after refining using CaO-SiO2 slag. Unexpectedly, the content of calcium in silicon increased from 0.015% to 0.042% in the study by DIETL [22]. Obviously, the calcium derived from refining slag should not be ignored using CaO-SiO2 system. However, it can be removed using hydrometallurgical treatment or vacuum volatilization [23,24].
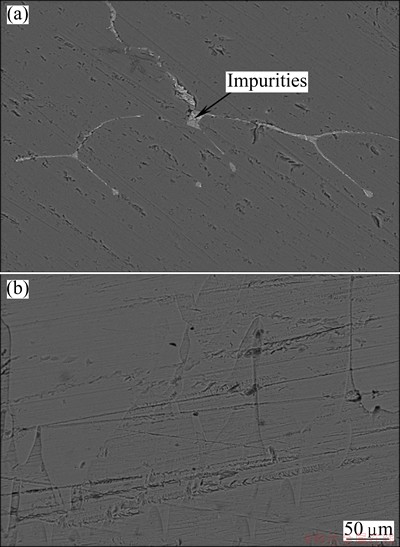
Fig. 7 SEM images of refined silicon using CaO-SiO2slag before (a) and after (b) refining
3.3 Effect of technological conditions on boron removal
In order to display the oxidizing ability of CaO-SiO2 slag for boron removal, the distribution of boron between CaO-SiO2 slag and silicon was studied with different slag compositions. The mass ratios of slag to silicon and the refining time are set as 1:1 and 3 h, respectively, at 1600 °C. The distribution coefficient of boron was calculated according to Eq. (7), as shown in Fig. 8.
It is found that the distribution coefficient of boron increases with the increase of CaO proportion in slag. It reaches 1.57 when the mass ratio of CaO/SiO2 is 1.5, the slag composition is 60%CaO-40%SiO2. Unexpectedly, the distribution coefficient of boron reduces when CaO/SiO2 composition is exceeded to 1.5, which is in accordance with the studies by SUZUKI et al [25]. It is presumed that the increase of CaO/SiO2 composition results in the decrease of SiO2 activity, which is crucial to the oxidizing ability of CaO-SiO2 slag. Therefore, the distribution coefficient of boron declines as well with the continuous increase of CaO in slag.
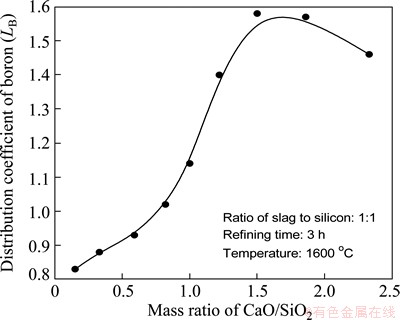
Fig. 8 Distribution coefficient of boron (LB) with different CaO/SiO2 compositions
Figure 9 shows the effects of slag/silicon mass ratio on boron removal. The slag composed of 60%CaO- 40%SiO2 is used to refine MG-Si. The refining time and temperature are set as 3 h and 1600 °C, respectively. It is found that the removal efficiency of boron increases with the increase of ratios for slag to silicon. The boron content in silicon is reduced from 18×10-6 to 1.8×10-6 and the removal efficiency reaches 90% when the mass ratio is 2.5. However, the trend is unobvious when the ratio is larger than 2.5. It is thought that the superfluous CaO-SiO2 slag is non-effective for boron removal in refining process. The optimal ratio of slag to silicon for boron removal is about 2.5 using a 60%CaO-40%SiO2 slag refining at 1600 °C for 3 h.
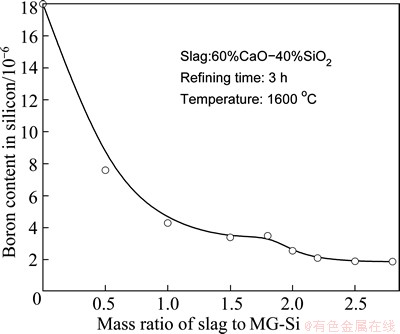
Fig. 9 Effects of mass ratio of CaO-SiO2 slag to MG-Si on boron removal
Figure 10 shows the effects of refining time on boron removal from MG-Si. The 60%CaO-40%SiO2 slag was used and the mass ratio of slag to silicon and the refining temperature were set as 2.5 and 1600 °C, respectively. It is found that the boron content in refined silicon decreases rapidly with the increase of refining time. It is reduced to 2.2×10-6 for 2.5 h and 1.8×10-6 for 3 h, respectively. However, the decreasing trend for boron removal becomes unobvious after longer refining time. It is thought that the driving force of boron diffusion towards slag phase decreases and the equilibrium for boron in slag and silicon is reached. Therefore, it is optimal for boron removal at 1600 °C for 3 h in the intermediate frequency inductive furnace using 60%CaO-40%SiO2 slag refining.
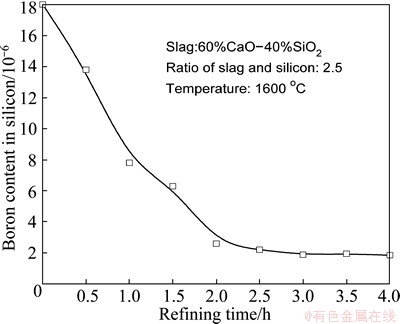
Fig. 10 Effects of refining time on boron removal
4 Conclusions
1) Boron removal from metallurgical grade silicon using a CaO-SiO2 slag refining was studied. It is non-effective basically to remove boron using a pure SiO2 refining. However, the removal efficiency of boron is greatly improved with the addition of basic CaO in slag, which is consistent with the thermodynamic calculation.
2) The oxidizing ability of CaO-SiO2 slag for boron removal was calculated and characterized. The distribution coefficient of boron between slag and silicon increases when the mole fraction of CaO in slag rises.
3) The mass fraction of boron in MG-Si can be reduced from 18×10-6 to 1.8×10-6 using 60%CaO- 40%SiO2 slag refining for 3 h when the mass ratio of slag to MG-Si is set as 2.5.
References
[1]  A, Ghosh M, Sonnenschein R, Woditsch P. Silicon for photovoltaic applications [J]. Materials Science and Engineering B, 2006, 134(2-3): 257-262.
A, Ghosh M, Sonnenschein R, Woditsch P. Silicon for photovoltaic applications [J]. Materials Science and Engineering B, 2006, 134(2-3): 257-262.
[2] Kaminski A, Vandelle B, Fave A, Boyeaux J P, Nam L Q, Monna R, Sarti D, Laugier A. Aluminium BSF in silicon solar cells [J]. Solar Energy Materials and Solar Cells, 2002, 72: 373-379.
[3] Aulich H A, Schulze F W. Crystalline silicon feedstock for solar cell [J]. Progress in Photovoltaics: Research and Applications, 2002, 10(2): 141-147.
[4] Nordstrand E F, Tangstad M. Removal of boron from silicon by moist hydrogen gas [J]. Metallurgical and Materials Transactions B, 2012, 43(4): 814-822.
[5] Wu J J, Ma W H, Yang B, DaI Y N, MORITA K. Boron removal from metallurgical grade silicon by oxidizing refining [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 463-467.
[6] Noriyoshi Y, Kazuhiro H, Yoshiei K. Removal of metal impurities in molten silicon by directional with electron beam heating [J]. Materials Transactions, 2004, 45(3): 850-857.
[7] Istratov A, Buonassisi T, McDonald R J, Smith A R, Schindler R, Kalejs J P, Weber E R. Metal content of multicrystalline silicon for solar cells and its impaction minority carrier diffusion length [J]. Journal of Applied Physics, 2003, 94: 6552-6559.
[8] Wolf S De, Szlufcik J, Delannoy Y, Périchaud I,  C, Einhaus R. Solar cells from upgraded metallurgical grade (UMG) and plasma-purified UMG multicrystalline silicon substrates [J]. Solar Energy Materials and Solar Cells, 2002, 72: 49-58.
C, Einhaus R. Solar cells from upgraded metallurgical grade (UMG) and plasma-purified UMG multicrystalline silicon substrates [J]. Solar Energy Materials and Solar Cells, 2002, 72: 49-58.
[9] Osinniy V, Bomholt P, Larsen A N, Enebakk E, Soiland A K, Tronstad R, Safir Y. Factors limiting minority carrier lifetime in solar grade silicon produced by the metallurgical route [J]. Solar Energy Materials and Solar Cells, 2011, 95: 564-572.
[10] Alemany C, Trassy C, Pateyron B. Refining of metallurgical-grade silicon by inductive plasma [J]. Solar Energy Materials and Solar Cells, 2002, 72(1-4): 41-48.
[11] Delannoy Y, Alemany C. Plasma-refining process to provide solar-grade silicon [J]. Solar Energy Materials and Solar Cells, 2002, 72(1-4): 69-75.
[12] Weis T, Schwerdfeger K. Chemical equilibria between silicon and slag melts [J]. Metallurgical and Materials Transactions B, 1994, 25(4): 497-504.
[13] Liaw H M, Secco F. Purification of metallurgical grade silicon by slagging and impurity redistribution [J]. Solar Cells, 1983, 10(2): 109-118.
[14] Khattak C P, Schmid F. Growth of the world's largest sapphire crystals [J]. Journal of Crystal Growth, 2001, 225: 572-579.
[15] Johnston M D, Barati M. Distribution of impurity elements in slag-silicon equilibria for oxidative refining of metallurgical silicon for solar cell applications [J]. Solar Energy Materials and Solar Cells, 2010, 94: 2085-2090.
[16] Luo D W, LIU N, LU Y P, ZHANG G L, LI T J. Removal of boron from metallurgical grade silicon by electromagnetic induction slag melting [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1178-1184.
[17] Teixeira L A V, Morita K. Removal of boron from molten silicon using CaO-SiO2 based slags [J]. ISIJ International, 2009, 49(6): 783-787.
[18] Johnston M D, Barati M. Effect of slag basicity and oxygen potential on the distribution of boron and phosphorus between slag and silicon [J]. Journal of Non-Crystalline Solids, 2011, 357: 970-975.
[19] Teixeira L A V, Tokuda Y, Yoko T, Morita K. Behavior and state of boron in CaO-SiO2 slags during refining of solar grade silicon [J]. ISIJ International, 2009, 49(6): 777-782.
[20] Nogami M, Moriya Y. Glass formation of the SiO2-B2O3 system by the gel process from metal alkoxides [J]. Journal of Non-Crystalline Solids, 1982, 48: 359-366.
[21] Zou Y X, Chou J C, Chao P. Activities in liquid CaO-SiO2 and CaO-Al2O3 slag [J]. Scientia Sinica, 1963, 12(8): 1249-1250.
[22] DIETL J. Silicon processing for photovoltaics II [R]. Proc 8th E C Photovoltaic Solar Energy Conf., Vol. 1. Dordrecht, the Netherlands: Kluwer Academic Publishers, 1988: 599-603.
[23] YU Z L, XIE K Q, ZHENG Y F, CHEN J H, MA W H, XIE G. Kinetics of aluminum removal from metallurgical grade silicon with pressure leaching [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2970-2976. (in Chinese)
[24] PENG X, DONG W, TAN Y, JIANG D C. Removal of aluminum frommetallurgical grade siliconusing electron beam melting [J]. Vacuum, 2011, 86(4): 471-475.
[25] Suzuki K, Sugiyama T, Takan K, Sano N. Thermodynamics for removal of boron from metallurgical silicon by flux treatment [J]. Journal of Japan Institute Metals, 1990, 54(2): 168-172.
伍继君1,2,3,李彦龙1,3,马文会1,2,3,魏奎先1,2,3,杨 斌1,2,戴永年1,2,3
1. 昆明理工大学 复杂有色金属资源清洁利用省部共建国家重点实验室/真空冶金国家工程实验室,昆明 650093;
2. 昆明理工大学 云南省有色金属真空冶金重点实验室,昆明 650093;
3. 昆明理工大学 云南省高校硅冶金与硅材料工程研究中心,昆明 650093
摘 要:利用CaO-SiO2熔渣去除冶金级硅(MG-Si)中的杂质硼。热力学分析和实验结果表明:纯SiO2基本上不能去除冶金级硅中的杂质硼。通过建立硼的分配系数与熔渣中SiO2和CaO活度之间的关系,从热力学上对CaO-SiO2熔渣的除硼能力进行表征。结果表明:随着渣中CaO配比的升高,硼的分配系数和去除效率大大提高。当熔渣组成为60%CaO-40%SiO2(质量分数)时,硼的分配系数达到最大值1.57。当渣硅比为2.5,精炼温度为1600 °C以及精炼时间为3 h时,利用60%CaO-40%SiO2熔渣可以将冶金级硅中的硼含量从18×10-6降低至1.8×10-6,去除效率达到90%。
关键词:冶金级硅;除硼;热力学;分配系数;熔渣精炼
(Edited by Chao WANG)
Foundation item: Projects (51104080, u1137601) supported by the National Natural Science Foundation of China; Project (14118557) supported by the Personnel Training Foundation of Kunming University of Science and Technology in China
Corresponding author: Ji-jun WU; E-mail: dragon_wu213@126.com
DOI: 10.1016/S1003-6326(14)63183-6

