
Effects of holding temperature and time on semi-solid isothermal heat-treated microstructure of ZA84 magnesium alloy
YANG Ming-bo(杨明波)1, 2, PAN Fu-sheng(潘复生)2, CHENG Ren-ju(程仁菊)2, SHEN Jia(沈 佳)1
1. College of Materials Science and Engineering, Chongqing Institute of Technology, Chongqing 400050, China;
2. College of Materials Science and Engineering, Chongqing University, Chongqing 400030, China
Received 3 September 2007; accepted 24 December 2007
Abstract: The feasibility of fabricating ZA84 magnesium alloy with non-dendritic microstructure by a semi-solid isothermal heat treatment process and the effects of holding temperature and time on the semi-solid isothermal heat-treated microstructure of the alloy were investigated. The results indicate that it is possible to produce ZA84 alloy with non-dendritic microstructure by suitable semi-solid isothermal heat treatment. After being treated at 560-575 ℃ for 120 min, ZA84 magnesium alloy can obtain a non-dendritic microstructure with 14.2%-25.6% liquid fraction and an average size of 56-65 μm of the unmelted primary solid particles. With the increasing holding time from 30 to 120 min or holding temperature from 560 to 575 ℃, the average size of unmelted primary solid particles decreases and globular tendency becomes more obvious. Under the experimental condition, the microstructural evolution of ZA84 alloy during semi-solid isothermal treatment is mainly composed of three stages of initial coarsening, structural separation and spheroidization. The subsequent coarsening of spheroidal grains is not observed.
Key words: ZA84 magnesium alloy; semi-solid state; isothermal heat treatment
1 Introduction
During the past two decades, magnesium alloys have been applied widely, especially in the automotive industry due to the requirement to reduce the mass of car components, partially as a result of the introduction of limiting emission legislation[1]. At present, high pressure die cast(HPDC) is the dominant method in manufacturing commercial parts of magnesium alloys. However, die-cast Mg components suffer low engineering performance due to the existence of inherent defects, such as porosity, hot cracks and oxide inclusions introduced by the high pressure die-casting(HPDC) process. This has limited the application of magnesium alloys to the functional components like casings. Further extension of application of magnesium alloys in the more demanding components requires major development in processing technologies.
It is well known that the semi-solid metal(SSM) processing of magnesium alloys, which has low processing temperature, can resolve the problem of oxidation and burning effectively. Now it becomes an important forming process for the fabrication of parts with better mechanical properties compared with parts made by conventional casting. Therefore, SSM processing is thought to have a wide progress space in the mechanical properties and casting forming for magnesium alloys. But up to now, magnesium alloys currently used in SSM processing are restricted to a few commercial alloys such as AZ91 and AM50[2], and the research about the Mg-Zn-Al based magnesium alloys SSM is very scarce. Actually, Mg-Zn-Al based alloys, such as ZA84 and ZA104 alloys, which are thought as a potential elevated magnesium alloys with high creep resistance and good castability[3-4], have broader solidification temperature range than the Mg-Al-Zn and Mg-Al-Mn based alloys. Therefore, the investigation of Mg-Zn-Al based magnesium alloys SSM is of great theoretical and practical significance. In general, the semi-solid metal(SSM) processing is mainly composed of three main processes, such as production of semi-solid material with non-dendritic microstructure, partial remelting and thixoforming. Among these, the production of semi-solid material with non-dendritic microstructure is the most important one. At present, the methods of obtaining semi-solid material with non-dendritic microstructure include mechanical stirring, electro- magnetic stirring, strain-induced melt activation, spray deposition, liquidus cast and semi-solid isothermal heat treatment[5-6]. Among these methods, the semi-solid isothermal heat treatment is a new way found in the middle 1990s, which omits the special procedure to fabricate the semi-solid billet but fulfils the semi-solid non-dendritic microstructure during heating prior to thixoforming. Due to the above-mentioned reasons, in the present work, the feasibility of fabrication of semi- solid ZA84 magnesium alloy by an isothermal heat treatment and the effects of holding temperature and time on the microstructure and grain size of the alloy were investigated.
2 Experimental
ZA84(Mg-8Zn-4Al-0.25Mn) alloy was prepared by adding the following materials: commercial AM60 alloy, pure Mg, Al, and Zn (>99.9%, mass fraction). The experimental alloy was melted in a crucible resistance furnace and protected by a flux addition. After being treated by C2Cl6, the melt was held at 740 ℃ for 20 min and then poured into a permanent mould with a cavity of size of d8 mm×120 mm. The actual composition of experimental alloy was given as follows (mass fraction, %): 87.94 Mg, 7.9 Zn, 3.91 Al and 0.25 Mn.
The differential scanning calorimetry (DSC) experiment was carried out by using a NETZSCH STA 449C system in order to obtain the semi-solid isothermal temperature. Sample of around 30 mg was heated in a flowing argon atmosphere from room temperature to 700 ℃ for 5 min before cooling down to 100 ℃. The heating curve was recorded at a controlling speed of 15 ℃/min. Fig.1 shows the DSC heating curve of the experimental alloy. According to Fig.1, the semi-solid isothermal temperatures, 560 ℃, 565 ℃, 570 ℃ and 575 ℃, were selected. Samples with the dimensions of 15 mm in length and 8 mm in diameter were cut from the initial as-cast alloy and put into a box-like chamber of resistance furnace at selected temperatures, held for 30 min, 60 min, 90 min and 120 min, respectively, and then taken out for water quenching quickly.

Fig.1 DSC heating curves of as-cast ZA84 alloy
Both the as-cast and/or semi-solid samples were etched in a 8% nitric acid solution in distilled water, and then were examined by Olympus optical microscope with an image analysis system and JOEL JSM-6460LV type scanning electron microscope(SEM) equipped with Oxford energy dispersive X-ray spectrometer(EDS). The phases in the experimental alloy were also analyzed by D/Max-1200X type analyzer operated at 40 kV and 30 mA.
3 Results and discussion
3.1 As-cast microstructure
Fig.2 shows the XRD pattern of the as-cast alloy. In general, in ZA84 alloy, two main ternary phases are reported[7]. One is identified as Mg32(Al,Zn)49 phase which has the cubic crystal structure (space group 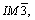 a=1.416 nm), and the other is Mg5Zn2Al2 phase having primitive orthorhombic structure (space group Pbcm, a=0.897 9 nm, b=1.698 8 nm and c=1.934 0 nm)[7]. As shown in Fig.2, the experimental alloy is also composed of α-Mg, Mg32(Al,Zn)49 and Al2Mg5Zn2 phases, similar to the ZA84 alloy reported by WANG et al[8].
a=1.416 nm), and the other is Mg5Zn2Al2 phase having primitive orthorhombic structure (space group Pbcm, a=0.897 9 nm, b=1.698 8 nm and c=1.934 0 nm)[7]. As shown in Fig.2, the experimental alloy is also composed of α-Mg, Mg32(Al,Zn)49 and Al2Mg5Zn2 phases, similar to the ZA84 alloy reported by WANG et al[8].
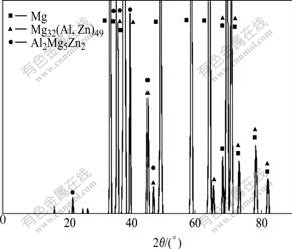
Fig.2 XRD pattern of as-cast ZA84 alloy
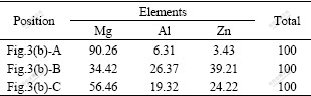
Fig.3 shows respectively the optical and SEM images of the as-cast experimental alloy. As shown in Fig.3(a), the experimental alloy exhibits typical dendritic microstructure. Furthermore, it is found from Fig.3(b) that two types of precipitates having different morphologies are present in the microstructure of the alloy. One precipitate has continuous and quasi-continuous network shape, and the other has an isolated shape. Obviously, the dark phase present randomly in the microstructures is α-Mg matrix. According to the EDS result (Table 1), the continuous and quasi-continuous phase has the composition equivalent to Mg32(Al, Zn)49 and the isolated phase has the stoichiometry composition as Mg5Al2Zn2. These results agree well with the XRD result.
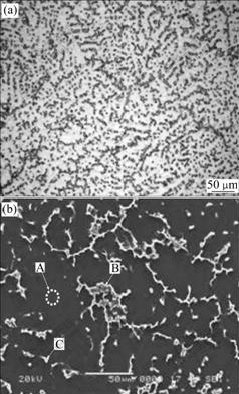
Fig.3 Optical and SEM images of as-cast ZA84 alloy: (a) Optical image; (b) SEM image
Table 1 EDS results of as-cast ZA84 alloy (molar fraction, %)
3.2 Semi-solid microstructures
Figs.4 and 5 respectively show the semi-solid microstructures of the experimental alloy after being treated at 570 ℃ for different holding time and at different holding temperatures for 120 min. It is found from Fig.4 that when the holding time is 30 min, the liquid in the semi-solid ZA84 alloy is dispersed discontinuously, and the “entrapped liquid” pool is also present inside the primary solids. With the holding time increasing from 60 to 120 min, the amount of liquid phase inside the grains decreases and that between the grains increases, and the liquid is dispersed continuously along grain boundaries. Finally, the initial as-cast alloy ultimately evolves into the semi-solid non-dendritic microstructure with unmelted primary solid particles. By combining Figs.4 and 5, it is found that with the increase of holding time or temperature, the unmelted primary solid particles in the semi-solid ZA84 alloy become relatively finer and more regular. Using the image analysis system, the number of unmelted primary solid particles in a selected area, and the perimeter and area of selected particles can be measured. Normally, the average size and the shape of a particle is respectively characterized by the equivalent diameter d0 and the shape factor F0 defined as[9]
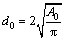 ,
, 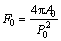 (1)
(1)
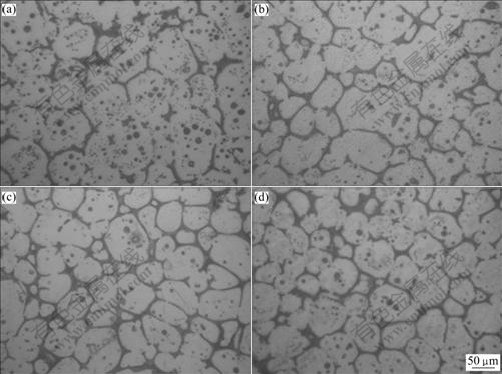
Fig.4 Microstructures of ZA84 alloy treated at 570 ℃for different holding time: (a) 30 min; (b) 60 min; (c) 90 min; (d) 120 min

Fig.5 Microstructures of ZA84 alloy treated at different holding temperatures for 120 min: (a) 560 ℃; (b) 565 ℃; (c) 570 ℃; (d) 575 ℃
where A0 and P0 represent the area and perimeter of the unmelted primary solid particle, respectively. The effects of holding time and temperature on the equivalent diameter and the shape factor of unmelted primary solid particles are shown in Fig.6. It is found from Fig.6 that with the increase of holding time from 30 to 120 min or holding temperature from 560 to 575 ℃, the equivalent diameter decreases and the shape factor increases, respectively, indicating that the average size of unmelted primary solid particles decreases and globular tendency becomes more obvious. Then it is inferred that it is possible to produce ZA84 alloy with non-dendritic microstructure by suitable semi-solid isothermal heat treatment.
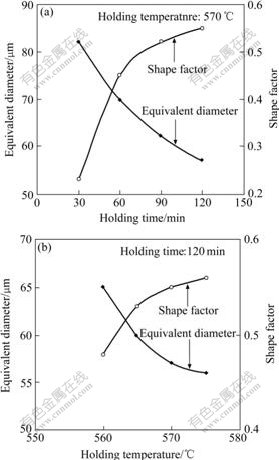
Fig.6 Effects of holding time and temperature on equivalent diameter and shape factor of unmelted primary solid particles in semi-solid ZA84 alloy: (a) Holding time; (b) Holding temperature
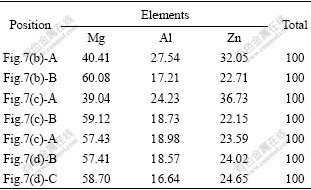
Fig.7 and Table 2 respectively show the SEM images and the EDS results of the experimental alloy treated at 575 ℃ for 120 min. In Fig.7, the morphology of precipitates in the secondary solidification microstructure is clearly exhibited. The precipitates both inside the grains and between the grains are found to respectively exhibit granule and sawtooth shapes, which are consistent with the results of the Mg-Al based alloys[10-12]. According to the EDS results, the white precipitates (“B” in Figs.7(b) and 7(c), and “A”, “B” and “C” in Fig.7(d)) both inside grains and between grains have similar contents of elements, which could correspond to the Al2Mg5Zn2 phase. The grey precipitates (“A” in Figs.7(b) and 7(c)) have similar contents of elements, which could correspond to the Mg32(Al,Zn)49 phase. This phenomenon is possibly related to the following reactions during the secondary solidification process[13]: L1(liquid)→α+Mg5Zn2Al2+L2 (liquid), L2+Mg5Zn2Al2→α+Mg32(Al,Zn)49+L3(liquid), and L3→α+Mg32(Al,Zn)49. However, the exact reason is not clear. It is a subject for further study.
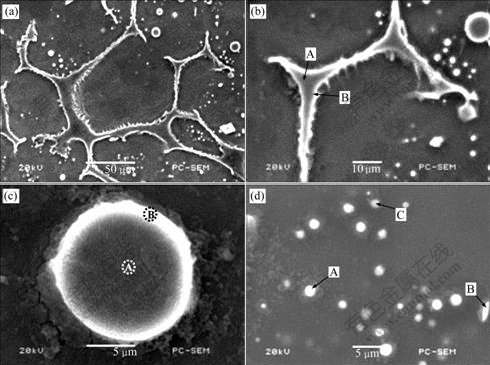
Fig.7 SEM images of ZA84 alloy treated at 575 ℃ for 120 min: (a) Low magnification; (b), (c) and (d) Local magnification in Fig.7(a)
Table 2 EDS results of ZA84 alloy treated at 575 ℃ for 120 min (molar fraction, %)
3.3 Discussion
The previous investigation showed that the non-dendritic microstructure evolution of as-cast alloys generally experienced four stages during semi-solid isothermal heat treatment, that is, the initial coarsening, separation, spheroidization and final coarsening [5,14-15], as illustrated in Fig.8. However, under the experimental condition of this work, the semi-solid microstructure evolution of ZA84 alloy is mainly composed of three stages of initial coarsening, separation and spheroidization, as shown in Fig.4. The coarsening of unmelted primary solid particles by mechanisms of coalescence and Ostwald ripening[16], which is obvious during the investigation of other alloys[17-18], is not observed. In addition, it is found from Figs.4 and 5, the structure separation and spheroidization of ZA84 alloy during the semi-solid isothermal treatment are not very sufficient. Apparently, the incomplete structure separation and spheroidization would possibly retard the final coarsening. Since the stages of structure separation and spheroidization are always accompanied by the increase of liquid fraction resulting from the remelting, the possible reason for the above-mentioned phenomenon is the low liquid fraction in the semi-solid microstructure of ZA84 alloy. According to the SCHEIL equation[19], the volume fraction of liquid φL in the semi-solid microstructure should be a constant value at a given temperature during semi-solid isothermal heat treatment, with the assumption that the homogenization of the liquid is complete and no diffusion is in the solid:
 (2)
(2)
where TM is the melting point of pure metal, TL is the liquidus temperature of an alloy, T is semi-solid isothermal temperature, k is the equilibrium distribution coefficient.

Fig.8 Schematic illustration of non-dendritic evolution during semi-solid isothermal treatment: (a) Dendrite; (b) Initial coarsening; (c) Separation; (d) Spheroidization; (e) Final coarsening
Assuming that the interactions among various alloying elements are neglected, then k of multi- component is treated using the equivalent pseudo-binary method, which is expressed as[20]
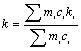 (3)
(3)
where ci is the initial composition of the i alloying element, mi and ki are the liquidus slope and partition coefficient of i element in the Mg-i binary alloys, respectively. Under the experimental condition of this work, i is Zn or Al element. Based on the DSC result in the present experiment, the value of TL is 612.6 ℃, and the value of TM is 650 ℃[17,19]. According to the Ref.[21], m(Al)=-6.87; m(Zn)=-6.04; k(Al)=0.37; k(Zn)= 0.12; c(Al)=3.91% and c(Zn)=7.9%, respectively. So the liquid fraction is 0.329, 0.354, 0.382 and 0.414 at 560 ℃, 565 ℃, 570 ℃ and 575 ℃ according to Eqns.(2) and (3). While in the present experiment, the practical values of φL measured by the auto image analysis system respectively are 0.142, 0.19, 0.22 and 0.256 for the samples treated at 560 ℃, 565 ℃, 570 ℃ and 575 ℃ for 120 min. The reason for the difference of calculated and measured liquid fraction might be that the liquid-solid diffusion equilibrium is not reached due to short holding time and/or low holding temperature in the present experiment. Considering that a 40%-60% liquid content is generally defined as a working range of SSM in order to obtain adequate flowability for a semi-solid slurry[16], then in further study a longer isothermal holding time than 120 min and more appropriate temperature range of semi-solid isothermal treatment should be considered for the experimental alloy. It is estimated that if higher holding temperature and/or longer holding time than the present work were adopted, the liquid-solid diffusion would become quicker, and the liquid fraction resulting from the remelting would increase, which would be beneficial to promoting the non-dendritic microstructural evolution such as structural separation and spheroidization.
4 Conclusions
1) It is possible to produce ZA84 magnesium alloy with non-dendritic microstructure by suitable semi-solid isothermal heat treatment.
2)After being treated at 560-575 ℃ for 120 min, ZA84 alloy can obtain a non-dendritic microstructure with a 14.2%-25.6% liquid content and an average size range of 56-65 um of the unmelted primary solid particles. Thereinto with the increase of holding time from 30 to 120 min or holding temperature from 560 to 575 ℃, the average size of unmelted primary solid particles decreases and globular tendency becomes more obvious.
3) Under the experimental condition, the microstructural evolution of ZA84 alloy during semi-solid isothermal treatment, is mainly composed of three stages of initial coarsening, structural separation and spheroidization. The subsequent coarsening of spheroidal grains is not observed.
4) In order to obtain adequate flowability for a semi-solid slurry produced by semi-solid isothermal heat treatment, in further study, longer holding time than 120 min and more appropriate holding temperature range of semi-solid isothermal treatment need to be considered for ZA84 alloy.
References
[1] DU X H, ZHANG E L. Microstructure and mechanical behaviour of semi-solid diecasting AZ91D magnesium alloy [J]. Mater Letters, 2007, 61: 2333-2337.
[2] ZHANG Q Q, CAO Z Y, LIU Y B, WU J H, ZHANG Y F. Study on the microstructure evolution and rheological parameter of semi-solid Mg-10Al-4Zn alloys [J]. Mater Sci Eng A, 2008, 478: 195-200.
[3] LUO A, PEKG?LERY?Z O. Review of cast magnesium alloys for elevated temperature applications [J]. J Mater Sci, 1994, 29: 5259-5271.
[4] PEKG?LERY?Z O, ARSLAN A K. Creep magnesium alloys for power train applications [J]. Advanced Eng Mater, 2003(12): 31-38.
[5] CHEN T J, MA Y, HAO Y, LU S, XU G J, SUN J. Structural evolution of ZA27 alloy during semi-solid isothermal heat treatment[J]. Trans Nonferrous Met Soc China, 2001, 11(1): 98-102.
[6] KIM J M, KIM K T, JUNG W J. Effects of isothermal heating procedure and strontium addition on semisolid forming of AZ91 magnesium alloy[J]. Mater Sci Technol, 2002, 18: 698-701.
[7] BALASUBRAMANI N, PILLAI U T S, PAI B C. Optimization of heat treatment parameters in ZA84 magnesium alloy [J]. J Alloys Compd, 2008, 457:118-123.
[8] WANG Y X, GUAN S K, ZENG X Q. Effects of RE on the microstructure and mechanical properties of Mg-8Zn-4Al magnesium alloy [J]. Mater Sci Eng A, 2006, 416: 109-118.
[9] QIN Q D, ZHAO Y G, CONG P J, ZHOU W, XU B. Microstructure evolution of in situ Mg2Si/Al-Si-Cu composite in semisolid remelting processing [J]. Mater Sci Eng A, 2007, 444: 99-103.
[10] CZERWINSKI F, ZIELINSKA-LIPIEC A. The microstructure evolution during semisolid molding of a creep-resistant Mg-5Al-2Sr alloy [J]. Acta Mater, 2005, 53: 3433-3444.
[11] CHEN T J, MA Y, LI B, LI Y D, HAO Y. Wear behavior of thixoformed AZ91D magnesium alloy: A comparison with permanent mould cast alloy [J]. Mater Sci Eng A, 2007, 445/446: 477-485.
[12] ZHANG X L, LI T J, TENG H T, XIE S S, JIN J Z. Semisolid processing AZ91magnesium alloy by electromagnetic stirring after near-liquidus isothermal heat treatment [J]. Mater Sci Eng A, 2008, 475: 194-201.
[13] ZHANG J, GUO Z X, PAN F S, LI Z S, LUO X D. Effect of composition on the microstructure and mechanical properties of Mg-Zn-Al alloys [J]. Mater Sci Eng A, 2007, 456: 43-51.
[14] WANG J L, SU Y H, TSAO C Y A. Structural evolution of conventional cast dendritic and spray-cast nonodendritic structures during isothermal holding in the semi-solid state [J]. Scripta Mater, 1997, 37: 2003-2007.
[15] ZOQUI E J, ROBERT M H. Contribution to the study of mechanisms involved in the formation of rheocast structure [J]. J Mater Proc Technol, 2001, 109: 215-219.
[16] CZERWINSKI F. The processing phenomena of semisolid Mg-9Al-1Zn alloy at ultra high contents of the unmelted phase [J]. Mater Sci Eng A, 2005, 392: 51-61.
[17] WANG J G, LIN H Q, LI Y Q, JIANG Q C. Effect of initial as-cast microstructure on semisolid microstructure of AZ91D alloy during the strain-induced melt activation process [J]. J Alloys Compd, 2008, 475: 251-258.
[18] CHEN T J, HAO Y, SUN J, LI Y D. Effects of Mg and RE additions on the semi-solid microstructure of a zinc alloy ZA27 [J]. Sci Technol Adv Mater, 2003, 4: 495-502.
[19] ZHANG Q Q, CAO Z Y, ZHANG Y F, SU G H, LIU Y B. Effect of compression ratio on the microstructure evolution of semisolid AZ91D alloy [J]. J Mater Proc Technol, 2007, 184: 195-200.
[20] LI D Z, HU Z Y, LI Q, LI Y Y. Microstructure simulation and process optimization of turbine blade castings [J]. Acta Metallurgical Sinica (English Letters), 1998, 11: 383-390
[21] ZENG X Q, WANG Y X, DING W J. Effect of strontium on the microstructure, mechanical properties, and fracture behavior of AZ31 magnesium alloys [J]. Metall Mater Trans A, 2006, 37: 1333-1341.
Foundation item: Project(50725413) supported by the National Natural Science Funds for Distinguished Young Scholar in China; Project(2007CB613704) supported by the National Basic Research Program of China; Projects(2006AA4012-9-6, 2007BB4400) supported by the Chongqing Science and Technology Commission of China
Corresponding author: YANG Ming-bo; Tel: +86-23-68667455; E-mail: yangmingbo@cqit.edu.cn
(Edited by YANG Bing)