网络首发时间: 2017-03-29 13:56
稀有金属2018年第4期
MoS2纳米片在催化及生物领域的应用研究进展
范培栋 嵇天浩
北京工商大学理学院
摘 要:
随着纳米材料的研究逐渐深入, 具有类石墨烯结构的二维纳米材料, 如MoS2纳米片, 已经成为被广泛关注的研究热点。本文围绕MoS2纳米片在很多领域的具体应用研究, 从简单介绍MoS2纳米片的结构和主要合成方法开始, 重点论述了MoS2纳米片及其相关材料的四个应用研究成果。首先, 作为催化剂, 与MoS2纳米片相关的材料在光或电催化析氢反应和催化氧化还原反应等方面的应用研究进展被详细阐述, 其中涉及的与MoS2纳米片复合的材料主要有还原氧化石墨烯、碳纳米管、Ti O2纳米粒子、金属纳米粒子、半导体纳米粒子等;然后, 详细介绍了与MoS2纳米片相关的材料在生物传感器和医药领域的应用研究进展。在生物传感器方面, 人们发现MoS2纳米片对生物分子有较灵敏的响应;在医药领域方面, 人们发现MoS2纳米片可以作为光吸收剂或载体得到应用。最后, 对这种材料在未来的研究探索和可能发展提出了展望。
关键词:
应用研究;MoS2纳米片;纳米复合材料;二维材料;
中图分类号: TB383.1
作者简介:范培栋 (1994-) , 男, 石家庄人, 硕士研究生, 研究方向:纳米功能材料的制备与应用;E-mail:904644402@qq.com;;嵇天浩, 教授;电话:010-68985545;E-mail:tianji66@126.com;
收稿日期:2017-02-19
基金:国家“十二五支撑”计划项目 (No 2012BAJ02B08) 资助;
Application Research of MoS2 Nanosheets in Catalysis and Biology
Fan Peidong Ji Tianhao
College of Science, Beijing Technology and Business University
Abstract:
With the research development of nanomaterials, graphene-like 2D materials, such as molybdenum disulfide (MoS2) nanosheets, have received extensive attention.In this review, MoS2-nanosheet structure and preparation methods were first simply introduced, and subsequently four application researches of MoS2 nanosheets and related composite were emphatically elucidated.First of all, MoS2 nanosheets and related composite as catalysts in the fields of catalytic hydrogen evolution reaction and catalytic oxygen reduction reaction were presented in detail.In the composite, other materials combined with MoS2 nanosheets mainly included reduced graphene oxide, carbon nantube, TiO2 nanoparticles, metal nanoparticles or semiconductor nanoparticles.Then, MoS2 nanosheets and related composite as biosensors and biomedical materials were also described in detail.MoS2 nanosheets as biosensors could be applied in a sensitive test of biomolecules, and also as biomedical materials for their application in medical support or light absorber.In the last, the applications and some problems of such nanosheets in future research works and other possible developments were prospected.
Keyword:
application research; MoS2 nanosheets; nanocomposites; 2D materials;
Received: 2017-02-19
近10年来, 具有二维片状结构的石墨烯及类石墨烯材料受到了人们广泛关注。二硫化钼 (Mo S2) 纳米片是一种类石墨烯材料, 由人工合成制得, 而其前驱物则是在自然界中存在的具有片层结构的块材Mo S2, 其主要存在形式是辉钼矿, 该晶体有不同种晶型, 分别属于六方或三方晶系, 具有金属光泽, 且呈现叶片或鳞片形状。
这种Mo S2矿是由单层Mo S2纳米片通过范德华力相互作用叠加而成, 而单层Mo S2纳米片又是由三层原子层构成, 外面两层为硫原子, 中间夹有一层钼原子 (S-Mo-S) [1,2]。由于范德华力的弱相互作用, 使得层与层间很容易滑移或分离[3], 因而导致这种块材Mo S2的摩擦系数很小, 具有良好的润滑性能[4]。同时, 又由于Mo S2本身性质, 决定其在光学、电子学或电化学领域有一定程度的应用。为了扩大该材料的应用范围, Mo S2研究转向了纳米尺寸方向[5]。当Mo S2的尺寸达到纳米范围时, 会出现其宏观材料所不具备的特有性质, 如小尺寸效应、表面效应或量子尺寸效应等, 因此, 纳米Mo S2的应用性能主要与其自身的尺寸和形状有关[5,6]。
对纳米Mo S2形貌的研究始于1993年。在当时已经制备出具有富勒烯结构的碳纳米材料之后, 科研人员又采用各种合成方法, 如水热法、化学气相沉积法或气固合成法等不同制备手段, 获得了不同形貌, 如球状、棒状、多面体状或片状等的纳米Mo S2[5]。因为最近石墨烯受到了广泛而特别的关注, 所以作为类石墨烯材料的Mo S2纳米片也受到了足够的重视。当然, 这也与它独特“三明治”结构、良好光电性能、特定催化性能以及大比表面积等密切相关。与石墨烯的零或接近零的能带隙相比, Mo S2纳米片有可部分调控的能带隙, 因而在光电器件领域有着更大的优势。在电子设备领域, 相比于常见的硅半导体材料, Mo S2纳米片的制备成本更低, 有可能获得性能更高的电子芯片, 因而它也是一种提升电子器件性能的潜在电子材料。
Mo S2纳米片因其具有可调变的能隙和非凡的光学或电学性能, 以及自身的低毒性、高热稳定性和高化学耐蚀性等优良性能, 必将吸引越来越多的研究者从事相关领域的研究, 也将在电化学、光学、催化、纳米电子学或者生物和医学等领域获得更广泛应用。正是基于其美好的应用前景, 本文在简单介绍制备方法之后, 从光或电催化析氢、氧化还原催化、生物传感器和生物医疗等四个方面, 对Mo S2纳米片的研究和应用现状做出了较详细的综述和总结, 并对Mo S2纳米片在未来的潜在应用前景进行了展望。
1 Mo S2纳米片的制备
目前, 制备纳米Mo S2纳米片的合成方法主要有:机械剥离法、液相剥离法、锂插层法、水热法等[7]。机械剥离法是由Geim等于2005年最先报道的, 制备的纳米片包括单层石墨烯、氮化硼以及Mo S2等材料[8];Zhang等改进和优化了该方法, 并制得了Mo S2纳米片光电晶体管[9]。液相剥离法是由Coleman等[10]在液相剥离石墨烯的研究基础上所提出, 他们对制备过程进行了改进, 以不同类型的有机溶剂作为液相, 从而获得单层或少层的高品质Mo S2纳米片。对于锂插层法, 早在1986年就由Joensen等[11]所报道, 后来又由Rao等在2010年进行改进后成功制备了1~3层Mo S2纳米片[12];随后在2011年, Zeng等[13]对该方法做了优化处理, 引入电化学方法制得单层Mo S2纳米片;同年, Eda等[14]通过Li嵌入法大量剥离块材Mo S2制得单层Mo S2纳米片, 可是该方法却改变了Mo S2结构, 使得半导体性能有明显下降, 但同时他们也证实, 使用正丁基锂和己烷制得的Mo S2纳米片, 其电学性能基本上没有损失。在2004和2009年, Li等[15]和Ma等[16]分别报道了采用水热化学反应和离子液体的制备过程, 获得多种形貌的Mo S2纳米材料。
2 Mo S2纳米片的应用
作为在许多领域有潜在应用价值的一类纳米材料, 类石墨烯片状材料已经受到了广泛重视和深入研究[17,18]。众所周知, 石墨烯具有极高载流子迁移率以及超强和超韧性能, 但它的缺点和不足也很明显, 即它的带隙是零或接近零, 这就限制并影响了它在光电领域的具体应用;而Mo S2纳米片可在一定程度上弥补石墨烯的不足, 其单层纳米片是带隙约为1.9 e V的直接带隙半导体材料[19]。当然, 通过控制Mo S2纳米片的层数, 或者对纳米片掺杂或吸附其他物质[20,21], 也可以改变其光电性质。例如, Lee等[22]经实验研究证实, 若调节Mo S2纳米片层数, 其带隙、电子迁移率和光学性能等都能发生改变, 其中单层或双层结构Mo S2纳米片有较好的电子迁移率和不饱和电流输出, 对绿光较敏感, 而三层结构Mo S2则呈现电性能下降现象, 但对红光则表现出优秀的光电转换性能。由此可见, 通过对Mo S2层数控制, 可以应用在对不同波长光有一定响应的光电材料中。另外, 由于Mo S2纳米片具有大比表面积且其双面含有S原子层, 有利于与部分有机分子、金属离子或无机纳米材料复合, 形成多功能复合材料。针对Mo S2纳米片所具有的这些特性, 下面将从催化析氢反应、催化氧化还原反应、生物传感器和生物医用材料等四方面应用进行详细综述。
2.1催化析氢反应
太阳能是各种可再生能源中最重要的基本能源之一, 也是最充足的可利用能源, 可以说是“取之不尽, 用之不竭”, 但目前如何利用太阳能已成为人们需要面对的主要问题。现在利用太阳能的主要形式是将其存储或转化, 其中光催化分解水析H2就是太阳能的重要应用领域, 但实际上仍存在可见光利用率低或反应效率小等问题。
Mo S2纳米片在可见光范围内有较强吸收能力, 是一种有价值的光解水析H2材料。由于这种材料的电子-空穴对分离程度偏小以及有效利用的电子数量相对偏少, 它本身对水的光解作用很弱, 只有在与其他材料复合形成了复合材料后, 它才能展现出高光解水作用[23,24,25]。在2010年, Splendiani等[26]发现Mo S2单层纳米片在可见光范围内有光致发光现象, 同时他们也证实, 发光强度随层数减少而增加, 且单层时最强。利用它对可见光的有效响应及相对较高的边缘电位, 在与被选择的金属或半导体纳米材料复合后, 就可以催化应用在可见光光解水析H2上了。
在2012年, Min和Lu[27]通过对比石墨、Au、碳纸或碳纳米管等多种材料之后发现, 还原氧化石墨烯 (RGO) 作为一种二维碳结构纳米材料可以成为Mo S2纳米片的理想载体。这种Mo S2-RGO纳米复合材料表现出在460 nm可见光下, 量子有效利用率高达24%的高活性光催化性能, 有望代替光解水中所使用的贵金属;同年, Xiang等[28,29]使用一种新型光催化剂, 即将Ti O2纳米粒子沉积在Mo S2和RGO组装而成的纳米片上所制成的复合催化剂, 对光解水性能进行了测试, 结果表明该复合材料具有较高的光催化活性。在2013年, Bernardi等[30]研究了由Mo S2和石墨烯堆叠而成的约1 nm厚太阳能电池的可行性, 发现厚度小于1 nm的Mo S2纳米片可吸收5%~10%的入射太阳光, 吸收量比半导体Ga As或Si还要高出一个数量级。在2014年, Yang等[31]研究了Cr和Ag分别负载到Mo S2纳米片上形成的共催化剂的光解水性能, 发现这种异质结构催化剂能够有效减少电子-空穴对复合, 使产氢量远高于纯Mo S2纳米片;同年, Zhang等[32]采用溶剂热法制备了p-n异质结构的Cd S纳米粒子沉积Mo S2纳米片, 这种光催化剂在可见光范围内有较好吸收, 可见光下的产氢率已达到137 mmol·h-1, 在450 nm波长下的表观量子效率为10.5%。
除了Mo S2纳米片及其复合纳米材料在可见光下表现出良好的光催化光解水析氢性能以外, 它们也具有良好的电催化分解水析氢性能。在2013年, Chang等[33]在Ni泡沫的表面负载上石墨烯, 以便提高泡沫材料的稳定性, 再将催化剂Mo S2纳米片沉积在上面, 形成3D多孔电催化析氢结构的复合材料, 其SEM照片如图1所示。在0.2 V电压作用下, 析氢速率可达302 ml·g-1·cm-2·h-1。纳米级的Mo S2和WS2无机催化剂相较于传统催化剂成本更低, 化学性质更稳定, 并且有优良的光催化和电催化性能。同年, Yan等[34]通过溶剂热法制得了Mo S2/CNT纳米复合材料, 其电镜照片如图2所示。为了促进Mo S2纳米片在碳纳米管 (CNT) 上的有效生长, 在制备过程中他们使用了葡萄糖或L-半胱氨酸, 同时在制备复合物之前, 需要对CNT进行酸处理。实验结果表明, 在复合催化剂中, 低结晶度Mo S2有利于提高析氢反应效率。这一结果也说明, Mo S2纳米片上的表面缺陷可增加析氢催化反应的活性位点, 进而提高析氢反应效率。
2.2催化氧化还原反应
随着社会对能源需求不断增大, 太阳能电池、锂离子电池或燃料电池等能源存储转换设备一直受到人们广泛关注和大量研究[35,36]。其中, 燃料电池是一种将化学能转化成电能的发电装置, 而氧化还原反应 (ORR) 是燃料电池中提供电能的核心反应, 因而是研究和应用时重点考虑的对象[37,38]。
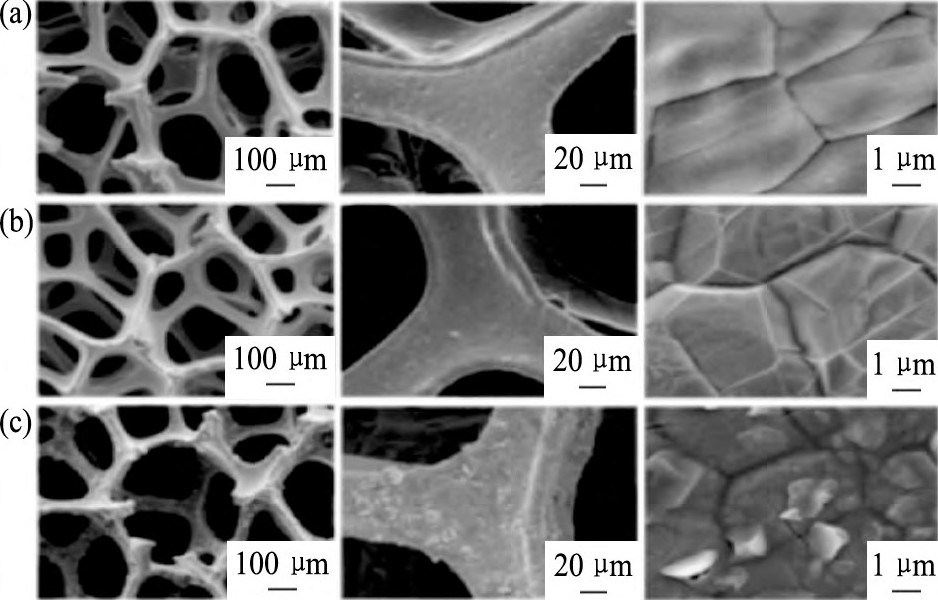
图1 样品的SEM照片Fig.1 SEM images of samples[33]
(a) As-obtained Ni foam; (b) Ni foam surfaces with graphene layers; (c) Graphene-protected Ni foam with Mo Sxnanosheets
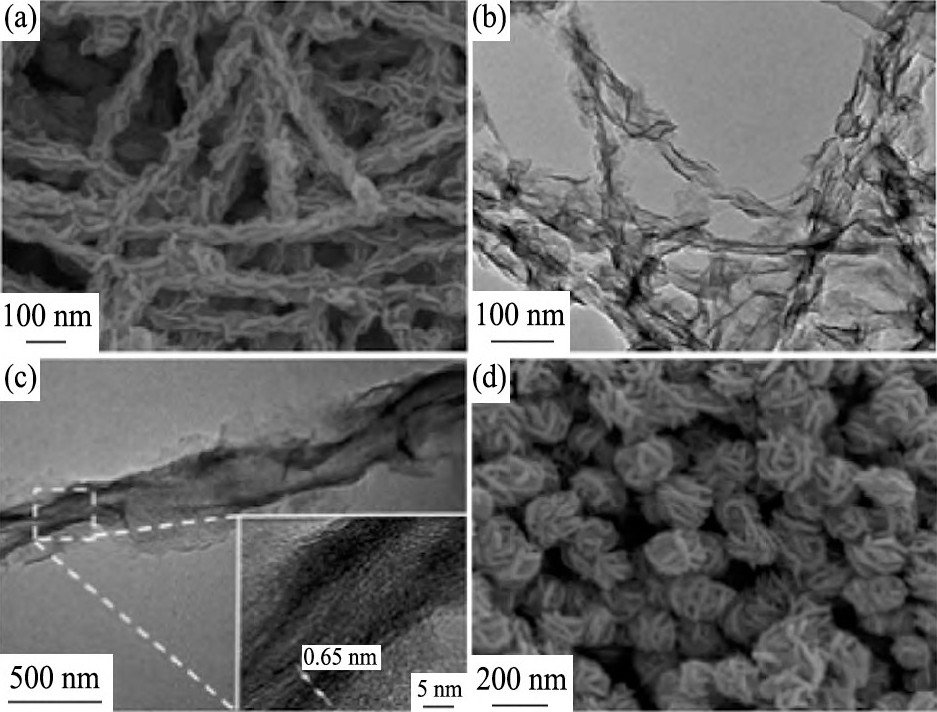
图2 样品的电镜照片Fig.2 SEM and TEM images of samples[34]
(a) FESEM image of Mo S2/CNTs; (b) and (c) TEM images of Mo S2/CNTs (Inset showing a HRTEM image of Mo S2nanisheets) ; (d) FESEM image of Mo S2nanospheres without CNTs
与惰性石墨烯或BN相似[39,40], 通过在Mo S2纳米片的结构中掺杂原子可以增加其活性位点, 提高其ORR的催化活性。Xiao等[41]制备出了Co或Ni原子掺杂的Mo S2纳米片, 经实验测试结果证实, 这种掺杂材料可以作为有效催化剂在电催化ORR中发挥作用, 主要是因为掺杂Mo S2能够有效吸附氧气。在研究中发现, Co-Mo S2的电催化活性低于Fe掺杂石墨烯, 但在碱性介质中, Co-Mo S2则表现出了优越的电催化活性, 而Ni-Mo S2的活性与Co掺杂石墨烯基本相同。Wang等[42]使用涂布法在Au膜上制备了Mo S2纳米粒子沉积的复合材料, 测试结果证实, 该种材料表现出极好的ORR催化活性, 因此这也预示Mo S2纳米片沉积的Au/Mo S2也应具有相近的电催化活性。最近, Zhao等[43]采用超声波法简单制得氮掺杂石墨烯 (NG) 与Mo S2纳米片复合而成的廉价ORR电催化剂, 其电镜照片如图3所示。经实验测试结果证实, 这种纳米片复合材料展现优良ORR催化能力, 其中NG在催化过程中发挥了重要作用, 它既是Mo S2的载体, 同时也起到降低电阻的作用, 因而使得ORR电催化活性得到显著提升。由此可见, Mo S2纳米片与其他材料恰当而有效复合, 不仅可以替代燃料电池中所使用的昂贵ORR催化剂, 大大降低燃料电池的成本, 而且还拓展和深化了Mo S2纳米片在能量存储和转换领域的应用。

图3 氮掺杂石墨烯 (NG) , Mo S2纳米片和Mo S2/NG的电镜照片Fig.3SEM and TEM images of NG, Mo S2nanosheets and Mo S2/NG[45]
(a, c) SEM images for NG, Mo S2nanosheets and Mo S2/NG, respectively; (b, d) TEM images for NG, Mo S2nanosheets and Mo S2/NG, respectively
2.3生物传感器
生物传感器是指对生物物质敏感, 且能通过特定的响应而对其进行检测的装置。由于Mo S2纳米片具有超大比表面积的二维结构以及优良的导电性、热或化学稳定性和生物相容性等特性, 它在生物传感器上展现出美好的应用前景。
在2013年, Zhu等[44]首次发现Mo S2单层纳米片对单链和双链DNA分子有不同的作用力, 因此他们利用Mo S2纳米片的荧光淬灭效应组装了DNA传感器, 实现了简单而低成本的DNA分子诊断。随后, 依Mo S2传感器对不同生物分子的检测研究和报道纷至沓来。在2016年, Kim等[45]在以L-半胱氨酸修饰的金电极表面, 沉积上Mo S2-石墨烯 (MG) 复合材料, 进而制得依靠酶联免疫吸附法检测的传感器电极, 通过氧化还原循环伏安测试可以检测甲状旁腺激素 (PTH) 和碱性磷酸酶 (ALP) 的微含量。这种MG传感器表现出很高的灵敏度和重复性, 可对人体血清中浓度在1~50 pg·ml-1的PTH进行定量分析。同年, Chekin等[46]采用一种简单快捷方法获得了新型Mo S2-RGO杂化复合材料, 并将其负载在玻碳电极表面, 在对叶酸检测中显示出很高的灵敏度;同时, 他们使用这种电极传感器对人体血清中的叶酸含量进行了检测, 结果表明, 该传感器不仅灵敏度较高, 而且检测数据可靠。在2017年, Parlak等[47]使用Au纳米粒子负载Mo S2纳米片复合材料, 对葡萄糖分子进行了电化学生物传感检测, 其中的Au纳米粒子主要起到提供电子作用, 测试结果表明, 这种复合材料对分析检测葡萄糖分子有着简单、低价和敏感高等优点。
2.4生物医用材料
生物医用材料又简称生物材料, 是用来对生物体进行诊断、治疗、修复或替代其病损组织、器官或增强其功能的材料, 因而无论是用于诊断或检测人体病灶的分析剂或传感器, 还是治疗人体病灶的药物或药物载体, 都是生物医用材料。Mo S2纳米片及其相关的复合材料就可以当作生物医用材料应用在人体病灶的治疗上。
目前, 人们已经开始关注和应用副作用小、治疗效果好的微创治疗方法, 例如近红外光吸收的光热治疗法 (PTT) [48]。与可见光相比, 近红外光的有效穿透性更强, 能量损失更小。通过光吸收剂对近红外光吸收并转变为热能, 能够起到烧死肿瘤细胞的作用。有研究表明, 无论是Mo S2纳米粒子或纳米管[49,50], 还是Mo S2纳米片[51,52], 对人体基本上无毒, 且与人体组织的相容性也较好, 他们通过对人体唾液腺细胞或人体肺腺癌细胞的毒性检查分析发现, 这些Mo S2纳米材料经过长时间使用后, 细胞的存活率仍然较高。Chou等[53]因而将Mo S2纳米片应用在人体上, 他们报道, Mo S2纳米片可以作为近红外光吸收剂应用在光热转换和药物运输上, 但是在PTT治疗过程中, 只有微量的Mo S2纳米片累积在肿瘤组织上, 而大量纳米片在经血管到达肝、脾或肺等器官时, 危害到了这些器官的安全。因此, 在减少副作用、增大对肿瘤组织的靶向性或增强光热转换效率等方面还有许多研究工作需要进行详细而深入探索。
Mo S2纳米片在作为红外光吸收剂的同时, 还可以兼作药物载体。例如, Wang等[54]采用简单混合制备过程获得了乳酸-羟基乙酸共聚物 (PLGA) 包覆Mo S2纳米片和阿霉素的油溶胶复合物, 其结构如图4所示。Mo S2纳米片的作用既是光吸收剂也是药物载体。这种复合物的制备过程如下:先将PLGA、Mo S2纳米片和阿霉素加入到甲基吡咯烷酮 (NMP) 溶剂中进行混合, 再不断搅拌, 进而将Mo S2和阿霉素包覆在聚 (乳酸-乙醇酸) 中形成固体复合材料。这种结构的复合物不仅有利于降低药物输运时的损失和对其他器官的毒副作用, 而且也提高了对病灶的综合治疗效果, 因而在肿瘤治疗上有较大的潜在应用价值。

图4 样品的结构表征Fig.4FESEM image of structural characterizations of PLGA sample[54]
3展望
作为一种新兴的类石墨烯无机材料, Mo S2纳米片以及相关纳米材料已经受到越来越多研究者关注。其原因是由于单层或少数层Mo S2纳米片既具有超大比表面积、热或化学稳定性、生物相容性等性质, 同时也具有优良的光学、电学、力学等性能, 因而这种材料在上面所述的多个领域有着广泛的应用。然而, 相关研究工作只是刚刚开始, 还需要在以上所述的4个领域研究基础上, 深入细致地进行更广泛探索, 以便尽快实现产业化;与此同时, 也需要拓展这种材料在其他领域的新颖应用, 如探讨有机分子对Mo S2纳米片的共价修饰及其应用[55]、纳米电子器件[56,57]等。就其目前的实际应用研究现状, 提出以下几点具体建议:
1.为了实现在纳米电子器件上的应用, 完美结构或缺陷可控的Mo S2纳米片的大尺寸、大面积制备还需要深入研究。
2.寻找有效制备方法和过程, 获得层数可控, 且电学或光学性质可调的Mo S2纳米片。
3.对Mo S2纳米片进行深度有效改性, 包括掺杂或复合, 以满足更多领域的广泛应用。
4.对Mo S2纳米片进行可控裁剪, 并与其他材料复合组装, 以达到所需要的目的。
5.加大Mo S2纳米片在生物医用领域的研究力度, 如扩大Mo S2的光波吸收范围、增强Mo S2的光热转换效率、增加Mo S2的生物多功能性, 及其与其他生物材料复合的协同作用。
总之, 由于Mo S2纳米片的综合内禀性能显示许多引人注目的优点, 它在未来的一段时间内还将继续受到人们的关注, 并将被持续研究开发, 以期获得更有价值的应用。
参考文献
[1] Ma N, Chen S, Huo D H, Zhao J Y, Ji T H.Porous Ag nanoparticle-deposited Mo S2monolith:preparation and adsorption capacity[J].Chinese Journal of Rare Metals, 2017, 41 (10) :1099. (马楠, 陈爽, 霍冬昊, 赵京彦, 嵇天浩.Ag纳米粒子沉积Mo S2多孔复合块材的组装及其吸附性能[J].稀有金属, 2017, 41 (10) :1099.)
[2] Ji T H, Zou L F, Xia H K, Wu Y.3D Cd-Mo S2porous monolith fabricated through a self-assembly between Cd2+cations and Mo S2nanoflakes[J].Nanotechnology, 2016, 11 (5) :1650053.
[3] Hu K H, Huo H Z, Han X G, Hu X G.Survey of preparing nanoscaled molybdenum disulfide[J].Modern Chemical Industry, 2003, 23 (8) :14. (胡坤宏, 沃恒洲, 韩效钊, 胡献国.纳米二硫化钼制备现状与发展趋势[J].现代化工, 2003, 23 (8) :14.)
[4] Zhou K Q, Jiang S H, Bao C L, Song L, Wang B B, Tang G, Hu Y, Zhou G.Preparation of poly (vinyl alcohol) nanocomposites with molybdenum disulfide (Mo S2) :structural characteristics and markedly enhanced properties[J].RSC Advances, 2012, 2 (31) :11695.
[5] Xue S F, Lan X Z, Zhou J, Song Y H, Zhang Q L, Yang Y, Ren P.Advances in the preparation of different morphology nano-sized molybdenum disulfide[J].Ordnance Material Science and Engineering, 2010, 33 (3) :88. (薛首峰, 兰新哲, 周军, 宋永辉, 张秋利, 杨勇, 任萍.不同形貌纳米二硫化钼制备研究进展[J].兵器材料科学与工程, 2010, 33 (3) :88.)
[6] Cai C C, Huang Z J, Liu M L, Wang G, Duan Y P.Study on structure conversion of graphene-like Mo S2[J].Journal of Synthetic Crystals, 2015, 44 (9) :2422. (蔡聪聪, 黄仲佳, 刘明朗, 王刚, 段园培.类石墨烯二硫化钼结构演变行为研究[J].人工晶体学报, 2015, 44 (9) :2422.)
[7] Cui X H, Chen H Y, Chen T.Research progress on the preparation and application of nano-sized molybdenum disulfide[J].Acta Chimica Sinica, 2016, 74 (5) :392. (崔向红, 陈怀银, 杨涛.纳米尺寸二硫化钼的制备与应用研究进展[J].化学学报, 2016, 74 (5) :392.)
[8] Novoselov K S, Jiang D, Schedin F, Booth T J, Khotkevich V V, Morozov S V, Geim A K.Two-dimensional atomic crystals[J].Proceedings of the National Academy of Sciences of the United States of America, 2005, 102 (30) :10451.
[9] Yin Z Y, Li H, Li H, Jiang L, Shi Y M, Lu G, Zhang Q, Chen X D, Zhang H.Single-layer Mo S2phototransistors[J].ACS Nano, 2012, 6 (1) :74.
[10] Coleman J N, Lotya M, O'Neill A, Bergin S D, King P J, Khan U, Young K, Gaucher A, De S, Smith R J, Shvets I V, Arora S K, Stanton G, Kim H Y, Lee K, Kim G T, Duesberg G S, Hallam T, Boland J J, Wang J J, Donegan J F, Grunlan J C, Moriarty G, Shmeliov A, Nicholls R J, Perkins J M, Grieveson E M, Theuwissen K, Mc Comb D W, Nellist P D, Nicolosi V.Two-dimensional nanosheets produced by liquid exfoliation of layered materials[J].Science, 2011, 331 (6017) :568.
[11] Joensen P, Frindt R F, Morrison S R.Single-layer Mo S2[J].Materials Research Bulletin, 1986, 21 (4) :457.
[12] Matte H S S R, Gomathi A, Manna A K, Late D J, Datta R, Pati S K, Rao C N.Mo S2and WS2analogues of graphene[J].Angewandte Chemie-International Edition, 2010, 49 (24) :4059.
[13] Zeng Z Y, Yin Z Y, Huang X, Li H, He Q, Lu G, Boey F, Zhang H.Single-layer semiconducting nanosheets:high-yield preparation and device fabrication[J].Angewandte Chemie-International Edition, 2011, 50 (47) :11093.
[14] Eda G, Yamaguchi H, Voiry D, Fujita T, Chen M, Chhowalla M.Photoluminescence from chemically exfoliated Mo S2[J].Nano Letters, 2011, 11 (12) :5111.
[15] Li X L, Li Y D.Mo S2nanostructures:synthesis and electrochemical Mg2+intercalation[J].The Journal of Physical Chemistry B, 2004, 108 (37) :13893.
[16] Ma L, Chen W X, Li H, Xu Z D.Synthesis and characterization of Mo S2nanostructures with different morphologies via an ionic liquid-assisted hydrothermal route[J].Materials Chemistry&Physics, 2009, 116 (2-3) :400.
[17] Chernikov A, Am V D Z, Hill H M, Rigosi A F, Velauthapillai A, Hone J, Heinz T F.Electrical tuning of exciton binding energies in monolayer WS2[J].Physical Review Letters, 2015, 115 (12) :126802.
[18] You Y M, Zhang X X, Berkelbach T C, Heinz T F.Observation of biexcitons in monolayer WSe2[J].Nature Physics, 2015, 11 (6) :477.
[19] Mak K F, Lee C G, Hong J, Shan J, Tony F, Heinz T F.Atomically thin Mo S2:a new direct gap semiconductor[J].Physical Review Letters, 2010, 105 (13) :136805.
[20] Nguyen E P, Carey B J, Ou J Z, Embden J V, Gaspera E D, Chrimes A F, Spencer J S M, Zhuiykov S, Kalantar Z K, Daeneke T.Electronic tuning of 2D Mo S2through surface functionalization[J].Advanced Materials, 2015, 27 (40) :6225.
[21] Liu B, Zhao W J, Ding Z J, Verzhbitskiy I, Li L J, Lu J P, Chen J Y, Eda J, Loh K P.Engineering bandgaps of monolayer Mo S2and WS2on fluoropolymer substrates by electrostatically tuned many-body effects[J].Advanced Materials, 2016, 28 (30) :6457.
[22] Lee H S, Min S W, Chang Y G.Mo S2nanosheet phototransistors with thickness-modulated optical energy gap[J].Nano Letters, 2012, 12 (7) :3695.
[23] Lukowski M A, Daniel A S, Meng F, Forticaux A, Li L, Jin S.Enhanced hydrogen evolution catalysis from chemically exfoliated metallic Mo S2nanosheets[J].Journal of the American Chemical Society, 2013, 135 (28) :10274.
[24] Xu Y, Bu X M, Wang P P, Wang D, Wang X Y.Synthesis and photocatalysis of 2D Mo S2based nano-materials[J].Electronic Components and Materials, 2015, 34 (9) :7. (许颖, 卜修明, 王朋朋, 王丁, 王现英.二维Mo S2纳米材料的制备及在光催化中的应用进展[J].电子元件与材料, 2015, 34 (9) :7.)
[25] Huang F, Pu X C, Ran M, Liang Q, Zhao H, Qi M, Yan A H.Advances in research of molybdenum disulfide nanomaterials used for photocatalysis[J].Materials Review, 2016, 30 (15) :12. (黄飞, 蒲雪超, 冉濛, 梁琦, 赵辉, 齐敏, 闫爱华.二硫化钼纳米材料在光催化应用中的研究进展[J].材料导报, 2016, 30 (15) :12.)
[26] Splendiani A, Sun L, Zhang Y B, Li T, Kim J, Chim C Y, Galli G, Wang F.Emerging photoluminescence in monolayer Mo S2[J].Nano Letters, 2010, 10 (4) :1271.
[27] Min S X, Lu G X.Sites for high efficient photocatalytic hydrogen evolution on a limited-layered Mo S2cocatalyst confined on graphene sheets-the role of graphene[J].Journal of Physical Chemistry C, 2012, 116 (48) :25415.
[28] Li Y G, Wang H L, Xie L M, Liang Y Y, Hong G S, Dai H J.Mo S2nanoparticles grown on graphene:an advanced catalyst for the hydrogen evolution reaction[J].Journal of the American Chemical Society, 2011, 133 (19) :7296.
[29] Xiang Q J, Yu J G, Jaroniec M.Synergetic effect of Mo S2and graphene as cocatalysts for enhanced photocatalytic H2production activity of Ti O2nanoparticles[J].Journal of the American Chemical Society, 2012, 134 (15) :6575.
[30] Bernardi M, Palummo M, Grossman J C.Extraordinary sunlight absorption and one nanometer thick photovoltaics using two-dimensional monolayer materials[J].Nano Letters, 2013, 13 (8) :3664.
[31] Yang L, Zhong D, Zhang J Y, Yan Z P, Ge S F, Du P W, Jiang J, Sun D, Wu X J, Fan Z Y, Dayeh S A, Xiang B.Optical properties of metal-molybdenum disulfide hybrid nanosheets and their application for enhanced photocatalytic hydrogen evolution[J].ACS Nano, 2014, 8 (7) :6979.
[32] Zhang J, Zhu Z P, Feng X L.Construction of two-dimensional Mo S2/Cd S p-n nanohybrids for highly efficient photocatalytic hydrogen evolution[J].Chemistry-A European Journal, 2014, 20 (34) :10632.
[33] Chang Y H, Lin C T, Chen T Y, Hsu C L, Lee Y H, Zhang W, Wei K H, Li L J.Highly efficient electrocatalytic hydrogen production by Mo Sxgrown on graphene-protected 3D Ni foams[J].Advanced Materials, 2013, 25 (5) :756.
[34] Yan Y, Ge X M, Liu Z L, Wang J Y, Lee J M, WangX.Facile synthesis of low crystalline Mo S2nanosheetcoated CNTs for enhanced hydrogen evolution reaction[J].Nanoscale, 2013, 5 (17) :7768.
[35] Feng Y, Yu S M, Zhang X W, Qiu L H, Chu F Q, You J B, Lu J M.Enhanced proton conduction in polymer electrolyte membranes as synthesized by polymerization of protic ionic liquid-based microemulsions[J].Chemistry of Materials, 2009, 21 (8) :1480.
[36] Liang Y, Wang H, Zhou J, Li Y, Wang J, Regier T, Dai H.Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts[J].Journal of the American Chemical Society, 2012, 134 (7) :3517.
[37] Deng D H, Yu L, Chen X Q, Wang G X, Jin L, Pan X L, Deng J, Sun J Q, Bao X H.Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction[J].Angewandte Chemie-International Edition, 2013, 52 (1) :371.
[38] Liu R L, Wu D Q, Feng X L, Mullen K.Nitrogendoped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction[J].Angewandte Chemie-International Edition, 2010, 49 (14) :2565.
[39] Zhang X F, Guo J J, Guan P F, Chisholm M F.Catalytically active single-atom niobium in graphitic layers[J].Nature Communications, 2013, 4 (5) :1924.
[40] Lyalin A, Nakayama A, Uosaki K, Taketsugu T.Theoretical predictions for hexagonal BN based nanomaterials as electrocatalysts for the oxygen reduction reaction[J].Physical Chemistry Chemical Physics, 2013, 15 (8) :2809.
[41] Xiao B B, Zhang P, Han L P, Wen Z.Functional Mo S2by the Co/Ni doping as the catalyst for oxygen reduction reaction[J].Applied Surface Science, 2015, 354:221.
[42] Wang T Y, Zhuo J Q, Chen Y, Du K Z, Papakonstantinou P, Zhu Z W, Shao Y H, Li M X.Synergistic catalytic effect of Mo S2nanoparticles supported on gold nanoparticle films for a highly efficient oxygen reduction reaction[J].Chemistry and Catalysis Chemistry, 2014, 6 (7) :1877.
[43] Zhao K, Gu W, Zhao L Y, Zhang C L, Peng W D, Xian Y Z.Mo S2/nitrogen-doped graphene as efficient electrocatalyst for oxygen reduction reaction[J].Electrochimica Acta, 2015, 169:142.
[44] Zhu C F, Zeng Z Y, Li H, Li F, Fan C H, Zhang H.Single-layer Mo S2-based nanoprobes for homogeneous detection of biomolecules[J].Journal of the American Chemical Society, 2013, 135 (16) :5998.
[45] Kim H U, Kim H Y, Kulkarni A, Ahn C, Jin Y H, Kim Y, Lee K, Lee M, Kim T.A sensitive electrochemical sensor for in vitro detection of parathyroid hormone based on a Mo S2-graphene composite[J].Scientific Reports, 2016, 6:1.
[46] Chekin F, Teodorescu F, Coffinier Y, Pan G H, Barrasa A, Boukherroub R, Szunerits S.Mo S2/reduced graphene oxide as active hybrid material for the electrochemical detection of folic acid in human serum[J].Biosensors&Bioelectronics, 2016, 85:807.
[47] Parlak O, Incel A, Uzun L, Turnera A P, Tiwari A.Structuring Au nanoparticles on two-dimensional Mo S2nanosheets for electrochemical glucose biosensors[J].Biosensors&Bioelectronics, 2017, 89:545.
[48] Ma Y, Liang X L, Tong S, Bao J, Ren Q S, Dai Z F.Gold nanoshell nanomicelles for potential magnetic resonance imaging, light-triggered drug release, and photothermal therapy[J].Advanced Functional Materials, 2013, 23 (7) :815.
[49] Wu H H, Yang R, Song B M, Han Q S, Li J Y, Zhang Y, Fang Y, Tenne R, Wang C.Biocompatible inorganic fullerene-like molybdenum disulfide nanoparticles produced by pulsed laser ablation in water[J].ACS Nano, 2011, 5 (2) :1276.
[50] Goldman E B, Zak A, Tenne R, Kartvelishvily E, Zaidman S L, Neumann Y, Cohen R S, Palmon A, Hovav A H, Aframian D J.Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells[J].Tissue Engineering Part A, 2015, 21 (5-6) :1013.
[51] Teo W Z, Chng E L K, Sofer Z, Pumera M.Cytotoxicity of exfoliated transition-metal dichalcogenides (Mo S2, WS2and WSe2) is lower than that of graphene and its analogues[J].Journal of the American Chemical Society, 2014, 20 (31) :9627.
[52] Appel J H, Li D O, Podlevsky J D, Debnath A, Green A A, Wang Q H, Chae J.Low cytotoxicity and genotoxicity of two-dimensional Mo S2and WS2[J].Biomaterials Science and Engineering, 2016, 2 (3) :361.
[53] Chou S S, Kaehr B, Kim J, Foley B M, De M, Hopkins P E, Huang J, Brinker C J, Dravid V P.Chemicallyexfoliated Mo S2as near-infrared photothermal agents[J].Angewandte Chemie-International Edition, 2013, 52 (15) :4160.
[54] Wang S G, Chen Y, Li X, Gao W, Zhang L L, Liu J, Zheng Y Y, Chen H R, Shi J L.Injectable 2D Mo S2integrated drug delivering implant for highly efficient NIR-triggered synergistic tumor hyperthermia[J].Advanced Materials, 2015, 27 (44) :120.
[55] Presolski S, Pumera M.Covalent functionalization of Mo S2[J].Materials Today, 2016, 37 (3) :140.
[56] Radisavljevic B, Whitwick M B, Kis A.Integrated circuits and logic operations based on single-layer Mo S2[J].ACS Nano, 2011, 5 (12) :9934.
[57] Wang X T, Huang L, Peng Y T, Huo N J, Wu K D, Xia C X, Wei Z M.Enhanced rectification, transport property and photocurrent generation of multilayer Re Se2/Mo S2p-n heterojunctions[J].Nano Research, 2016, 9 (2) :507.






