
Dynamics in simultaneous electro-generative leaching for sphalerite-MnO2
XIAO Li(肖 利)1, 3, QIU Guan-zhou(邱冠周)2, FANG Zheng(方 正)1, LIU Jian-she(柳建设)2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China;
3. Hunan Metallurgic Professional Technical College, Zhuzhou 412000, China
Received 2 April 2007; accepted 10 September 2007
Abstract:
The principle for the electro-generative leaching was applied to simultaneous leaching of sphalerite-MnO2. A galvanic system for the bio-electro-generative leaching was set up. The effects of grain size and temperature on rate of zinc extraction from sphalerite under the conditions of presence and absence of Acidithiobacillus ferrooxidans (A.ferrooxidans) were studied, respectively. The results show that with bacteria, the maximum extraction of zinc from the ores with grain size of 16.6 μm can reach 32.01% after leaching for 12 h, while to obtain the same extraction ratio in the traditional bio-leaching route (i.e. not electro-generative one) 10 d is needed to ore granules with same size. The unreacted shrinking core model was used for describing the reaction-relative and diffusion-relative phenomena presented in the process of the electro-generative leaching with and without bacteria, which is considered to be diffusion controlled. The activation energies of the anodic reaction for leaching system in the presence and absence of bacteria are 11.97 and 14.39 kJ/mol, respectively, indicating that leaching rate can be decreased by A. ferrooxidans. SEM was used to study the effect of A. ferrooxidans on the ores in the simultaneous electro-generative leaching, which indicates that the produced sulfur on the surface of the sulfides can be oxidized by A. ferrooxidans after bio-electro-generative leaching for 24 h, and the transferred charge due to the bacterial oxidation is up to 17.86%, which is an important part of the output electric quantity.
Key words:
sphalerite mineral; electro-generative leaching; bio-oxidation; activation energy;
1 Introduction
There are many reports about hydrometallurgical treatment of sphalerite minerals[1-4]. CRUNDWELL[1] presented that the rate of dissolution of sphalerite was proportional to the iron content in the process of ferric ion leaching of the minerals. WANG and FANG[3] indicated that sphalerite leaching was controlled by diffusion through a layer of sulfur formed on the surface recently.
Some new technology such as electro-generative leaching(EGL)[3] has been applied to leaching of sulfides. In the process performed by electro-generation, the Gibss free energy could transform to an applicable electrical work[4] and leaching products simultaneously are acquired. Here, the galvanic cell is designed as a system with anolyte and cathlyte compartments separated by an anion membrane[5-6], which would make cations in anolyte and cathlyte separate and anions migrate freely. The anode is designed as a gather of electrolyte and electrode of sulfide powder, and the cathode as another gather of strong acid and electrode of MnO2 powder. Instead of the produced substances that pollute environment in traditional leaching, such as gaseous H2S[7] and SO2[8], the new technique would not only simplify the purified process of leaching solution[5], but also precipitate the element sulfur. WANG and FANG[6] presented the technology to the simultaneous leaching of chalcopyrite concentrate with MnO2[6]. Their results indicated that the surface of leached sulfides was covered by the accumulated sulfur, greatly reducing the output current and voltage and thwarting the anodic reaction to take place. As a result, it is necessary to develop a proper technique, which could be employed to eliminate the accumulated sulfur on the surface of the sulfides.
Acidithiobacillus ferrooxidan (A. ferrooxidans) plays an important role in the aspect of the biochemical treatment of sulfur and oxidation of ferrous ions into ferric ones[9-11]. Under normal aerobic conditions, the bacteria can utilize ions in solution as an energetic substance and can also obtain energy from direct oxidation of the solid substrate of the sulfur[12]. If the microbes could be added into anodic electrolyte of the electro-generative leaching cell, and oxidize the produced sulfur to sulfuric acid in the sulfide leaching, the leaching resistance will be decreased.
In the present study, a new technology, where bacteria are added to oxidize sulfur in electro-generative leaching, will be used for sphalerite leaching. It is called as bio-electro-generative leaching(BEGL). The behaviors of sulfide anode were reported.
2 Experimental
2.1 Reactions of simultaneous electro-generative leaching of sphalerite-MnO2
A system was designed to a galvanic cell for simultaneous leaching of sphalerite and MnO2. The overall reaction in the anolyte is
ZnS(s)+MnO2(s)+4H+(aq)=Mn2+(aq)+Zn2+(aq)+S0(s)+2H2O (1)
The anodic reaction is represented as
ZnS(s)=Zn2+(aq)+S0(s)+2e (2)
When there exists A. ferrooxidans in anolyte, the overall reaction can be represented as
4MnO2(s)+8H+(aq)+ZnS(s)=4Mn2+(aq)+4H2O+Zn2+(aq)+![]() (3)
(3)
The anodic reaction with A. ferrooxidans is as follows:
ZnS(s)+4H2O=Zn2+(aq)+![]() +8H++8e (4)
+8H++8e (4)
Except the dissociated ZnS, FeS, an intergrowth of ZnS in sphalerite is dissociated to form ferrous ions, which can be oxidized to the ferric by A. ferrooxidans. The ferric ions as an oxidative agent could accelerate the leaching of sphalerite[11] and themselves are reduced to ferrous ions again. It is ferrous ions that form the substrate for microbial growth according to reaction[12- 13]:
![]() (5)
(5)
Anyway, in the leaching process, iron ions continuously change from ferrous to ferric by A. ferrooxidans action and to ferrous again by the leaching of sphalerite, producing a cycle of iron ions.
2.2 Materials
The selected sphalerite was a natural hand-sorted ores from a domestic mine. Its chemical composition is listed in Table 1.
Table1 Chemical composition of sphalerite (mass fraction, %)
![]()
The XRD analysis shows that ZnS and FeS coexist in the minerals. Four kinds of size, 33.9, 26.1, 16.6 and 10.4 μm, were selected.
2.3 Electro-generative set-up
The set-up used was an electrolysis cell made of PVC in water bath, which was divided into two compartments connected by anion membrane, anolyte and cathlyte, each of 200 mL capacity, as shown in Fig.1.
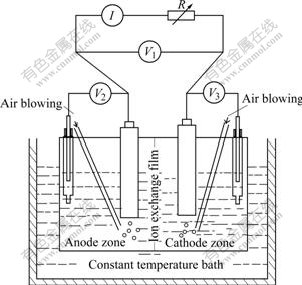
Fig.1 Schematic diagram of experimental apparatus
The anode and cathode were made of the powder of sphalerite and MnO2 respectively. The structure of the electrodes is shown in Fig.2. To increase the conductivity of the electrode, the semi-conductive powder of sphalerite (2.0 g) and MnO2 concentrate (2.0 g) were respectively mixed first with carbon paste consisted of acetylene black (AB) (0.2 g) as the conducting medium. The sphalerite-contained mixture and MnO2-contained mixture were then respectively compacted in an acid-resisting nylon filter net with aperture size of 45 μm, in which the bacteria could attach on the surface of sulfide mineral and the dissociated ions could diffuse freely. Two carbon rods with a diameter of 2.48 cm were separately connected to the two mixtures to gather electric current respectively.
Fig.2 Sketch of anode and cathode structure
Two air-blowing tubes were inserted into anolyte and catholyte, respectively, to supply the oxygen for bacteria and to agitate.
The pH value, the electrode potentials (vs SCE) and the output voltage of the leaching cell were measured with a PHS-3C digital acidimeter, the output current of the galvanic with an ampere meter and the concentration of oxygen with a Degussa oxygen meter. All of the measured instruments were calibrated before running.
2.4 Solutions and bacteria
To compare BEGL with EGL, the bacteria culture medium ((NH4)2SO4 3.0 g/L, KCl 0.1 g/L, K2HPO4 0.5 g/L, MgSO4?7H2SO4 0.5 g/L, Ca(NO3)2 0.01 g/L) acidified to pH 1.8 by H2SO4 was taken as anolyte in EGL process, and the same nutrient medium with exponential growth phase bacteria was used as anolyte in the BEGL.
The bacterial culture used was a pure strain Yunnan 3 (PQ321745) of A. ferrooxidans that was suited for growth on sphalerite-contained ores and was obtained from Yunnan Province of China. The bacteria were cultivated and grown in the sulfur-contained medium ((NH4)2SO4 3.0 g/L, KCl 0.1 g/L, K2HPO4 0.5 g/L, MgSO4?7H2SO4 0.5 g/L, Ca(NO3)2 0.01 g/L, S 10 g/L) at 303 K prior to electro-generative leaching experiment. In order to avoid the influence of metabolite produced by bacteria, A. ferrooxidans was gathered by centrifugation and then scattered in clear bacteria culture medium, whose number in solution was up to 108/mL at the beginning of each run of the bio-oxidation-electro- generative leaching.
As well known, the anions such as chloride ions in the system could migrate freely through the anion membrane, and A. ferrooxidans has little tolerance for Cl-. 1 mol/L H2SO4 was used as catholyte, instead of HCl.
The solutions used for experiments were prepared using analytic grade reagents and distilled water. Oxygen concentration in the solution was measured to be 5.9 mg/L.
3 Results and discussion
3.1Effect of grain size on zinc extraction
The ores with four grain sizes were used to study the effect of grain size on zinc extraction. According to Faraday’s law, the current output of cell is in direct ratio to ions dissociated in electrolyte. In order to assure the zinc ions dissociated as soon as possible, we adopted a rather small exterior load.
The output voltage and current obviously decrease for the sphalerite with four sizes after leaching for 12 h whether or not inoculate the bacteria in anolyte. But the falling rate of the current is slow in the presence of A. ferrooxidans compared with that in the absence of the bacteria. Sample was used to analyze the conversion rate of Zn2+ in anolyte every 30 min.
The plots of the conversion rate vs time are shown in Figs.3 and 4. The relation between them can be expressed as xB=kBt, which agrees with one of the characteristic equations of the unreacted shrinking core model[14-15]. These characteristic equations related to the rate-determining steps are listed in Table 2, where xB is the fraction of the leaching product B; t is leaching time and kB is the rate constant in min-1.
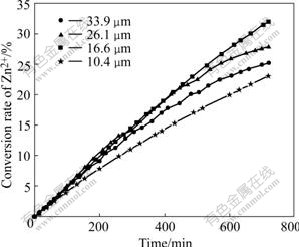
Fig.3 Relationship between conversion rate of Zn2+and time with A. ferrooxidans
Table 2 Correspondence between rate-determining step and equation of conversion vs time


Fig.4 Relationship between conversion rate of Zn2+ and time without A. ferrooxidans
According to the model, the leaching controlling steps for both EGL and BEGL all are diffusion, i.e. reactants from the bulk solution to the interaction interface through the product layer and the product species outward the bulk solution through the layer.
It can be seen from Fig.3 that the change in the conversion rate as particle size in presence of A. ferrooxidans is more evident than that in the absence of the bacteria shown in Fig.4, which indicates that the effect of the particle size on the zinc extraction in BEGL is more remarkable. Furthermore, the effect of the bacteria on conversion of zinc is larger than that without it under the same conditions of grain size of ores and temperature, which indicates that A. ferrooxidans can oxidize part of sulfur produced in the electro-generative leaching.
Experimental results in Fig.3 show that in BEGL, the maximum conversion of zinc leaching for 12 h, 32.01%, comes from the ores with grain size of 16.6 μm, that is to say, the optimum particle size should be 16.6 μm when the bacteria are used in the electro-generative leaching. The particle size favors the bacteria that oxidize sulfur on the ore surface. The extraction rate increases with the decrease of the particle size ranging from 33.9 to 16.6 μm, indicating that the sulfur- contained product layer may be more easily oxidized by A. ferrooxidans in the small particle size range. However, when the particle size is 10.4 μm, the bacteria have little influence on the extraction. However, to the ores of the size of 16.6 μm, the same conversion rate of zinc in the traditional bio-leaching with the same bacteria needs 10 d, which indicates that BEGL is a high-performance leaching technique.
3.2 Activation energy of electro-generative leaching for sphalerite
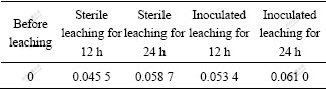
As mentioned above, the five temperature points of A. ferrooxidans, 299, 301, 303, 305 and 307 K were favored. The effect of temperature on extraction of zinc in two systems, both BEGL and EGL, at these temperatures was studied. The extraction rate of zinc leaching for 12 h in BEGL and EGL was measured under the conditions of the small external resistance, and the rate constant kB at different temperatures was calculated by Eqn.(8), as listed in Table 3.
Table 3 Rate constant kB and extraction rate of zinc for 12 h in BEGL and EGL
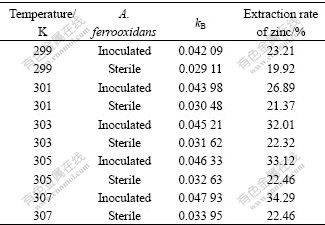
The extraction rate increases from 23.21% to 34.29% as the temperature changes from 299 to 307 K in BEGL. The relatively large conversion rate of zinc and its dependance on temperature is in BEGL. It would be explained by the fact that A. ferrooxidans could promote the oxidation of sulfur. The experiments of EGL shows that the extraction rate of zinc gradually increases with increasing temperature, indicating that temperature has little effect on the dissociation of sphalerite because no bacteria could oxidize sulfur formed at this case.
According to the Arrhenius equation:
lg kB=lgk0-Ea/(2.303RT) (9)
where kB is the constant of leaching rate; k0 is the pre- exponential factor; Ea is the activation energy; R is the universal gas constant; and t is temperature in K. For inoculating and sterile solution, plotting lg kB vs 1/T, respectively, two straight-lines are obtained with the slope (-Ea/2.303R), as shown in Fig.5. The activation energies for BEGL and EGL are calculated as 11.97 and 14.39 kJ/mol, respectively. The difference of activation energy between two leaching systems suggests that A. ferrooxidans can decrease the reaction energy. It has been reported that the activation energy of sphalerite leaching in acidic ferric chloride solution was 46.9 kJ/mol[11]. Compared with this experiment, except the effect of bacteria, the obviously low activation energies in BEGL and EGL systems showed the electric catalytic activity of acetylene black, which could also reduce the whole apparent activation energy[16].

Fig.5 lg kB-1/T curves with and without A. f
Generally, when the activation energy is lower than 20 kJ/mol, the process is considered to be diffusion controlled[17]. In other words, whether existing A. ferrooxidans in the anolyte or not, the oxidization of sphaleriate is controlled by diffusion. Because the element sulfur on the ore surface is a main substance with diffusion resistance, the fact that the system is still diffusion controlled shows that the solid sulfur is not entirely oxidized by bacteria in 12 h.
3.2 XRD analysis before and after electro-generative leaching of sphaleriate
The phases of sphaleriate before and after BEGL and EGL leaching for 12 h and 24 h were respectively characterized by XRD to study the effect of two leaching methods on the ores, as shown in Fig.6. It can be seen that the element sulfur exists in the residue. The lattice aberrance, ε of sphalerite samples before and after leach- ing is listed in Table 4, which reveals that the leaching time has influence on the lattice change of samples. In the same period of leaching, A. ferrooxidans can change in lattice more.
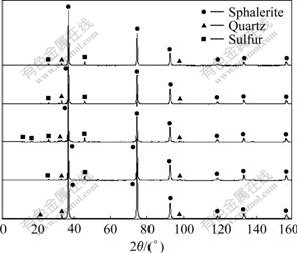
Fig.6 XRD patterns of sphalerite before and after leaching: 1 Before EGL; 2 After EGL for 12 h; 3 After EGL for 24 h; 4 After BEGL for 12 h; 5 BEGL for 24 h
Table 4 Lattice aberrance ε of sphalerite samples before and after BEGL and EGL (%)
3.3 SEM analysis of residue in electro-generative leaching for sphaleriate
SEM images of the samples before and after BEGL and EGL for 12 h and 24 h are shown in Fig.7.
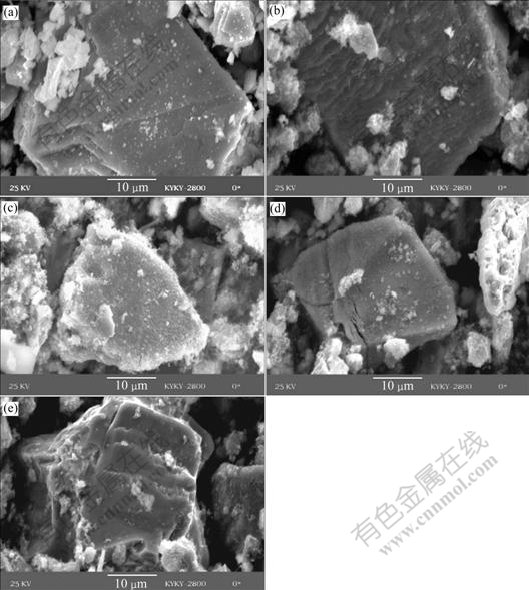
Fig.7 SEM images of sphalerite before and after leaching: (a) Before electro- generative leaching; (b) EGL for 12 h; (c) EGL for 24 h; (d) BEGL for 12 h; (e) BEGL for 24 h
It can be seen from Fig.7 that there is an obvious change on the surface of sphalerite before and after leaching. Before BEGL and EGL, the surface is clear. After EGL for 24 h, a large quantity of floccules accumulates on the surface of ores, while the surface turns to smooth with few floccules for BEGL. However, sulfur accumulates on the surface of sphalerite for 12 h in BEGL and EGL.
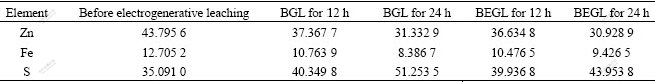
The molar fraction of Zn, Fe and S measured by AES is listed in Table 5. The quantity of element sulfur on the sample in 24 h EGL is larger than that in BEGL, while the quantity for 12 h is almost the same. This indicates that the produced sulfur can be more easily oxidized by A. ferrooxidans as time increases. This conclusion has been also confirmed by SEM results.
Table 5 Molar fraction of main elements for ferri-sphalerite before and after leaching (%)
3.4 Output electric quantity in EGL and BEGL processes
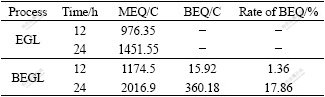
Assume that the all-transferred charge is due to S2- to S0 and is considered as the theoretic electric quantity (TEQ), which can be calculated by the Faraday’s law. However, the measured electric quantity(MEQ) in BEGL process is larger than the theoretic one. This means that the transferred charge is not only S2- to S0 but also part of S0 to sulfate group that is called as biologic electric quantity. The biologic electric quantity is defined as the difference between the measured and the theoretic one. The ratio of biologic electric quantity to the measured one can be used to predict the progress of BEGSL process. It can be seen from Table 6 that after 12 h in BEGL, the rate is 1.36%, which shows that the bacterial oxidation on the surface of pyrite has not initiated. Subsequently, the increase in the rate of BEQ with the increase in time, is up to 17.86% for 24 h, which indicates that the transferred charge due to the fact that the bacterial oxidation is an important part of the output electric quantity.
Table 6 Relationship between time and MEQ in EGL and such indexes as MEQ, BEQ and rate of BEQ in BEGL
4 Conclusions
1) The trend of BEGL enhances with increasing temperature from 299 to 307 K, but reduces in EGL. With BEGL, the time for obtaining the maximum extraction of zinc smartly shortens compared with traditional bio-leaching route (i.e. not electro-generative one).
2) The leaching product layer in anode contains sulfur with the diffusion resistance. The sulfur produced in the leaching is almost completely oxidized in BEGL process for 24 h.
3) The unreacted shrinking core model is used for describing the reaction-relative and diffusion-relative phenomena presented in the both EGL and BEGL processes. The activation energies of BEGL and EGL systems are 11.97 and 14.39 kJ/mol respectively due to bacterial actions and the electric catalytic activity of acetylene black as conductive component of electrodes.
4) BEGL technique is able to eliminate the accumulated sulfur on the surface of the sulfides, and in favor of the leaching of sphalerite. After 24 h, the transferred charge due to the bacterial oxidation is an important part of the output electric quantity.
References
[1] CRUNDWELL F K. Kinetics and mechanisms of the oxidative dissolution of a zinc sulphide concentrate in ferric sulphate solutions [J]. Hydrometallurgy, 1987, 19(1): 227-242.
[2] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71(1): 57-66.
[3] WANG Shao-fen, FANG Zheng. Simultaneous electro-generative leaching of chalcopyrite concentrate and MnO2 [J]. J Cent South Univ Technol., 2006, 13(1): 49-52.
[4] LANGER S H, YURCHAK S. Electrochemical reduction of the benzene ring by electrogenerative hydrogenation [J]. Journal of the Electrochemical Society, 1969, 116(5): 1228-1230.
[5] FANG Z, ZHANG Q R. Thermoelectrochemistry and its application to metallurgical research [J]. J Mater Sci Technol, 2001, 17(1): 20-24.
[6] WANG S F, FANG Z. Electrogenerative leaching of galena with ferric chloride [J]. Minerals Engineering, 2003, 16(3): 869-872.
[7] RAMACHANDRA R S, HEPLER L G. Equilibrium constants and thermodynamics of ionization of aqueous hydrogen sulfide [J]. Hydrometallurgy, 1977, 2(2): 293-299..
[8] DALEWSKI F. Removing arsenic from copper smelter gases [J]. JOM, 1999, 51(1): 24-26.
[9] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: experiments with a controlled redox potential indicate no direct bacterial mechanism [J]. Appl Environ Microbiol, 1998, 64(12): 3570-3575.
[10] HANSFORD G S, VARGAS T. Chemical and electrochemical basis of bioleaching process [J]. Hydrometallurgy, 2001, 59(1): 135-145.
[11] BOBECK G E, SU H. Kinetics of dissolution of sphalerite in ferric chloride solution [J]. Metallurgical Transactions B, 1985, 16(3): 413-424.
[12] CHOI W K, TORMA A E, OHLINE R W. Electrochemical aspects of zinc sulphide leaching by thiobacillus ferrooxidans [J]. Hydrometallurgy, 1993, 33(1): 137-152.
[13] GABRIEL D S. Relative importance of diffusion and reaction control during the bacterial and ferric sulphate leaching of zinc sulphide [J]. Hydrometallurgy, 2004, 73(2): 313-324.
[14] LIZAMZA H M, FAIRWEATHER M J, DAI Z, ALLEGRETTO T D. How does bioleaching start [J]. Hydrometallurgy, 2003, 69(1/3): 109-116.
[15] GUDYANGA F P, MAHLANGU T. Reductive oxidative pretreatment of a stibnite flotation concentrate: thermodynamic and kinetic considerations [J]. Minerals Engineering, 1998, 11(3): 563-580.
[16] WANG Shao-fen, FANG Zheng, WANG Yun-yan. Application of carbon paste electrode on the electro-generative leaching process of sulfide minerals [J]. Electrochemistry, 2005, 11(1): 77-82.
[17] FOWLER T A, HOLMES P R, CRUNDWEDD F K. 2001. On the kinetics and mechanism of the dissolution of pyrite in the presence of Thiobacillus ferrooxidans [J]. Hydrometally, 2001, 59(2): 257-270.
Foundation item: Project(2004CB619204) supported by the National Basic Research Program of China; Project(50374077) supported by the National Natural Science Foundation of China; Project(07D069) supported by the Education Department Foundation of Hunan Province, China
Corresponding author: QIU Guan-zhou; Tel: +86-731-8660356; E-mail: xiaoli_csu@163.com


