J. Cent. South Univ. (2017) 24: 1929-1933
DOI: https://doi.org/10.1007/s11771-017-3600-z
Effect of sintering atmosphere on corrosion resistance of NiFe2O4 ceramic in Na3AlF6-Al2O3 melt
TIAN Zhong-liang(田忠良), YANG Kai(杨凯), LAI Yan-qing(赖延清), ZHANG Kai(张凯), LI Jie(李劼)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract: A comparative study on the corrosion resistance of NiFe2O4 ceramic inert anode for aluminum electrolysis prepared in the different sintering atmosphere was carried out in Na3AlF6-Al2O3 melt. The results show that the corrosion rates of NiFe2O4 ceramic inert anodes prepared in the vacuum and the atmosphere with oxygen content of 1×10-2 are 6.08 cm/a and 2.59 cm/a, respectively. A densification layer is formed at the surface of anode due to some reactions which produce aluminates. For the anode prepared in the atmosphere with oxygen content of 1×10-2, the thickness of the densification layer (about 50 μm) is thicker than that (about 20 μm) formed at the surface of anode prepared in the vacuum. The content of NiO and Fe(II) in Ni(II)xFe(II)1-xFe(III)2O4 increases with the decrease of the oxygen content of sintering atmosphere, which reduces the corrosion resistance of the material.
Key words: sintering atmosphere; corrosion; NiFe2O4 ceramic; inert anode; aluminum electrolysis
1 Introduction
The past years have seen renewed interest in the potential use of inert anodes in aluminum electrolysis cells [1]. Because replacing consumable carbon anodes with inert anodes would drastically reduce the emission of greenhouse gases, perfluorocarbon(PFC) byproducts and other polluting emissions such as PAHs associated with the production of primary aluminum and the manufacture of carbon anodes [1, 2]. In addition, there would also be a positive impact on sulphur emissions. So, the development of inert anode is a long standing dream of researchers. Indeed, HALL [3] and SADOWAY [4] lamented that, in the absence of an inert anode, aluminum would have difficulty in competing with steel as a structural metal. The Aluminum Association of America once regarded it as one of the highest research priorities for the primary Al producers [5].
Though the search for this material has taken more than 100 years, no acceptable inert anode material has yet been found for long-term use in industrial aluminum cells [1, 6]. In recent years, the research focused on the development of NiFe2O4-based cermets and Ni/Fe-based alloys [1, 6, 7]. Among them, NiFe2O4-based cermets, which possess not only good electrical conductivity of metal but also good corrosion resistance of ceramic to the molten cryolite, were regarded as one of the promising materials for inert anode of aluminum electrolysis. A considerable work about NiFe2O4-based cermet material was conducted. A 6 kA pilot cell and a 20 kA pilot cell were also carried out in America and China, respectively [6]. And the results show that both the mechanical properties and the corrosion resistance of NiFe2O4-based cermet could not meet requirements of the thermal shock and corrosion rate.
To improve the mechanical properties of material, modifying agent or sintering assistant was used during the preparation process, and some progresses were made [8, 9]. The effects of sintering atmosphere on the mechanical properties of NiFe2O4 ceramic were also studied in our previous studies, and the results showed that the alteration of oxygen content in the sintering atmosphere could affect its microstructure and mechanical properties [10]. However, the main challenge for the material as inert anode is still on its anti-corrosion performance under the condition of electrolysis. Because it is not only related to the life of inert anode, but also affects the quality of metal Al.
In this work, NiFe2O4 ceramic inert anodes were prepared in the different sintering atmosphere by cold pressing-sintering process. The influence of oxygen content in the sintering atmosphere on its corrosion resistance to Na3AlF6-Al2O3 melt was studied. And the phase composition and microstructure were also discussed. The purpose is to explore how much impact the sintering atmosphere has on the corrosion resistance of NiFe2O4 ceramic inert anode for aluminum electrolysis.
2 Experimental
2.1 Fabrication of anodes
The samples of NiFe2O4 ceramics were prepared based on the previous work [10]. The raw materials, NiO and Fe2O3 were all of reagent grade. Firstly, a proper amount of Fe2O3 and NiO was mixed by using a ball mill and then were calcined in a muffle furnace at 1200 °C for 6 h to obtain NiFe2O4 ceramic powder. And then, the obtained NiFe2O4 ceramic powder was ground again and statically pressed with a pressure of 2×108 Pa. Finally, these green specimens were sintered at 1350 °C for 4 h in the vacuum or an atmosphere with the oxygen content of 1×10-2 respectively to obtain the desired NiFe2O4 ceramic inert anode samples.
2.2 Chemicals
The bath was prepared by mixing Na3AlF6 with AlF3, CaF2 and Al2O3. AlF3 was prepared by subliming in the laboratory and its purity was above 99.0%. The others were analytically grade and were obtained from Sinopharm Chemical Reagent Co., Ltd. All reagents were vacuum dried at 150 °C for at least 48 h before experiment. The electrolyte composition was similar to that used in industrial aluminum electrolysis cells. The molar ratio of n[NaF]/n[AlF3] was 2.3, the concentration of Al2O3 was 2.5% and the concentration of CaF2 was 5.0%. The initial mass of electrolyte was 800 g.
2.3 Electrolytic cell and electrolysis procedure
The laboratory electrolysis cell system is presented in Fig. 1. A high purity graphite crucible with a bottom open alumina lining, which was adopted to prevent the current flowing through the cell side, was used as simulated electrolysis cell. To obtain a steady cathode surface during the test, 90 g of metal aluminum was placed in the hole which was drilled at the bottom of carbon crucible. The graphite crucible with about 800 g electrolyte and the NiFe2O4 ceramic inert anode were placed in a vertical tube furnace to obtain the extra heat to reach the desired temperature.
Metal aluminum was added prior to electrolysis. The cell with NiFe2O4 ceramic inert anode was heated to the required temperature 960 °C in the vertical furnace under argon atmosphere, and kept for 2 h before immersing the anode and electrifying 20 min afterward. The temperature of the furnace was controlled to ±1 °C by TCE-II programmable temperature control unit. The temperature of Na3AlF6-AlF3-CaF2-Al2O3 melt was maintained at 960 °C and measured with Pt/Pt-10%Rh thermocouple once an hour and maintained over a range of ±3 °C during testing.
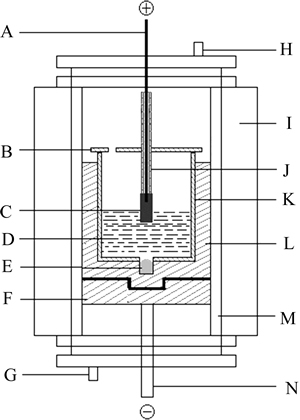
Fig. 1 Schematic of experimental cell (A-Stainless steel anode rod; B-Alumina cover; C-NiFe2O4 based cermet inert anode; D-Electrolyte; E-Metal aluminum cathode; F-Graphite mechanical support; G-Gas inlet; H-Gas outlet; I-Vertical tube furnace; J-Alumina tube; K-Alumina lining; L-Graphite crucible; M-Furnace tube; N-Stainless steel cathode rod)
The anode-cathode distance was kept about 4.0 cm and the anode-bath contacting area was controlled by immersing the anode into the electrolyte about 1.0 cm. The current density based on the bottom area of the inert anode was 1.0 A/cm2. And the current and the cell voltage were supplied and monitored by a Multi-Purpose Potentiostat/Galvanostat (model 273A/10, Perkin-Elmer Instruments). To keep its concentration constant during the test, Al2O3 was added at 15 min intervals based on the current efficiency 80%.
At the end of the test, the inert anode was raised out of the electrolyte to stop the electrolysis while maintaining polarization so as to prevent the corrosion of the anode material by metal aluminum dissolved in the bath. The cell was left to cool with the anode resting above the electrolyte. The metal aluminum recovered at the cathode was analyzed by X-rays fluorescence spectrum for getting the content of Ni and Fe. The surface of the NiFe2O4 ceramic inert anode was inspected by X-ray diffraction(XRD). The cross-section of the anode sample was examined by XRMA (JSM-5600LV) using a quantitative energy dispersive spectrometer (EDS) connected to the SEM.
3 Results and discussion
3.1 Material performance
The results from our previous studies indicated that it took approximately 4 to 6 h for stoichiometric NiFe2O4 in Na3AlF6-AlF3-Al2O3 melt to reach a steady state concentration, which was taken to be the solubility [11]. And the study carried out by OLSEN et al [12, 13] also confirmed this. Therefore, in present work, all electrolysis experiments lasted 10 h.
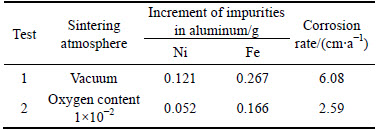
After testing, the contents of Ni and Fe in the metal aluminum recovered at the cathode were analyzed by XRF. The increments of impurities Ni and Fe are listed in Table 1. As can be seen, for the test 1, the increment of impurity Ni in aluminum is 0.121 g. While for test 2, the value is only 0.052 g. It can be calculated that the increment of Ni is decreased significantly (by 57.0%) due to the change of sintering atmosphere during preparing. The same is true for the impurity Fe, although its increment is decreased only 37.8%. This indicates that the alteration of oxygen content in the sintering atmosphere is helpful to improve the quality of the metal aluminum.
Table 1 Increment of impurities Ni and Fe and corrosion rate of anode
The study carried out by OLSEN et al [12] shows that the corrosion rate of anode is nearly the same as the deposition rate of impurity into the aluminum cathode after the corrosion reaches a steady-state. Therefore, the corrosion rate of inert anode can be calculated according to the deposition rate of some kind of impurity at the cathode. As for NiFe2O4 ceramic inert anode, its corrosion rate can be calculated by the change of Ni content in the metal aluminum recovered at the cathode based on the following equation [14]
 (1)
(1)
where Vc(cm/a) is the corrosion rate of anode, ΔCNi(Al)(g) is the increment of Ni in the metal aluminum at the cathode, CNi(cer)(%) is content of Ni in the anode, ρ(g/cm3) is the density of anode, S(cm2) is the surface of the anode immersed in the bath, τ(h) is the time of electrolysis.
The corrosion rate based on Eq. (1) is calculated to be 6.08 cm/a for NiFe2O4 ceramic inert anode prepared in the vacuum. However, for the inert anode prepared in the atmosphere with the oxygen content of 1×10-2, its corrosion rate is 2.59 cm/a. This indicates that the corrosion resistance of NiFe2O4 ceramic to Na3AlF6-AlF3-Al2O3 melt can be improved by changing the oxygen content of sintering atmosphere.
3.2 Microstructure analysis
The XRD analysis results of NiFe2O4 ceramic prepared in the different atmospheres are shown in Fig. 2. Though there is no other impurity in NiFe2O4 ceramic powder obtained by calcining the mixture of Fe2O3 and NiO with the molar ratio of 1:1 during preparing, it is found that both the anodes obtained in the vacuum and in the atmosphere with oxygen content of 1×10-2 contain the phase NiO besides the phase NiFe2O4. Furthermore, the characteristic peak of NiFe2O4 is more obvious for the anode prepared in the atmosphere with oxygen content of 1×10-2 than in the vacuum.
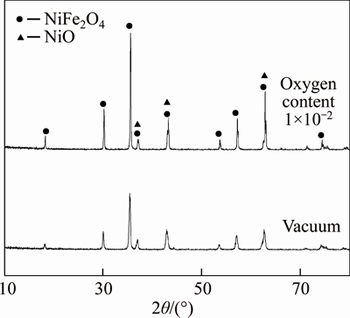
Fig. 2 X-ray diffraction patterns of NiFe2O4
The reasons of formation new phase NiO in the ceramics may be explained by the following reaction:
NiFe2O4→Ni(II)xFe(II)1-xFe(III)2O4+NiO+O2 (2)
This means that the ceramic NiFe2O4 will be decomposed into Ni(II)xFe(II)1-xFe(III)2O4 and NiO if the oxygen content in the atmosphere decreases. Therefore, the new phase NiO appears. When the oxygen content in the sintering atmosphere decreases, Fe(III) in NiFe2O4 is reduced to Fe(II) and the content of Fe(II) increases. Of course, Fe(II) can not be detected in NiFe2O4 ceramic because the stoichiometric compound NiFe2O4 and the nonstoichiometric compound Ni(II)xFe(II)1-xFe(III)2O4 can not be distinguished by XRD. In Na3AlF6-AlF3- Al2O3 melt, the low valence state Fe(Ⅱ) in NiFe2O4 may dissolve more easily than Fe(III) [15]. Therefore, the corrosion resistance of NiFe2O4 ceramic prepared in the vacuum decreases.
At the same time, the reaction (2) also indicates that the content of NiO decreases and the content of NiFe2O4 increases if the anode is sintered in the high oxygen content. The results of Ref. [16] show that the corrosion resistance of NiFe2O4 is better than that of NiO. So, if the ceramic inert anode is prepared in the oxygen content of 1×10-2, its corrosion resistance is better than that prepared in the vacuum.
To get more information, SEM pictures of cross-sections of the anode surfaces after electrolysis are obtained and shown in Fig. 3. There are some visible structural changes for the anodes sintered in the different atmosphere when going from the unchanged interior of the electrode to the surface. A distinct densification layer is formed at the surface of anode. The results from EDS analysis of the densification layer show that there is the elements Al, which is not added to the anode during the preparation process, existed after electrolysis besides the elements Ni, Fe, O. This indicates that there are new phases generated during electrolysis process. This phenomenon may be attributed to the effect of electrolyte composition during electrolysis process [13, 17].

Fig. 3 SEM pictures of anodes after electrolysis:(Note: The numbered markers indicate points at which quantitative analysis was performed (mass fraction, %))
The stoichiometric formula of NiFe2O4 phase in the as-sintered sample are Ni(II)yFe(II)1-yFe(III)2O4. Under electrolysis conditions, the oxide will decompose and generate FeO [17], and then the reaction (3) takes place and the aluminate FeAl2O4 may appear.
FeO+Al2O3=FeAl2O4 (3)
In addition, NiO can also react with Al2O3 which exists in the bath and generate the aluminate NiAl2O4.
NiO+Al2O3=NiAl2O4 (4)
These aluminates such as FeAl2O4 and NiAl2O4 with a spinel structure will have better chemical stability and thermal stability than that of the original substances of NiO. At the same time, the solubility of these aluminates in Na3AlF6-AlF3-Al2O3 melt is low and their anti-corrosion is stronger than that of the anode body [18]. Therefore, it can be inferred that such substances generated are beneficial on improving the corrosion resistance of the anode. Of course, further studies should be carried out to reveal the reason that the densification layer forms.
It is very obvious for the anodes prepared in the different atmosphere that the thickness of the densification layer at the surface is different. The densification layer of about 50 μm thickness is formed for the anode prepared in the atmosphere with the oxygen content 1×10-2 after electrolysis 10 h. However, the thickness is no more than 20 μm for the anode prepared in the vacuum. Since the surface layer of this anode is denser than the anode material itself which contained some pores due to its poor sintering characteristics, it is helpful for NiFe2O4 ceramic inert anode to improve its corrosion resistance against Na3AlF6-AlF3-Al2O3 melt. Therefore, the corrosion rate of the anode prepared in the atmosphere with the oxygen content of 1×10-2 is lower than that prepared in vacuum.
4 Conclusions
1) The corrosion rate of NiFe2O4 ceramic during electrolysis can be reduced by improving properly the oxygen content of sintering atmosphere. Based on the change of Ni in the metal aluminum recovered at the cathode, the corrosion rate of anode prepared in the atmosphere with the oxygen content of 1×10-2 is 2.59 cm/a. However, for the anode prepared in vacuum, its corrosion rate is 6.08 cm/a.
2) The decrease of the oxygen content in the sintering atmosphere will cause the increase of the content of Fe(II) in Ni(II)xFe(II)1-xFe(III)2O4, which may dissolve more easily than that of Fe(III). Meanwhile, the content of NiO also increases. It is adverse to improve the corrosion resistance of the anode during electrolysis.
3) A distinct densification layer is formed at the surface. The thicknesses of the densification layer for the anodes prepared in the different atmosphere are about 50 μm and 20 μm. Further studies should be conducted to reveal the reason that the distinct layer forms.
References
[1] PAWLEK R P. Inert anodes: An update [C] //GRANDFIELD J. Light Metals. Hoboken, NJ, USA: TMS, 2014: 1309-1313.
[2] KVANDE H, HAUPIN W. Inert anodes for Al smelters: Energy balances and environmental impact [J]. JOM, 2001, 53: 29-33.
[3] HALL C M. Process of reducing aluminum from its fluoride salts by electrolysis: USA, 0400664 [P]. 1889.
[4] SADOWAY D R. Inert anodes for the Hall-Héroult cell: The ultimate materials challenge [J]. JOM, 2001, 53: 34-35.
[5] GOUPIL G, HELLE S, DAVIS B, GUAY D,  L. Anodic behavior of mechanically alloyed Cu-Ni-Fe and Cu-Ni-Fe-O electrodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte [J]. Electrochimica Acta, 2013, 112: 176-182.
L. Anodic behavior of mechanically alloyed Cu-Ni-Fe and Cu-Ni-Fe-O electrodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte [J]. Electrochimica Acta, 2013, 112: 176-182.
[6] TIAN Zhong-liang, LAI Yan-qing, LI Zhi-you, CHAI Deng-peng, LI Jie, LIU Ye-xiang. Further development on NiFe2O4-based cermet inert anodes for aluminum electrolysis [J]. JOM, 2014, 66(11): 2229-2234.
[7] KHRAMOV A P, KOVROV V A, YU. ZAIKOV P, CHUMAREV V M. Anodic behaviour of the Cu82Al8Ni5Fe5 alloy in low-temperature aluminium electrolysis [J]. Corrosion Sci, 2013, 70: 194-202.
[8] LI J, WANG Z G, LAI Y Q, WU Y Y, YE S L. Effect of structural parameters on the thermal stress of a NiFe2O4-based cermet inert anode in aluminum electrolysis [J]. ActaMetallSin(Engl.Lett.) 2007, 20(2): 139-147.
[9] DU Jin-jing, LIU Yi-han, YAO Guang-chun, LONG Xiu-li, ZHANG Xiao. Effect of MnO2 addition on early-stage sintering behavior and properties of NiFe2O4 ceramics [C]// SUAREZ C E. Light Metals. Washington, PA, USA: TMS, 2012: 1385-1388.
[10] TIAN Zhong-liang, ZHANG Teng, LIU Kai, LAI Yin-qing, LI Jie. Effect of sintering atmosphere on composition and properties of NiFe2O4 ceramic [J]. Journal of Central South University of Technology, 2015, 22: 450-454. (in Chinese)
[11] LAI Yan-qing, TIAN Zhong-liang, QIN Qing-wei, ZHANG Gang, LI Jie. Solubility of composite oxide ceramics in Na3AlF6-Al2O3 melts [J]. Journal of Central South University of Technology, 2003, 34(3): 245-248. (in Chinese)
[12] OLSEN E, THONSTAD J. Nickel ferrite as inert anodes in aluminium electrolysis: Part I material fabrication and preliminary testing [J]. Journal of Applied Electrochemistry, 1999, 29: 293-299.
[13] OLSEN E, THONSTAD J. Nickel ferrite as inert anodes in aluminium electrolysis: Part II material performance and long-term testing [J]. Journal of Applied Electrochemistry, 1999, 29: 301-311.
[14] BLINOV V, POLYAKOVP, THONSTADJ, IVANOVV, PANKOV E. Behaviour of inert anodes for aluminium electrolysis in a low temperature electrolyte, Part I [J]. Aluminium,1997, 73(12): 906-910.
[15] HE Han-bing, WANG Yuan, LONG Jia-ju, CHEN Zhao-hui. Corrosion of NiFe2O4-10NiO-based cermet inert anodes for aluminium electrolysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3816-3821.
[16] LI Jie, DUAN Hua-nan, LAI Yan-qing, TIAN Zhong-liang, LIU Ye-xiang. Effect of NiO content on corrosion behaviour of Ni-xNiO-NiFe2O4 cermets in Na3AlF6-Al2O3 melts [J]. Transactions of the Nonferrous Metals Society of China, 2004, 14(6): 1180-1186.
[17] TIAN Zhong-liang, LAI Yan-qing, YANG Shu, LI Jie, HWANG J Y, LIU Ye-xiang. Anodic corrosion behavior of NiFe2O4-Based cermet in Na3AlF6-K3AlF6-AlF3 for aluminum electrolysis [J]. Metall Mater Trans. B, 2015, 46: 1257-1261.
[18] ZHANG Yun-shu, WU Xiao-xia, RAPP R A. Modeling of the solubility of NiO/NiAl2O4 and FeO/FeAl2O4 in cryolite melts [C]// PAUL N. CREPEAU. Light Metals. San Diego, California, USA: TMS, 2003: 415-421.
(Edited by HE Yun-bin)
Cite this article as: TIAN Zhong-liang, YANG Kai, LAI Yan-qing, ZHANG Kai, LI Jie. Effect of sintering atmosphere on corrosion resistance of NiFe2O4 ceramic in Na3AlF6-Al2O3 melt [J]. Journal of Central South University, 2017, 24(9): 1929–1933. DOI: https://doi.org/10.1007/s11771-017-3600-z.
Foundation item: Projects(51474238, 51334002) supported by the National Natural Science Foundation of China
Received date: 2016-03-22; Accepted date: 2016-05-16
Corresponding author: LAI Yan-qing, Professor; Tel: +86-13875851590; E-mail: cuslyqmelt@126.com