Characterization of RuO2+SnO2/Ti anodes with high SnO2 concentrations
来源期刊:中国有色金属学报(英文版)2004年第6期
论文作者:王欣 唐电 周敬恩
文章页码:1111 - 1116
Key words:titanium anode; sol-gel; nanometer coatings
Abstract: Two SnO2+RuO2/Ti anodes with high SnO2 concentrations were prepared by painting, sintering and annealing through a sol-gel technique. The microstructure, morphology and grain size of coatings and the electrochemical properties of the anodes were investigated by XRD, DTA, SEM, TEM and CV. It is demonstrated that the anodic coatings consist of solid solution (Sn, X)O2 (X represents Ru or Ti) phases. The average grain size of the coatings is about less than 30nm. When the annealing temperature increases from 450℃ to 600℃, the solid solutions decompose. The crystal of the coating is equiaxial. The morphology of TiO2+SnO2/Ti coatings is a mixture of mud-flat cracking with a kind of agglomerated structure.
WANG Xin(王 欣)1, 2, TANG Dian(唐 电)2, ZHOU Jing-en(周敬恩)1
(1. Department of Material Science and Engineering, Xian Jiaotong University, Xian 710049, China;
2. Institute for Materials Research, Fuzhou University, Fuzhou 350002, China)
Abstract: Two SnO2+RuO2 /Ti anodes with high SnO2-concentrations were prepared by painting, sintering and annealing through a sol-gel technique. The microstructure, morphology and grain size of coatings and the electrochemical properties of the anodes were investigated by XRD, DTA, SEM, TEM and CV. It is demonstrated that the anodic coatings consist of solid solution (Sn, X)O2 (X represents Ru or Ti) phases. The average grain size of the coatings is about less than 30nm. When the annealing temperature increases from 450℃ to 600℃, the solid solutions decompose. The crystal of the coating is equiaxial. The morphology of TiO2+SnO2/Ti coatings is a mixture of mud-flat cracking with a kind of agglomerated structure.
Key words: titanium anode; sol-gel; nanometer coatings CLC number: TQ138.21
Document code: A
1 INTRODUCTION
Titanium anodes (also known as dimensionally stable anodes) are described as one of the greatest technological breakthrough in the field of electrochemistry industry in the past 20th century[1-3]. A conclusion can be drawn that the success of titanium anodes is due to its high corrosion resistance, good conductibility and electrocatalytic activity for chlorine evolution reaction. Sn-containing oxide anodes are the most frequently used ones, especially for the ternary oxide anode TiO2+SnO2+RuO2/Ti materials used in the chloral-alkali industry[4, 5]. However, these coatings are usually prepared by thermal decomposition of the corresponding metal chlorides, which are brushed with the certain solvent onto the titanium substrates. Although such method is simple and not costly in production, a variety of micro analytical methods have revealed[6-8] that the distributions of the elements and the microstructure of the coatings are not homogeneous and the final composition of the coatings might not coincide with that of the starting materials for the loss of tin through heat-treating. The loss of tin through heat-treating might be the main reason that the tin concentrations of Sn-containing anode coatings for practical use are always lower than 15% (mass fraction).
As has been known, sol-gel method is an excellent route to obtain chemically homogeneous mixed oxide coatings on the metallic matrix, and it might be much easier to control its grain size on nanoscale[9, 10]. In our laboratory, we exerted ourselves into preparing the nanometer anodic oxide coatings and the nanostructured matrix materials including SnO2/Ti, SnO2+RuO2/Ti, SnO2+TiO2/Ti have been obtained through a sol-gel technique recently[11-14]. We found that not only highly dispersive and homogeneous microstructure but also high Sn-containing coating might be obtained by means of sol-gel method[11, 12]. In this paper, attempts are made to characterize the structural and property features of some binary anodes with their tin molar concentrations being higher than 20%.
2 EXPERIMENTAL
2.1 Preparation of samples
Commercial RuCl3 and the experimental AR grade SnCl4 were used as the source materials. The binary oxides coating sols were gained by using RuCl3 and SnCl4 as metal precursors dissolved in ethanol. Citric acid and ethylene glycol were used for chelating. Acetic acid was added to the stock solutions to improve the precursors solubility. Two citric solutions were prepared separately and the ratio of cation (Ru4+ or Sn4+), citric acid, and ehtylene glycol was 1∶4.65∶0.33. Ruthenium and tin citrate were prepared by the addition of ruthinium and tin ethanol solution into an ethanol solution of citric acid, respectively, with a constant stirring. After the complete homogenization, ethylene glycol was mixed into the two solutions, followed by heating at 100-140℃, to promote the polyesterification reaction. Two high viscous polyester resins were yielded after stirring continually for several days.
The industrial pure titanium plate(TA2) was used as the substrates. The plates were initially sandblasted and etched in boiling 20% oxalic acid for 2h to 3h and then cleaned by distilled water and kept in ethyl alcohol.
According to Table 1, certain amounts of Ru and Sn polyester resins were also diluted by ethanol and then mixed all together. Some additives were interfused when the blended solutions were being stirred continually. The needed binary compound coating sols were formed after several days.
Table 1 Molar ratio of metal elements of materials for samples

The prepared coating sols were brushed on the Ti plates, dried by an infrared lamp, oxidized in an oven at 450℃ for 10min and then cooled to the room temperature. The procedure was repeated till the sols were used up. The final annealing procedure was applied to the plate samples at 450℃ and 600℃ for 1h, respectively. To simplify the description, the different sol or coating with the different molar ratios of the metal elements was indicated only by the element and molar ratio. For instant, the coating of SnO2+RuO2/Ti with the molar ratio of Sn to Ru being 41∶18 was referred as Ru18Sn41 oxide coating.
2.2 Instrumental analyses
Differentia thermal analysis(DTA) for the dried gel was carried out by using a CRY-2P DTA instrument in the temperature range from 50℃ to 800℃ at 20℃/min under the condition of air flow rate of 40mL/min. Morphology of each coating was observed by scanning electron microscopy(SEM), and the molar composition of each coating was analyzed by electron probe micro-analysis(EPMA) and energy-disperse spectrometer(EDS), conducted in a JEOL JSM-5800 scanning electron microscope. X-ray diffraction(XRD) analysis was employed for the phase structures of the coatings using a D/max-3C X-ray diffractometer and the experimental conditions were: CuKα radiation, Ni filter, 40kV, 15mA. The crystal size and the morphology of the surface were examined by transmission electron microscopy(TEM) in a Hitachi HU-12 electron microscope with the acceleration voltage of 120kV.
3 RESULTS AND DISCUSSION
3.1 DTA analysis
DTA curve of Ru54Sn23 dried gel obtained is reported in Fig.1.
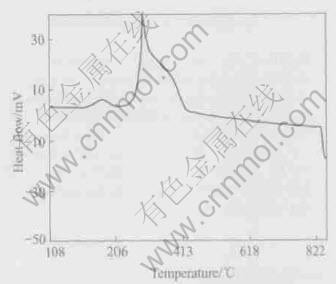
Fig.1 DTA curve of binary oxide coating dried gel Ru54Sn23
As shown in Fig.1, an obvious exothermic peak at the temperature of 168℃ along the Ru54Sn23 dried gel DTA curve may be attributed to some organic solvent with low molecular mass burning in the resins, and the more polyesterification in the resin may result in the nallow endothermal valley which is located at 234℃ along the curve. The oxidization of the organic functional groups and some other solvents whose burning point is higher may be the reason for a narrow peak at about 300℃. The metal oxides formation and their crystallization probably lead to the heat release at 367℃. The metal oxides dissolve mutually and an amourphous solid solution(Sn, Ru) O2 is initiated to form as well within the same range of temperature. After 450℃, some rutile phase metal oxides exist and a kind of solid solution (Sn, Ru)O2 is formed, which consists of rutile phases and will be proved by the subsequent XRD results. An endothermic process appearing at about 830℃ may indicate that RuO2 is decomposed into Ru and O2.
The structure of the binary gels is very sophisticated in the resins, in response to the frequent appearance of endothermic and exothermic processes presented on the DTA curve. The complex structure of cation citrates, polymerization degree and difference between two resins thermal stability can lead to complex shapes of DTA curve. In addition, interacting of the polymerized resins may also complicate the binary gels structure.
3.2 XRD analysis
XRD spectra of titanium anode samples prepared at 450℃ and 600℃ are shown in Fig.2. The XRD patterns indicate that the titanium anode coatings are mainly composed of rutilic phases. The main peaks are characterized at 2θ=30°, 44° and 64°, respectively.

Fig.2 XRD patterns of titanium anodes coatings
Brookite TiO2 is presented in the coatings of Ru18Sn41 at 450℃ and the characteristic peaks of rutile RuO2 and SnO2 shift a small angle from the common positions. The interplanar distance of rutile SnO2 is increased and those of brookite TiO2 are reduced for low indices crystal planes; on the contrary, rutile interplanar distance of SnO2 is reduced and that of RuO2 is increased for high indices planes. It is suggested that part of Ti substrate annealed at 450℃ is oxidized to form brookite TiO2. Because Sn4+ has the similar ion radius with Ti4+, the formed brookite TiO2 is dissolved partially into the rutile SnO2 and changes the crystal spacing between the planes of 2θ= 30°. Meanwhile, a kind of rutilic phase solid solution (Sn, Ru)O2 may be formed in the coating because rutile RuO2 and SnO2 present the same crystal structure and it is convenient for metallic ion to diffuse between the crystal planes those at 2θ= 64°. In addition, Ru4+ and Sn4+ have similar ion radii and the percentage composition of SnO2 is higher in the Ru18Sn41 coatings. It can be concluded that Ru4+ takes the place of Sn4+ in rutile phase to form the solid solution (Sn, Ru) O2.
The peaks in Ru18Sn41 XRD patterns are widened to some extent, which indicates that the crystal size of the coating is very small.
The main peaks of rutile SnO2 in Ru54Sn23 XRD pattern are very weak and may be explained by low SnO2 content in the coating. Rutile TiO2 is detected at 450℃, which may also be attributed to the oxidation of Ti substrate. RuO2 and rutile TiO2 are soluble in the coating and a kind of RuO2 based solid solution (Ru, Ti) O2 forms when Ti4+ substitutes for some of Ru4+.
When the annealing temperature increases, the phases at 600℃ do not differ a lot from those at 450℃ in both binary coatings. But the peak at 2θ = 30° in Ru18Sn41 splits into two peaks and rutile RuO2 appears. Because its thermal vibration amplitude and frequency enhance with the annealing temperature increasing, Ru4+ is separated from the equilibrium position to form the observed rutile RuO2 in the coating. It is found that the brookite TiO2 has changed into rutile in Ru18Sn41 at higher temperature.
Titanium phase is investigated in the patterns due to the thin binary oxide coatings on the substrate. But the characteristic peaks are weakened when the samples are annealed at 600℃ compared with those at 450℃. We can infer that the coating grows thicker when the Ti substrate is oxidized into rutile TiO2 at elevated annealing temperature.
It can be found that the structure of the binary is sophisticated. This is consistent with the result of DTA.
It seems that the grain size in the coating is very small. Using Sherry equation, the average crystal size of the samples can be determined and is shown in Table 2.
Table 2 Average crystal size of titanium anodes coating
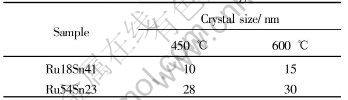
From Table 2 it can be concluded that the sample coatings consist of nanosized particles. Increasing in content of RuO2 coarsens the crystal grains greatly. RuO2 is one of the most expensive materials and rare metallic oxides. Hence, to choose the Ru18Sn41 oxide coatings instead is better than to select the Ru54sn23 as the anode material. It is good not only for cutting down expense but also for obtaining finer crystal size.
The particles have not been enlarged sharply when the annealing temperature increases and conversely it can not be investigated in TiO2, SnO2 and RuO2 simple system[6-8, 11, 12]. We can conclude that the structures of both samples are stable relatively compared with those of monosystem.
3.3 TEM analysis
The TEM image and diffraction pattern of Ru18Sn41 are shown in Fig.3(a). The ring-shaped diffraction pattern infers that the crystal size in the coating is very small. On the other hand, the particle size in Ru54Sn23 is widened and we can calculate it to be about 30nm from Fig.3(b). It is suggested that in binary oxide coatings the increase in amount of SnO2 will lead the crystal to nanometer size.
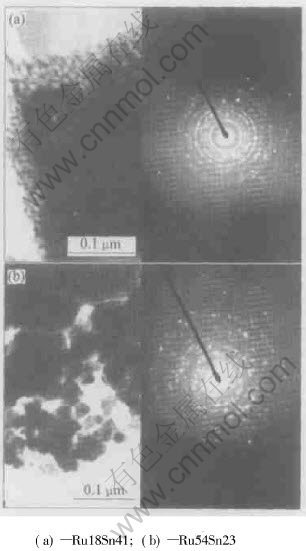
Fig.3 TEM images and diffraction patterns of titanium anode coatings
The doted-like crystals can be seen all over the field of vision in the binary coatings. It is convenient to calculate the average crystal size, which is below 30nm. These results fairly agree with those of XRD analysis for both binary oxide coatings.
The crystal morphology of the oxides is observed in the coatings. It is worth of noticing that the shapes of quadri-prism that are typical appearance of a rutile phase are never observed in these specimens. It seems that the nano-grains have not grown up to the normal dimension to form the shapes of quadri-prism. The doted-like equiaxial grains are good to improve the electrochemical property of titanium anodes because this kind of coating crystals can distribute homogeneously in the coatings, and as well as increase the number of the electrocatalytic reaction active centers.
In present experiments, the doted-like equiaxial grains can be found in both of the samples. But the amount of RuO2 in Ru18Sn41 coating is less than usual 30% molar ratio used generally in electrochemical industry. This suggests that higher amount of RuO2 is not necessarily required for gaining the ideal crystal morphology.
3.4 SEM and EDX analysis
Fig.4 shows the representative SEM micrographs of binary oxide coatings on two titanium anodes samples. The oxide surface is a rough morphology with a mixture of mud-flat cracking and a kind of agglomerated structure is also observed in the field. Since the only cracked-mud structure is observed in coatings prepared by the thermal decomposition approach, the conglobation morphology of the binary oxide coatings may be related to the sol-gel preparation method.
As the amount of SnO2 is increased from 0.18 to 0.54mol the cracked-mud coatings seem to become invisible and more accumulated parts are observed in the fields and the agglomerated structure may prevent Ti substrate below the cracks exposed to corrosive medium and thus improve titanium anodes samples electrochemical performance. The average size of the cracking is about 10μm.
The surfaces of the two samples are not covered heterogeneously probably because there is a lack of TiO2 as framework and bond material in the coatings.
In the coatings annealed at 450℃, the edges of the cracking are more uniform than those annealed at 600℃. It is suggested that the coatings are not separated from Ti substrate easily when the titanium anodes samples are annealed at the temperature of 450℃. But the cracked-mud warps up when annealed at 600℃ and the coatings are inclined to flaking off from the Ti substrate. From this point, we can conclude that the appropriate annealing process to prepare titanium anodes coatings is at 450℃for 1h. That is in agreement with the result of XRD.
Comparing the results of 450℃ and 600℃, an interesting phenomenon can be observed. The size of the cracked-mud in the coatings is degraded with the increasing of annealing temperature, which should be attributed to the coatings crushing into small portions when the temperature changes from 450℃ to 600℃.
EPMA and EDS results for the Sn-containing
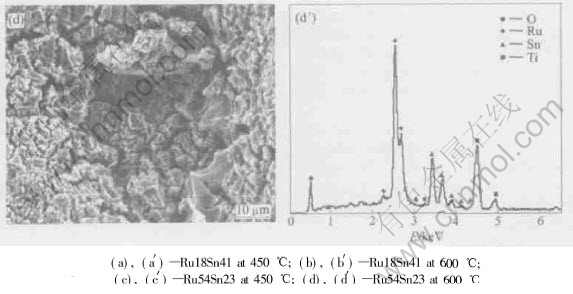
Fig.4 SEM images and EDA patterns of binary oxide titanium anode samples
coatings prepared by the sol-gel method were studied also in our previous publications[15]. In this experiment, we also find that the molar concentrations of starting materials are maintained till the final coatings are obtained. Fig.4 shows the molar ratio between Sn and Ru atoms obtained by EDX focused on the whole electrodes surface. Within the instrumental error, the analysis shows an excellent relationship between experimental and nominal composition of Sn and Ru atoms. Generally, the composition of coatings prepared by thermal decomposition method(TDM) is different far from the nominal composition and especially the loss of Sn is very great. On the contrary, Sn in the prepared coatings is high, for which one of the reasons may be that RuCl3 used in this experiment is industrial grade and so Ru in the stock materials is less than 38% (mass fraction). In addition, Sn atoms are almost the same during the samples preparation, i.e. the loss of Sn is much less than that of the usual method—Thermal Decomposing Methods. These two lead to that the molar ratio of Sn to Ru in the coatings is more than the nominal and Sn is above 60% in Ru18Sn41, and close to 30% in Ru54Sn23.
Ti atoms are detected in Ru54Sn23. The coating breaking off may be one of the reasons and Ru54Sn23 coating is too thin to prevent electron ray from penetrating it. On the other hand, O atoms increase sharply but Ti decreases acutely in this coating when the annealing temperature changes from 450℃ to 600℃, which indicates that Ti substrate oxides to form TiO2 and the coating becomes thicker. Increase in annealing temperature has no obvious effect on the molar ratio in Ru18Sn41, which suggests this coating is compact enough to protect the substrate from oxidation. These results are consistent with those of XRD.
4 CONCLUSIONS
1) The nanoscale oxide coatings are applied onto the titanium substrate by a sol-gel method with the molar ratio of Ru to Sn being 18∶41 and 54∶23 in binary oxide coatings, respectively. The molar ratios of the obtained coatings consist with that of nominal compositions.
2) The surface morphology of the high Sn-containing anodes is a mixture of mud-flat cracking with a kind of agglomerated structure. The results show that the oxide coatings consist of a rutile phase solid solution that is stable when the samples are annealed at 450℃.
3) The coating crystal of titanium anodes samples is equiaxial. The diameters of coating grains are less than 30nm. The increase in amount of RuO2 coarsens the particles. The size of crystal remains at the same level when the annealing temperature increases.
REFERENCES
[1]Hine F. Electrode materials for electrochemical industries [J]. Met Surf Tech, 1985, 36(8): 313-320. (in Japanese).
[2]Trasatti S. Electrocatalysis: understanding the success of DSA [J]. Electrochimica Acta, 2000, 45: 2377-2385.
[3]TANG Dian. Electrode materials for electrochemical industries [J]. Material Science and Technology, 1989, 6(1): 89-91. (in Chinese)
[4]Yasushi M, Minoru I, Hayato K, et al. Surface characterization of ruthenium-tin oxide electrodes [J]. Applied Surface Science, 1997, 121/122: 314-318.
[5]WEN S, CHEN S, TANG D. Preparation of nanometer SnO2 [J]. Chlor-Alkali Ind, 1996(8): 233-238. (in Chinese)
[6]Ardizzone S, Falciola M, Trasatti S. Effect of the nature of the precursor on the electrocatalytic properties of thermally prepared ruthenium oxide [J]. J Electrochem Soc, 1989, 136: 225-232.
[7]Battisti A D, Lodi G, Cappadonia M, et al. Influence of the valve metal oxide on the properties of ruthenium based mixed oxide electrodes [J]. J Electrochem Soc, 1989,136: 2596-2604.
[8]TANG D, YAN Q, CUI X. Microstructure of titanium anodes [J]. Chlor-Alkali Ind, 1994(8): 24-26. (in Chinese)
[9]LIN J, ZHAO M, CHEN K. Synthesis and electrocatalytic properties of complex oxides containing lanthanum [J]. J Rare Earths, 1999, 17(3): 231-233.
[10]Forti J, Olivi P, Andrade A. Characterization of DSA-type coatings with nominal composition of Ti/Ru0.3Ti0.7-xSnxO2 prepared via a polymeric precursor [J]. Electrochimica Acta, 2001, 47: 913-920.
[11]Panic V, Dekanski A, Milonjic S, et al. The influence of the aging time of RuO2 and TiO2 sols on the electrochemical properties and behavior for the Cl2-evolution [J]. Electrochimica Acta, 2000, 46: 415-421.
[12]TANG D, WEN S, CHEN S. Preparation of RuO2-SnO2 nanomaterial by a sol-gel technique [J]. Trans Nonferrous Met Soc China, 2000, 10(3): 337-339.
[13]WANG X, TANG D, ZHOU J. Effects of SnO2 on microstructure, morphology of RuO2+SnO2+TiO2/Ti anode [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(5): 920-924.(in Chinese)
[14]WANG X, TANG D, ZHOU J. Effects of TiO2 on microstructure, morphology of RuO2+SnO2+TiO2/Ti anode [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(3): 708-712.(in Chinese)
[15]WEN S, CHEN S, TANG D. Preparation of nanometer binary oxides of RuO2-SnO2 [J]. Chlor-Alkali Ind, 1993, (3): 7-11. (in Chinese)
Foundation item: Project (59682006) supported by the National Natural Science Foundation of China
Received date: 2004-03-17; Accepted date: 2004-07-26
Correspondence: WANG Xin, PhD; Tel: +86-29-82673261; E-mail: xinwx@163.com

