Trans. Nonferrous Met. Soc. China 23(2013) 208-218
Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue
Xiao-bo MIN1,2, Xian-de XIE1, Li-yuan CHAI1,2, Yan-jie LIANG1, Mi LI1, Yong KE1
1. Institute of Environmental Science and Engineering, School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Centre for Control & Treatment of Heavy Metal Pollution, Changsha 410083, China
Received 3 November 2011; accepted 19 March 2012
Abstract:
Four different methods, namely mineralogical analysis, three-stage BCR sequential extraction procedure, dynamic leaching test and Hakanson Potential Ecological Risk Index Method were used to access the environmental activity and potential ecological risks of heavy metals in zinc leaching residue. The results demonstrate that the environmental activit y of heavy metals declines in the following order: Cd>Zn>Cu>As>Pb. Potential ecological risk indices for single heavy metal are Cd>Zn>Cu>As>Pb. Cd has serious potential ecological risk to the ecological environment and contributes most to the potential toxicity response indices for various heavy metals in the residue.
Key words:
heavy metals; BCR sequential extraction; environmental availability; leaching toxicity; dynamic leaching; potential ecological risk index method; zinc leaching residue;
1 Introduction
Zinc leaching residue is a typical product generated from the zinc hydrometallurgy process. It was considered to be hazardous wastes due to their significant content of metals [1]. Studies on recycling of zinc concentrate from zinc leaching residue by hydrometallurgy methods had been reported. However, these processes cost much and caused potential environmental problems that affected the health and living environment of human beings [2,3], owing to the low grade of zinc in the residue. For the above reasons, a considerable amount of residues were randomly piled up. Apart from the aesthetic and economic problems associated with large areas of derelict land, tailings dumping can be an important source of water pollution and wind-borne, metal- containing dusts [4,5].
Recently, an increasing number of researches on the behavior of transportation and transformation of heavy metals in the environment were on the account of frequent incidents of heavy metal contamination. As an important carrier of heavy metals, the nonferrous metal mining areas became one of the hot issues on heavy metal contamination. WANG et al [6] studied the effects of heavy metal elements release in Yangshanchong tailing pool, Tongling, Anhui Province, China by the content of heavy metals of the surrounding mining area. LAUREL et al [7] reported the capability of transport, exposure, and bioavailability of zinc, lead, and cadmium in mine waste by sequence extraction of mining waste in different particle sizes. NAVARRO et al [8] investigated the contamination of abandoned mine sites in a semi-arid zone by the content and occurrence of heavy metals combining with the local climate factors. DANG et al [9] studied the mobility of heavy metals associated with the natural weathering of coal mine spoils. However, fewer researches were direct on smelting wastes, although the researches direct on smelting wastes were vitally important for pollution control from the source.
Therefore, the environmental activity and potential ecological risk of zinc leaching residue were studied in the present study, which would provide theoretical basis and technical support for source pollution control.
Environmental activity of heavy metals is the generalization of their toxicity, bioavailability and geochemical behavior. A considerable amount of studies on environment activity of heavy metals had been done by various researchers from different countries. TSIRIDIS et al [10] used the toxicity characteristic leaching procedure (TCLP) to study the leaching toxicity of coal fly ash. KAZI et al [11] studied the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. WONG et al [12] studied the toxicity of sewage sludge by the content of elements, speciation of heavy metals and seed germination test. While, WANG et al [13] studied the release of heavy metals in Pb/Zn tailings under acid leaching conditions. However, no systematic research had been done on environmental activity of heavy metals. As a result, the total content of heavy metals, mineralogical analysis, chemical speciation, leaching toxicity test and long term leaching test were applied to systematically evaluate environmental activities of heavy metals in zinc leaching residue, combining with environmental conditions.
Potential ecological risk index method which was proposed by Swedish scientist HAKANSON in 1980 [14], had been widely applied to evaluate the harm of heavy metals in the sediments and residues [15,16]. The total amount of heavy metals was used to evaluate the potential ecological risk of tailings, which would obviously exaggerate their ecological risks [17]. Thus, in this work, acidic exchangeable form of heavy metals, the most possible contents that do damage to the environment, was applied to evaluate the potential ecological risk of the residue.
The aim of this work was to conduct a systematic evaluation of environmental activity and potential ecological risk of the zinc leaching residue, and to provide theoretical basis for harmless recycling of this kind of residue.
2 Experimental
2.1 Materials
The residue used for testing was obtained from a lead and zinc plant, located in Zhuzhou city, China. It was produced in the sulfuric acid leaching process. After sampling, it was air-dried, then sieved to fraction less than 2 mm, and stored at 4 °C for use. The pH value of the residue was 3.97.
The total content of heavy metals could be obtained according to the following steps. The air-dried samples were oven-dried at 40 °C for 24 h, then crushed and sieved with 200-mesh screen. After that, the powders were placed in a clean porcelain crucible and heated in a muffle furnace at 800 °C for 5 h. About 0.2 g of the ashed sample was digested with 5 mL concentrated nitric acid and 1 mL perchloric acid in Teflon beakers. Breakers with fluid were first heated gently on a hot plate at temperature of 80 °C for 3 h and then heated at a raised temperature of 170 °C until the samples were reduced to moist solids. The solid samples were allowed to cool to room temperature and rinsed with a few drops of deionized water. Samples were then leached in 5 mL concentrated HCl. The solutions were diluted with deionized water in 50 mL volumetric flasks, and analyzed using an inductively coupled plasma atomic emission spectrometer (ICP-AES). By inserting three duplicates into each batch of the samples, the precision of the data thus acquired can be controlled.
Mineralogical composition of the samples was determined by X-ray diffraction analysis (Simens 2500X) using Cu Kα radiation with a Philips PW3040 diffracto- meter equipped with a digital recorder, automatic window and Ni filter. Morphology observation of the residue was carried out by SEM and the element composition in a particular point was determined by EDX.
2.2 Sequential extraction
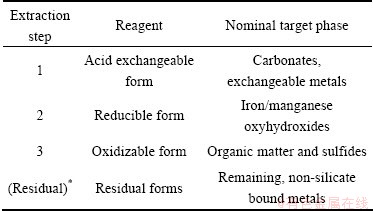
Three-step extraction procedure was first proposed by the Community Bureau Reference (BCR), and later it was modified by some scholars. In this work, Davidson’s three-stage BCR sequential extraction procedure was used to analyze the effective combination forms of heavy metals in the residue [18]. Each step was done in triplicate. The residual form was calculated by eliminating the acidic extractable form, reducible form as well as oxidisable form. Analytically pure and guarantee reagent, as listed in Table 1, were required in the extraction procedure.
Table 1 BCR three-stage sequential extraction scheme
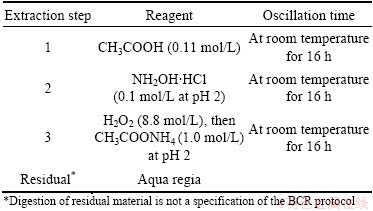
Step 1: 20 mL acetic acid (0.11 mol/L) was added to 0.5 g of dry “residue” in a 50 mL polypropylene wide-mouthed bottle. The bottle was shaken for 16 h at ambient temperature with 50 r/min. The extract was separated from the solid residue by centrifugation (5000 r/min) for 10 min and decanted into a polyethylene container and stored at 4 °C for analysis. The residue was washed with 10 mL distilled water by shaking for 60 min, centrifuged and the washings were discarded.
Step 2: The residue from Step 1 was slurried with a portion of 20 mL hydroxyammonium chloride (0.1 mol/L, adjusted to pH 2 with nitric acid) and transferred quantitatively back to the wide-mouthed bottle, where the remainder of the reagent was added. The extraction procedure was then performed as described above.
Step 3: 5 mL hydrogen peroxide (8.8 mol/L) was added in small aliquots to the residue from Step 2. The centrifuge tube was covered with a watch glass and the contents were digested at room temperature for 1 h with occasional manual shaking. Digestion was continued by heating the tube to 85 °C in a water bath for 2 h. The watch glass was removed and the tube content was evaporated to a small volume (1±0.5) mL. A second 5 mL aliquot of hydrogen peroxide was added and the digestion procedure was repeated. The cool moist residue was then returned to the 50 mL bottle and 25 mL of ammonium acetate (1.0 mol/L, adjusted to pH 2 with nitric acid) was added. The sample was shaken, centrifuged and the extract was separated as described in Step 1. The solid residue was retained for microwave digestion. And all of the samples were analyzed by ICP-AES.
2.3 Leaching toxicity test
The solid waste-extraction procedure of leaching toxicity-sulphuric acid and nitric acid method developed by the Ministry of Environmental Protection of China [19] was designed to simulate the leaching process of metals (and organic matters) from wastes by acid rainfalls.
Dry samples (50 g) were added to polyethylene extraction bottles along with a 10:1 ratio of liquid extractant to dry samples. The extraction fluid was the mixture of sulphuric acid and nitric acid with the mass ratio of 2:1 and was adjusted to pH=3.20±0.05. The extraction bottles were sealed, placed in a standard tumbler and tumbled for (18±2) h. After tumbling, the samples were pressure filtered through acid-treated (HNO3) 0.6 μm glass fiber filter paper. The solid phase was discarded and the eluant was placed in acid (HNO3)-rinsed polyethylene bottles. All samples were stored at 4 °C until use.
2.4 Dynamic leaching test
In order to simulate the long-term leaching effects of the residue in the acid rain environment, a long-term leaching experiment was designed with reference to ANS (1986) standard [20]. The residue used for testing was 200 g every time. The leaching agent (deionized water, adjusted to pH=3.20±0.05 with concentrated sulfuric and nitric acid solution of mass ratio of 2:1 was used to simulate the acid rain. Elution flow rate was calculated according to the hydrologic conditions of the origin of the residue, which was averaged from the rainfall of the past 10 years. Leaching period lasted for 12 d, and the monthly average was simulated by the daily flow in details. The ratios of the daily leachate and residue calculated by the rainfall are shown in Table 2.
Table 2 Simulation of flows in different months
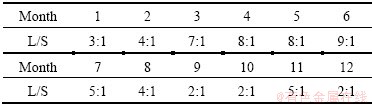
Heavy metals would hardly be leached out on account of metal re-adsorption when flow was low [21], which was in good agreement with the leaching effect when the residue was piled in the environment. Thus, this mode can accurately simulate the leaching effect when the residue was piled in the opening environment. And the specific operations were as follows.
About 2 cm thick quartz sand was placed into the PVC plastic tube with a diameter of 10 cm and a length of 30 cm. Then, 200 g of the residue which was mashed through 2 mm sieve was put into the polyethylene bottle. The BT 100-1L peristaltic pump was used to adjust the flow rate of leaching; the flow rate was calculated according to the ratio of liquid to solid (L/S). The experimental facilities are sketched in Fig. 1.
2.5 Potential ecological risk index method
Pollution index was used to evaluate the pollution of heavy metals in the residue. 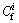 was used to reflect the pollution of single heavy metals in the residue. The formula for pollution index of single heavy metals is
was used to reflect the pollution of single heavy metals in the residue. The formula for pollution index of single heavy metals is
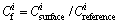 (1)
(1)
where 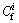 is the pollution coefficient for a certain heavy metal, which can reflect the pollution of the investigated region but cannot reveal the ecological effects and hazards;
is the pollution coefficient for a certain heavy metal, which can reflect the pollution of the investigated region but cannot reveal the ecological effects and hazards;  are the measured values of heavy metals in surface residue;
are the measured values of heavy metals in surface residue; 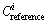 are the parameters for calculation, and with reference to the Grade III of the environmental quality standard for soil (GB 15618—1995) in this paper (Table 3).
are the parameters for calculation, and with reference to the Grade III of the environmental quality standard for soil (GB 15618—1995) in this paper (Table 3).
The formula for potential ecological risk index for the single heavy metal pollution is
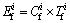 (2)
(2)
where  is the response coefficient for the toxicity of the single heavy metal. The formula reveals the hazards of heavy metals on human and aquatic ecosystem and reflects the level of heavy metal toxicity and ecological sensitivity to the heavy metal pollution. The standardized response coefficient for the toxicity of heavy metals, which was made by Hankanson, was adopted to be evaluation criterion. The corresponding coefficients based on its toxicity are shown in Table 4.
is the response coefficient for the toxicity of the single heavy metal. The formula reveals the hazards of heavy metals on human and aquatic ecosystem and reflects the level of heavy metal toxicity and ecological sensitivity to the heavy metal pollution. The standardized response coefficient for the toxicity of heavy metals, which was made by Hankanson, was adopted to be evaluation criterion. The corresponding coefficients based on its toxicity are shown in Table 4.
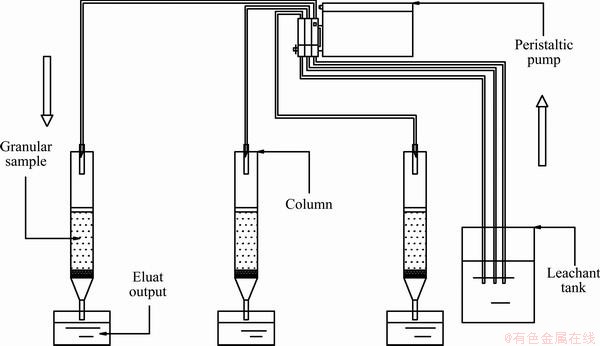
Fig. 1 Schematic of leaching test device
Table 3 Grade III standard of environmental quality standard for soil (mg/kg)
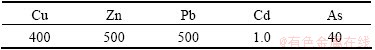
Table 4 Toxicity coefficient of heavy metals

The formula of potential toxicity response index for various heavy metals is
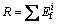 (3)
(3)
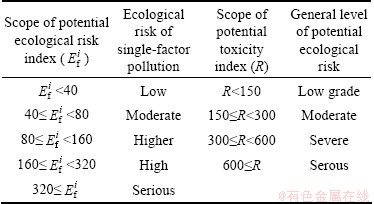
The grading standards of potential ecological risk of heavy metals are shown in Table 5.
Table 5 Relationship among R,  and pollution level
and pollution level
3 Results and discussion
3.1 Environmental activity assessment of heavy metals
3.1.1 Total concentration of heavy metals
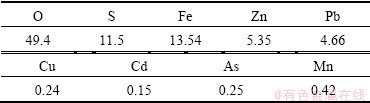
The total concentrations of major elements in the residue obtained by ICP-AES are presented in Table 6. It can be seen from Table 6 that, the contents of O, S and Fe are 49.4%, 11.5% and 13.54%, respectively. Si and C are also the important components of the residue. Zn has the highest content among all of the heavy metals (53547 mg/kg), and the second is Pb (46584 mg/kg). The concentrations of total Cu, Cd, As and Mn are 2400, 1500, 2500 and 4200 mg/kg, respectively.
Table 6 Compositions of residue (mass fraction, %)
3.1.2 Host minerals for heavy metals
The residue was analyzed by XRD pattern processing, identification and quantification software (Jade 6.0). The existence of heavy metals in the residue can be obtained by PDF card index method. The card used in this paper was PDF-2004. The results are shown in Fig. 2.
Carphosiderite, zinc sulfite hydrate, anglesite, dilead trioxide, lead sulfide and wurtzite were detected by XRD (Fig. 2). Among all of the high peaks, most of Fe exists in carphosiderite form, Zn exists in zinc sulfite hydrate form and Pb exists in anglesite form. Sulfate minerals are the main phase of the existing metals.
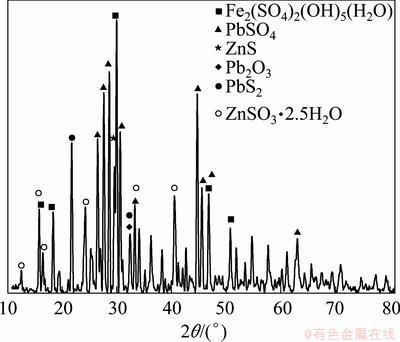
Fig. 2 XRD pattern of residue
Figure 3(a) shows that the crystals in the residue mainly exist in rod, lamellar, spherical, and other irregular forms with particle size mostly less than 1 μm.
EDX analyses for the residue were conducted at 3 different points in Fig. 3(a), namely a, b and c. Figures 3(c-d) show the EDX results of the compositions of points a, b and c, respectively. A considerable amount of C was detected in all sites of the residue according to the EDX result, indicating that C and other metals formed an important fraction in the residue. Carphosiderite and carbon are the main phases in point a, mixed with a small amount of zinc sulfate, silicates and other minerals. And C in this point accounts for 35% of the total amount, Fe for 14% and Zn for 1.7%. Carbon, carphosiderite and zinc sulfate are the main phases in point b. C accounts for 36% of the total amount, Fe for 16% and Zn for 2.7%. Carbon, carphosiderite and anglesite are the main phases in point c. C in this point accounts for 47% of the total amount, Pb for 3.4%, and anglesite is the main phase that Pb exists. The results of SEM/EDX demonstrate that heavy metals do not exist alone. Instead, they mix complicatedly with each other.
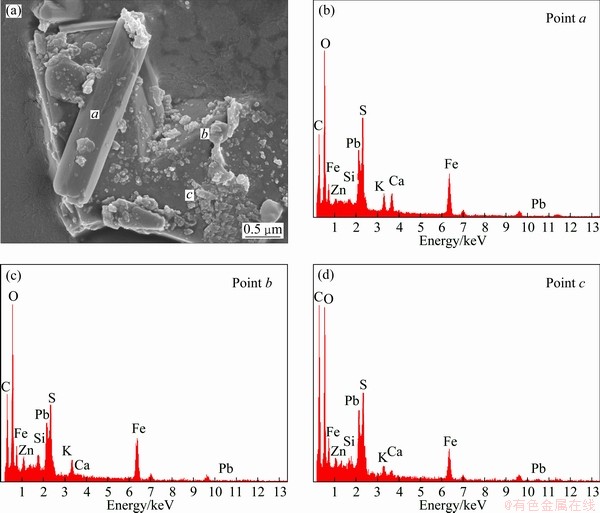
Fig. 3 SEM image and EDX patterns of zinc leaching residue
3.1.3 Chemical speciation of heavy metals
Study on the existing form of heavy metals in the residue is of great importance to analyze the environmental activity of heavy metals in the environment. The species of heavy metals from sequence extraction could furnish detailed information about the origin, chemical forms, biological and physico-chemical availability, mobilization and transport of trace metals [9]. The migration and transformation capabilities of elements in the environment can roughly be determined by comparing the content of heavy metals in various states in the residue. Meanwhile, the environmental activity of these elements can generally be determined. Each step of BCR test corresponds to the extraction of metals in different states, and the specific results are shown in Table 7 [22].
Table 7 Nominal target phases in BCR three-stage sequential extraction [22]
1) Acid exchangeable forms
The amount of acid exchangeable forms from BCR method is roughly equivalent to the sum of exchangeable and carbonate phase of the metal from the residue [23]. The carbonate form is a loosely bound phase and liable to change with environmental conditions, so this phase is susceptible to change along with pH change and generally obtained using a mild acid [23,24]. Besides, there is frequent acid rain in southern China [25]. Water soluble phase, exchangeable phase of acid exchangeable heavy metals will be easily released in acid rain environment. And now, it is widely believed that the exchangeable form of heavy metals is the direct phase that pollutes the environment [26,27].
Among the metals studied, the highest content of acid exchangeable forms is Cd and the lowest is Pb (Fig. 4), whereas the corresponding contents of Cd, Cu, Pb , Zn and As are 74%, 22%, 0.3%, 54% and 0.63%, respectively. It is revealed that the exchangeable and carbonate phase of Cd, Zn and Cu in the residue is as high as 1107.75, 29127.75 and 536.64 mg/kg, respectively. The results of exchangeable form of Cd, Zn and Cu in the residue show that all of them have high environmental activity. And some researchers have reported the enhancement of the leaching rate during the simultaneous leaching of metal oxides and metal sulfides [28], suggesting that a large amount of Cd, Zn and Cu would be released by the heavy rain.

Fig. 4 Speciation content of heavy metals in residue by BCR procedure
2) Reducible forms
The amount of reducible forms from BCR method is roughly equivalent to the content of Fe-Mn phase of the heavy metal in the residue [24]. The content of reducible form of Cd, Cu, Pb, Zn and As is 0.2%, 1.7%, 7%, 0.3% and 0.63% of the total mass, respectively (Fig. 4). Some researches suggested that Cd, Cu, Pb and Zn existing in crystalline state and amorphous state of Fe-Mn oxide phase by ionic bond will be released for ionic destroy when natural stockpiling is adopted [29,30].
3) Oxidizable forms
The amount of oxidizable forms from BCR method is roughly equivalent to the sum of organic matter and sulfide combination state of these heavy metals [24]. The organic fraction released under oxidizing conditions is not considered to be mobile and bioavailability; metals are incorporated into stable high molecular mass humic substances, which release small amount of metals over long time [31]. The toxic metals bounded to organic matters and sulfides can be easily released under oxidizing conditions [32]. The general equations of reactions are as follows:
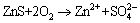 (4)
(4)
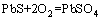 (5)
(5)
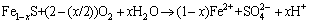 (6)
(6)
 (7)
(7)
The content of oxidizable forms of Cd, Cu, Pb, Zn and As is 0.6%, 2.4%, 10.5%, 2.3% and 1.18% of the total mass, respectively (Fig. 4). It reveals that there is a good deal of sulfide metal in the sludge.
4) Residual forms
Pb, As and Cu mainly exist in residual forms. The content of residual forms of Cd, Cu, Pb, Zn and As is 25.3%, 73.5%, 82.2%, 43.2% and 97.56% of the total mass, respectively (Fig. 4). The residual or non- extractable metals are retained within the crystal lattices of minerals and inside crystallized oxides. And this kind of metals would steadily exist in the residue for a long time [33,34]. Nonetheless, some researches suggested that there was a link between residual forms and other forms of heavy metals when environment changes [35,36].
3.1.4 Leaching toxicity analysis
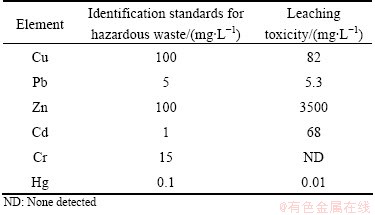
The overall purpose of this analysis was to classify waste as to its ultimate hazard and to predict the long-term behavior of contaminants. Leaching toxicity of Zn, Cd and Pb in the sludge was all higher than the Identification Standards for Hazardous Wastes— Identification for Extraction Toxicity (GB 5085.3—2007), especially for Zn (3500 mg/L) and Cd (68 mg/L) (Table 8). And the leaching toxicity for Cu, Pb, and Hg was 82, 5.3 and 0.01 mg/L, respectively. The results of leaching toxicity test suggested that metals in the residue were readily attacked by weak acid solutions and would be expected to migrate or dissolve by the rainfall, which had a good agreement with the BCR results and confirmed that Zn, Cd and Pb were the main pollution elements, and all of them had high environment activity to the surrounding environment.
Table 8 Leaching toxicity of residue
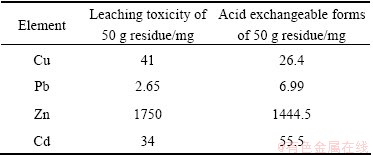
The content of acid exchangeable forms of Cu, Pb, Zn and Cd in 50 g residue was 26.4, 6.99, 1444.5 and 55.5 mg, respectively. And the leaching toxicity of Cu, Pb, Zn and Cd in 50 g residue was 41, 2.65, 1750 and 34 mg, respectively (Table 9). The amount of heavy metals existing as acid exchangeable forms was all close to the leaching toxicity values of the residue, suggesting that all of the acid exchangeable forms of heavy metals in this residue could be leached out under certain conditions.
Table 9 Heavy metals in acid exchangeable forms and leaching toxicity of residue
3.1.5 Release of heavy metals under acidic leaching conditions
The results of long-term leaching of main heavy metals in the residue showed that the emission rules of Zn, Cd and Cu in the leaching progress were similar (Fig. 5). The concentrations of Zn, Cd and Cu fluctuated as the flow changed, and declined to a stable level at the end of leaching process, indicating that the amount of elution was one of the most important factors that affected the leaching of heavy metals in the residue. Some researchers suggested that the release of heavy metals in the residue was a combination of two extreme behaviors: diffusion-controlled and solubility-controlled processes [37], illustrating that the leaching effect of elution and the diffusion of heavy metals were the main reasons that affected the heavy metals leaching out. Releasing of Zn, Cd and Cu (the concentrations significantly reduced after leaching for 48 h) showed that the contamination caused by the above three elements was serious in the first two days, and the trend would slow down with the increase of leaching time. And the new residue would cause more damage to the environment than the old one.
The leaching behavior of Pb was different from those of Zn, Cd and Cu in the residue (Fig. 5(d)). The concentration of Pb was low at the beginning, but increased with the extending of time. Maybe it was because Pb existed in different forms (mainly existed in residual forms) from Zn and Cd, which mainly existed in acid exchangeable forms. BCR analysis showed that 0.3% Pb existed in acid exchangeable forms, so it was hard to release at the beginning. Nonetheless, a considerable amount of Pb existed in reducible and oxidizable forms, accounting for 7% and 10.5% of the total content. The increasing concentration of Pb with the leaching time indicated that these two forms of Pb converted into acid exchangeable form [31,32,34].
Besides, the pH value of leachate ranged stably from 3.87 to 3.35 (Fig. 6), indicating that the residue had high acid buffering capacity. In addition, at the end of leaching, the concentration of Zn, Cd, Cu and Pb was kept at a stable level instead of 0, which suggested that the leaching of Zn, Cd, Cu and Pb from the residue was a long-term process and the environmental effect was accumulated.
3.1.6 Possible factors affecting release of heavy metals
The release of heavy metals of zinc leaching residue was mainly influenced by the occurrence of themselves, natural weathering, microbial activities and galvanic effect [38].
1) The occurrence of heavy metals
The study of the weathering sequence by GOLDICH indicated that the stability of minerals was related to their lattice energy [39]. Heavy metals in host minerals having higher lattice energy would be more stable in the residue. These minerals would not be decomposed and would remain in the leaching residue. However, with the release of other components such as clay minerals, these metals could easily be enriched in the residual. In other words, when the lattice energy got smaller, e.g., the sulfides were oxidized into sulphates, the crystal lattices of sulfides will break down immediately. Heavy metals would be released from the residue and entered into the environment.
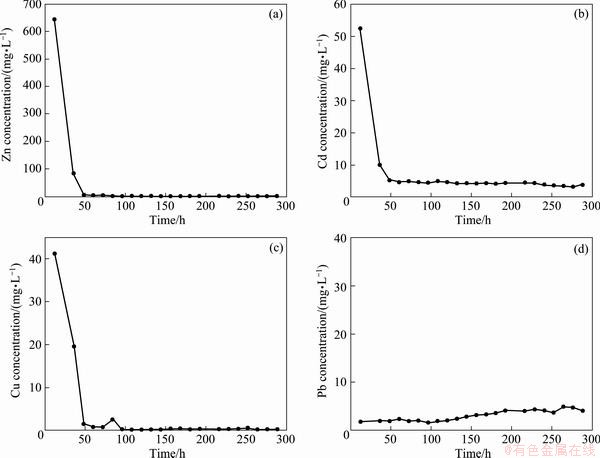
Fig. 5 Effect of leaching time on concentration of different heavy metals
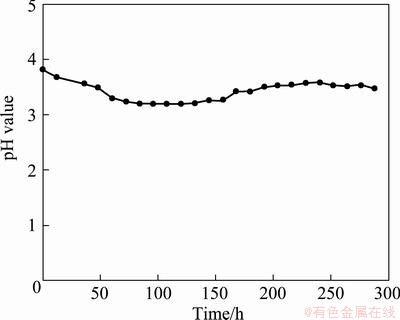
Fig. 6 Effect of leaching time on pH
In addition, chemical form was also one of the most important factors that characterized the occurrence of heavy metals. Different forms of heavy metals suggested different mechanisms of availability. In the “exchangeable fraction”, the heavy metals were specifically adsorbed and were likely to be released when the ionic composition of water was changed. In the “carbonate fraction” the metals were precipitated or co-precipitated and were susceptible to pH change. In the “Fe-Mn oxides”, the metals were unstable under reduction conditions. Under oxidizing conditions, organic matter can be degraded to result in the release of soluble metals. Metals in the “residual fraction” were held in the crystal structures of some primary and secondary minerals. It was reasonable to postulate that these metals cannot be released under conditions normally encountered in nature.
2) Natural weathering
Natural weathering conditions may cause exposed residues to small clay-size particles. Through this process, massive amount of fine particles can then be released to the environment within few weeks. Furthermore, with the help of acid rain, the acidification of acid precipitation will promote the release of heavy metals, such as Cd, Cu, Pb and Zn, and the acid exchangeable components of these metals in especial.
3) Microbial activities and galvanic effect
With the help of leaching and supersession of autotrophic and heterotrophic microorganisms, the environmental activity and toxicity of the reducible and oxidizable forms of heavy metals will probably go up. And the same effect could be performed by the chelation and methylation of ferroprotein [40,41]. The rate of oxidation of some sulfide minerals will be accelerated by galvanic effect [42], and the presence of coke can further strengthen the galvanic effect [43].
3.2 Potential ecological risk assessment results of heavy metals
The results of chemical speciation of heavy metals (Fig. 4) and the release of heavy metals under acidic conditions (Fig. 5) indicated that the environmental activities of heavy metals depended mostly on the occurrence of themselves. Researches showed that the exchangeable form of heavy metals was the direct phase that polluted the environment [28-30]. Therefore, it is unadvisable to evaluate the potential ecological risk by the total contents of heavy metals in the residue. And studies showed that the potential ecological risks would be exaggerated if it was evaluated by the mass fraction of heavy metals instead of the mass fraction of the effective state [19]. As a result, the potential ecological risk indexes of heavy metals were calculated by the fraction of acid exchangeable forms of heavy metals in this paper (Table 9). Potential ecological risk indices and potential toxicity response of heavy metals in the residue were shown in Table 10.
Table 10 Potential ecological risk assessment results of heavy metals in residue
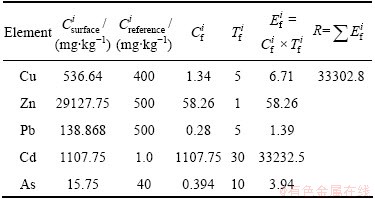
According to the calculation results (Table 9), the content of Pb was the lowest (138.868 mg/kg), which did not exceed the upper limits of the three standards for soil (500 mg/kg). The content of As was also low (15.75 mg/kg), which did not exceed the reference values. The content of Cu was not high too, comparing with the reference value. The contents of Zn and Cd exceeded the reference values, which were 58.26 and 1107.75 times of the three standards for soil. The above results showed that, Zn and Cd had the highest single pollution index of heavy metals in the residue. The single pollution indices of Cu, Pb and As were slight. So the pollution of Cu, Pb and As was low, while the pollution of Zn and Cd was serious.
In Table 9, the potential ecological risk indices of Cu, Pb and As were lower than 40, indicating slight potential ecological risk of all three metals in the residue. For its high content of the exchangeable forms of Zn, the potential ecological risk index was 58.26, which indicated the moderate potential ecological risk of Zn in the residue. The main element causing ecological hazards was Cd with 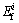 of 33232.5, which would have serious ecological risk. According to the evaluating standard, heavy metals in the zinc leaching residue had high potential ecological risk level with R>320.
of 33232.5, which would have serious ecological risk. According to the evaluating standard, heavy metals in the zinc leaching residue had high potential ecological risk level with R>320.
4 Conclusions
1) The residue was acidic with pH of 3.80. The main metallic elements in the residue were Fe, Zn, Pb, Cu and Cd and so on. Carphosiderite, zinc sulfite hydrate and anglesite were the main minerals of the residue. Pb and Cu mainly existed in residual forms, while Zn and Cd mainly existed in acid exchangeable forms in the residue.
2) This residue had high environment toxicity according to the leaching toxicity test, and the leaching toxicities of Zn, Cd and Pb were all higher than Identification Standards for Hazardous Wastes— Identification for extraction toxicity (GB 5085.3—2007). Long-term leaching tests showed that this kind of waste on the environment was a cumulative effect, and will cause great harm to the surrounding environment. Heavy metals in the residue would be leached out due to weathering, acid rain, microbial action and galvanic effect and other effects, which provides the theoretical basis for the reference of harmless and resources recycling technologies of valuable metals.
3) The environmental activity of the heavy metals declined in the following order: Cd>Zn>Cu>As>Pb, according to the leaching effect and occurrence of themselves.
4) Potential ecological risk indices for heavy metals were Cd>Zn>Cu>As>Pb. Cd had serious potential ecological risk to the ecological environment and contributed most to potential toxicity response indices for various heavy metals (R>320) in the residue.
References
[1] TURAN M D, ALTUNDOGAN H S, TUMEN F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75: 169-176.
[2] RAGHAVAN R, MOHANAN P K , PATNAIK S C. Innovative processing technique to produce zinc concentrate from zinc leach residue with simultaneous recovery of lead and silver [J]. Hydrometallurgy, 1998, 48: 225-237.
[3] ABDEL-AAL E A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore [J]. Hydrometallurgy, 2000, 55: 247-254.
[4] YE Z H, SHU W H, ZHANG Z Q, LAN C Y, WONG M H. Evaluation of major constraints to revegetation of lead/zinc mine tailings using bioassay techniques [J]. Chemosphere, 2002, 47: 1103-1111.
[5] GUO Jian-pin, WU Fu-chen, XIE Shu-rong, YAO Ling-ling, XIE Yu-hua. Environmental conditions and exploitation of lead-zinc tailings in Linxiang County, Hunan Province [J]. Chinese Journal of Soil Science, 2007, 38(3): 553-557. (in Chinese)
[6] WANG Shao-hua, YANG Jie, LIU Shu-ming, XU Zhao-wen, LU Xian-cai, WANG Bing, WANG Hao, BAI Kang-chen. Environmental effects of heavy metal elements release in Yangshanchong tailing pool, Shizishan, Tongling, Anhui Province [J]. Geological Journal of China Universities, 2011, 17(1): 93-100. (in Chinese)
[7] LAUREL A S, DAVID B S, DANIEL J B, KATHLEED D M, JAMES P S. Characterization of zinc, lead, and cadmium in mine waste: Implication for transport, exposure and bioavilibility [J]. Environmental Science and Technology, 2007, 41: 4164-4171.
[8] NAVARRO M C, SIRVENT C P, SANCHEZ M J, VIDAL J, TOVAR P J, BECH J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone [J]. Journal of Geochemical Exploration, 2008, 96: 183-193.
[9] DANG Z, LIU C Q, HAIGH M J. Mobility of heavy metals associated with the natural weathering of coal mine spoils [J]. Environmental Pollution, 2002, 118: 419-426.
[10] TSIRIDIS V, SAMARAS P, KUNGOLOS A, SAKELLARO- POULOS G P. Application of leaching tests for toxicity evaluation of coal fly ash [J]. Environmental Toxicity, 2006, 21(4): 409-416.
[11] KAZI T G, JAMALI M K, KAZI G H, ARAIN M B. Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test [J]. Analytical and Bioanalytical Chemistry, 2005, 383: 297-304.
[12] WONG J W C, LI K, FANG M, SU D C. Toxicity evaluation of sewage sludges in Hong Kong [J]. Environment Internationnal, 2001, 27: 273-380.
[13] WANG Lan, LIU Fang, WANG Jian, LI Jin-juan. Release characteristic of heavy metals in Pb/Zn tailing under acid leaching and the effects of leachate on plant seedings growth [J]. Chinese Journal of Ecological, 2010, 29(6): 1121-1126. (in Chinese)
[14] HAKANSON L. An ecological risk index for aquatic pollution control—A sedimentological approach [J]. Water Reserch, 1980, 14(1): 975-1001.
[15] FAN Wei-hua, BAI Zhong-ke, LI Hui-feng, QIAO Jun-wei, LI Xia. Potential ecological risk of heavy metals in the reclaimed soils [J]. Transcation of the Chinese Scoiety of Agriculture Engineering, 2011, 27(1): 123-130. (in Chinese)
[16] DELGADO J, BARBA-BRIOSO C, NIETO J M, BOSKI T. Speciation and ecological risk of toxic elements in the estuarine sediments affected by multiple anthropogenic contributions (Guadiana salt marshes, SW Iberian Penins): Surfical sediments [J]. Science of the Total Environmrent, 2011, 409(19): 3666-3679.
[17] KOU Shi-wei, CAI Su-ying, ZHANG Bo, HE Jia-min, HUANG Shi-yuan, ZHENG Jie-hui, YI Ru-han. Speciation dietribution and potential ecological risk assessment of Cd and Pb in Yunfu Pyrite area [J]. Journal of Jinan Uinversity: Natural Science, 2011, 32(1): 48-52. (in Chinese)
[18] PUEYO M, MATEU J, RIGOL A, VIDAL M, RAURET G. Use of modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils [J]. Environmental Pollution, 2008, 2(152): 330-341.
[19] HJ/T299-2007. Soild waste—Extraction procedure for leaching toxicity-Sulphuric acid & nitric acid method [S].
[20] American national standard measurements of the leachability of solidified low-level radioactive wastes by a short-term test procedure [S]. ANSI/ANS, 16.1. La Grange Park, Illinois: American Nuclear Society.
[21] PATRA A C, SUMESH C G., MOHAPATRA S, SAHOO S K, TRIPATHI R M, PURANIK V D. Long-term leaching of uranium from different waste matrices [J]. Journal of Environmental Management, 2011, 92: 919-925.
[22] DAVIDSON C M, DUNCAN A L, LITTLEJOHN D, ALLAN M U, LOUISE M G. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land [J]. Analytical Chimica Acta, 1998, 363: 45-55.
[23] MUKHERJEE D, MUKHERJEE A, KUMAR B. Chemical fraction of metals in freshly deposited marine estuarine sediments ecosystem, India [J]. Earth and Environmental Science, 2009, 58(8): 1757-1767.
[24] XIAO Hua-yun, JIANG Shui-ying, WU Dai-she, ZHOU Wen-bin. Risk element (As, Cd, Cu, Pb and Zn) contamination of soils and edible vegetables in vicinity of Guixi Smelter, South China [J]. Soil and Sediment Contamination: An International Journal, 2011, 20(2): 592-604. (in Chinese)
[25] JIANG Yi-min, ZENG Guang-ming, ZHANG Xian-bao, LIN Yu-peng. Main features of acid rain and controlling and preventing strategies in Hunan Province [J]. Journal of Hunan University: Natural Science, 200l, 28(3): 78-83. (in Chinese)
[26] RIEUWERTS J S, FARAGO M E. CIKRT M, VLADIMIR B. Differences in lead bioavailability between a smelting and a mining area [J]. Water, Air, & Soil Pollution, 2000, 122: 203-229.
[27] XIAN X, SHOKOHIFARD G I. Effect of pH on chemical forms and plant availability of cadmium, zinc and lead in pollution soils [J]. Water, Air, & Soil Pollution, 1989, 45: 265-273.
[28] KAI T, SUENAGA Y, MIGITA A, TAKAHASHI T. Kinetic model for simultaneous leaching of zinc sulfide and manganese dioxide in the presence of iron-oxidizing bacteria [J]. Chemical Engineering Science, 2000, 55: 3429-3436.
[29] GUO Zhao-hui, CHENG Yi, CHAI Li-yuan, SONG Jie. Mineralogical characteristics and environmental availability of non-ferrous slag [J]. Journal of Central South University: Science and Technology, 2007, 38(6): 1100-1105. (in Chinese)
[30] WONG S C, LI X D, ZHANG G, QI S H, MIN Y S. Heavy metals in agricultural soils of the Pearl River Delta, South China [J]. Environmental Pollution, 2002, 199(1): 33-44.
[31] MIRAGAYA J G, SOSA A M. Trace elements in the valencia lake (Venezuela) sediments [J]. Water, Air, & Soil Pollution, 1994, 77: 141-150.
[32] MARGFIY E, QUERALT I, CARVALHO M L. Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features [J]. Environment Pollution, 2007, 145(1): l79-184.
[33] WU Pan, LIU Cong-qiang, YANG Yuan-gen, ZHANG Guo-ping. Environmental impacts and geochemical partitioning of heavy metals (Pb, Zn) in the historical Zn smelting wastes [J]. Geochemical, 2003, 32(2): 139-145. (in Chinese)
[34] SINGH S P, TACK F M, VERLOO M G. Heavy metal fraction and extractability in dredged sediment derived [J]. Water, Air, & Soil Pollution, 1996, 87: 313-328.
[35] CLEVENGER T E. Use of sequential extraction to evaluate the heavy metals in mining wastes [J]. Water, Air, & Soil Pollution, 1990, 50: 241.
[36] GEE C, RAMSEY M H, MASKALL J. Mineralogy and weathering processes in historical smelting slags and their effect on the mobilization of lead [J]. Journal of Geochemical Exploration, 1997, 58(2-3): 249-257.
[37] LAURENT D W, RABIA B. Modelling of long-term dynamic leaching tests applied to solidified/stabilised waste [J]. Waste Management, 2007, 27: 1638-1647.
[38] AI-ABED S R, HAGEMAN P L, JEGADEESAN G. Comparative evaluation of short-term leach tests for heavy metal release from mineral processing waste [J]. Science of the Total Environment, 2006, 364(1-3): 14-23.
[39] GOLDISH S S. A study on rock weathering [J]. Journal of Geology, 1938, 46: 17-58.
[40] GADD G M. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization [J]. Current Opinion in Biotechnology, 2000, 11(1): 271-279.
[41] KLAUS B. Bioleaching: Metal solubilization by microorganisms [J]. FEMS Microbiology Reviews, 1997, 20(3-4): 591-604.
[42] YANG Xian-wan. Bio-hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2003. (in Chinese)
[43] LI Hong-xu, QIU Guan-zhou, HU Yue-hua. Galvanic effect on mixed sulfide bioleaching [J]. The Chinese Journal of Nonferrous Metals, 2003, l3(5): 1283-1287. (in Chinese).
锌浸出渣中重金属的环境活性和生态风险评价
闵小波,谢先德,柴立元,梁彦杰,李 密,柯 勇
中南大学 冶金科学与工程学院 环境工程研究所,长沙 410083
摘 要:采用矿物学分析、BCR三步连续浸提、动态淋洗实验以及Hakanson潜在生态风险评价4种方法对锌浸出渣重金属的环境活性以及生态风险进行评价。结果表明,锌浸出渣中重金属的环境活性大小依次为Cd>Zn>Cu>As>Pb。废渣中主要重金属的潜在生态风险评价表明,该种废渣对环境具有很高的生态风险,单个重金属的生态危害顺序为Cd>Zn>Cu>As>Pb。Cd有很严重的生态风险,是对生态环境造成毒性的主要原因。
关键词:重金属;BCR连续浸提;环境活性;浸出毒性;动态淋洗;生态危害指数法(PERI);锌浸出渣
(Edited by Sai-qian YUAN)
Foundation item: Project (50925417) supported by the National Natural Science Funds for Distinguished Young Scholar of China; Project (2010AA065203) supported by the High Technology Research and Development Program of China; Project (2010-609) Supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education, China; Project (ncet-10-0840) supported by Program for New Century Excellent Talents in University; Project (2012FJ1080) supported by Key Projects of Science and Technology of Hunan Province, China
Corresponding author: Xiao-bo MIN; Tel: +86-731-88830577; Fax: +86-731-88710171; E-mail: mxb@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62448-6
Abstract: Four different methods, namely mineralogical analysis, three-stage BCR sequential extraction procedure, dynamic leaching test and Hakanson Potential Ecological Risk Index Method were used to access the environmental activity and potential ecological risks of heavy metals in zinc leaching residue. The results demonstrate that the environmental activit y of heavy metals declines in the following order: Cd>Zn>Cu>As>Pb. Potential ecological risk indices for single heavy metal are Cd>Zn>Cu>As>Pb. Cd has serious potential ecological risk to the ecological environment and contributes most to the potential toxicity response indices for various heavy metals in the residue.


