DOI:10.19476/j.ysxb.1004.0609.2017.10.26
机械力活化Fe-MnO2稳定含砷废渣
徐 慧1,闵小波1, 2,梁彦杰1, 2,王云燕1, 2
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083)
摘 要:
以模拟含砷废渣为研究对象,考察机械活化铁锰稳定剂对砷稳定效果的影响,确定了最优参数:稳定剂种类为Fe-MnO2、稳定剂铁锰摩尔比为0.4:0.6、稳定剂用量为8%、球磨时间为1 h。在此最佳条件下,可将砷酸铁渣中As的浸出毒性由51.45 mg/L降至2.36 mg/L,优于国家危险废物鉴别标准浸出毒性鉴别(GB 5085—2007)。借助X射线衍射、粒度和比表面分析、扫描电镜等,从球磨过程中铁锰反应机制、铁锰复合稳定剂腐蚀过程特征以及砷吸附后的铁锰复合稳定剂形貌结构变化等方面,研究铁锰复合稳定剂稳定含砷废渣的机理。在机械力化学和MnO2复合作用下,促进零价铁粉表面的腐蚀过程,生成了大量高效吸附砷的水铁矿类物质,实现砷的高效稳定。
关键词:
文章编号:1004-0609(2017)-10-2170-10 中图分类号:X705 文献标志码:A
世界卫生组织将类金属砷污染列为环境污染的首位。砷一旦进入环境将发生化学或生物转化,以不同形态通过水体、土壤等介质对生态环境和人类健康产生持续影响。我国集中全球砷矿资源探明储量的70%,成为世界上受砷污染最为严重的国家。根据2013年《Science》发布的中国砷污染预警模型估算,我国1/3的省市出现了严重的地方性砷中毒,约有1960万人受到砷污染地下水的危害[1]。砷除赋存于雄黄、雌黄矿外,大多伴生于铜、铅、锌等有色金属矿石中。据统计,我国人为排放砷大约20万t/a,其中有色重金属冶炼行业占50%以上,是我国最主要的砷污染源[2-5]。由于除砷的工艺经济成本高,砷产物利用率低,我国仅有少数冶炼厂以白砷的形式回收少量砷,回收的砷不足进入冶炼系统总砷量的10%,其余20%以上的砷进入冶炼渣,60%~70%的砷以中间产品堆存[6]。我国对三废中的砷含量有严格限制,含砷废气和废水不能直接排放至大气和水体,在废水和废气的净化处理过程中砷常以含砷废渣的形态分离出来,故砷的三废处理问题终究归结于含砷固废的处理[7]。含砷废渣具体来源为金属冶炼(如黑铜泥、砷碱渣、砷烟灰)、处理含砷废水和废酸的沉渣(如砷滤饼、硫化砷渣)及电解过程中产生的含砷阳极泥等[8]。长期以来,含砷废渣大多采用囤积贮存的方法进行处置,易形成二次污染,已经构成了我国有色金属冶金企业最主要的环境污染源。
目前,国内外对含砷废渣的处理主要采用稳定化固化处理和资源化利用,如回收有价成分、生产建筑材料等[9]。在处理有毒砷渣和污泥时,大都采用化学方法将其稳定,即通过化学反应生成相对难溶的、自然条件下较稳定的金属砷酸盐和亚砷酸盐,包括常见的亚砷酸钙、砷酸钙、砷酸铁等[10-11]。因可溶性的砷能够与许多金属离子形成此类化合物,故沉淀法常以钙、铁、镁、铝盐及硫化物等作沉淀剂。用热水或酸碱等溶液将含砷废渣中的砷浸出,然后对浸出液进行钙盐沉淀法和铁盐沉淀法处理。目前常用的钙盐沉淀法处理成本低、工艺简单。但是钙盐的溶解度较大,必须使钙离子远远过量,砷浓度才能降至较低。铁盐沉淀法在高pH值下生成砷酸铁的同时还会产生大量氢氧化铁胶体,溶液中的砷酸根与氢氧化铁可发生吸附共沉淀,从而有较高的砷脱除率。当前,含砷废渣的处理主要采用的湿法回收等方法不仅成本较高,而且会增大污泥产量。因此,开发一种高效、经济、环保的含砷废渣处理技术显得十分迫切。
机械力化学技术在环保领域的应用是近年来的研究热点[12-18]。机械力化学在有机固体废物处理方面已有较成熟的应用,但在重金属固体废物处理方面却鲜有相关研究报道[19-21]。CHAI等[22-23]研究锌冶炼中和渣机械硫化时发现在添加单质硫磺情况下,机械力球磨过程能将氧化锌硫化生成硫化锌以达到回收重金属的目的。MONTINARO等[24-25]和STELLACCI等[26]认为机械力化学稳定重金属是由于在机械力诱导作用下,重金属离子进入到晶体网格里,从而形成稳定的化合物。
本文作者利用机械球磨制备用于稳定含砷废渣中砷的活性铁锰系材料,提出采用铁锰复合稳定剂处理含砷废渣工艺,并对其稳定机理进行探索,为含砷废渣的稳定化开辟了一种切实可行的方法。
1 实验
1.1 实验材料
1.1.1 砷酸铁渣
1) 砷酸铁渣的制备:砷酸铁渣为实验室制备的模拟含砷废水铁盐沉淀废渣。制备方法如下:将粗砷酸钠干渣(砷含量24%(质量分数))配成初始砷浓度为10~50 g/L的碱性高砷溶液,逐滴加入浓硫酸(AR,株洲市星空化玻有限公司),调节pH值至4。将配置好的含砷溶液水浴加热并机械搅拌,速度控制在(200±20) r/min。按铁砷摩尔比1.5将所需的七水硫酸亚铁(AR,国药集团化学试剂有限公司)配成亚铁溶液,pH值为3.5±0.1,将亚铁溶液加入到预热好的含砷溶液。继续加热至设定温度并搅拌,鼓入预热的空气,流量控制为≥120 L/h,反应7 h。静置冷却至60 ℃,过滤,洗涤,滤渣于60 ℃烘箱中烘干至恒定质量,研磨,备用。
2) 砷酸铁渣的性质:砷酸铁渣的SEM-EDS见图1。砷酸铁渣呈疏松颗粒状、大小不一。主要元素为砷、铁、硫、钠和氧。因此,推测砷酸铁渣中主要物相为FeAsO4和Na2SO4。砷酸铁渣XRD谱见图2。砷酸铁渣中没有晶型完整的物相,大部分物质以非晶态形式存在。所制备的砷酸铁渣中FeAsO4并非稳定存在,其中的砷易被浸出,浸出毒性为51.45 mg/L。因此,控制好砷的浸出毒性是实现硫化砷渣稳定化的主要目标。
1.1.2 机械力活化Fe-MnO2复合材料
按照一定摩尔比称取铁和MnO2,按照球料比10:1称取磨球,于行星球磨机(QM-QX4全方位行星式球磨机,单罐容积500 mL,罐体介质不锈钢,最大装料量为罐体容积3/4,额定转速公转250 r/min,自转530 r/min)中球磨一定时间后,取出复合吸附剂,备用。

图1 砷酸铁渣的SEM像和EDS谱
Fig. 1 SEM image(a) and EDS spectrum(b) of simulated arsenic bearing solid waste
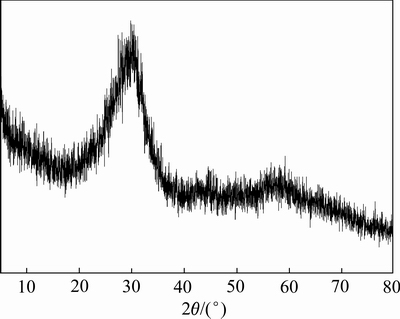
图2 砷酸铁渣的XRD谱
Fig. 2 XRD pattern of simulated arsenic bearing solid waste
1.2 实验方法
1.2.1 机械力活化Fe-MnO2对含砷固废的稳定化实验
分别开展了最佳稳定剂种类(Fe-MnO2、Fe-KMnO4、Fe-MnCO3、Fe-MnSO4)、铁锰摩尔比(0:1、0.2:0.8、0.4:0.6、0.6:0.4、0.8:0.2、1:0)、铁锰稳定剂浓度为2%、4%、6%、8%、10%和12%(质量分数)分别添加0.1、0.2、0.3、0.4、0.5和0.6 g、球磨时间(30、60、90、120、150、180、210、240 min)的单因素实验,以一定的添加量与含砷废渣混合均匀,进行浸出毒性实验。
1.2.2 机械力活化Fe-MnO2对含砷固废的稳定机制实验
行星球磨机中球磨0~1 h,每5 min取样一次,进行XRD、粒度、比表面积、SEM检测与分析;为了模拟砷渣的浸出环境,球磨后的产物分别放置于酸性溶液(醋酸溶液),酸性含砷溶液(pH=5.0,砷酸钠溶液)中浸泡,吸附达到平衡后检测样品性质。
1.3 分析方法
浸出毒性按照美国TCLP浸出毒性鉴别方法进行检测,浸出液中有害元素浓度采用ICP(IRIS Intrepid II XSP,美国热电公司)分析,废渣的物相采用XRD (D/max2550VB+,日本理学株氏会社生产)表征,废渣的微观形貌采用扫描电镜(Nano SEM 230,FEI公司生产)观察。
2 铁锰复合稳定剂处理含砷废渣工艺
2.1 稳定剂体系
稳定剂种类是影响机械力球磨稳定效果的决定性因素。有研究表明,铁类物质对砷的去除具有重要作用[27-29]。氧化锰比表面积大、表面活性强、电荷零点低、负电荷量高,不仅对许多过渡元素和重金属元素有很强的吸附固定能力,也能通过氧化 As3+、Cr3+、Se3+、U4+、Ce3+、Pu4+等变价元素而改变其毒性和形态。基于此,本文作者利用还原铁粉分别与MnO2、KMnO4、MnCO3、MnSO4以摩尔比为1:0.3组合作为稳定剂,单独用MnO2及还原铁粉作为对照组,在球料比为10:1、球磨时间1 h、添加量为2%条件下对含砷废渣进行稳定处理,其结果如图3所示。原渣浸出液、两组对照组及4组样品浸出液的pH值相差不大,变化不明显。原渣浸出砷浓度为51.45 mg/L,单独铁粉作用浸出砷浓度为41.51 mg/L,单独MnO2处理后砷浸出浓度为17.69 mg/L。加入Fe-KMnO4、Fe-MnO2稳定体系处理后,渣中砷的浸出浓度分别下降至6.308 mg/L和4.246 mg/L,国家危险废物鉴别浸出毒性鉴别标准小于5 mg/L。而Fe-MnCO3、Fe-MnSO4为稳定剂体系时砷的浸出浓度分别为39.23 mg/L和35.17 mg/L,仍然远远超过限值。这是由于锰系促进剂不同的氧化能力所致。铁与砷稳定化合物一般在氧化性条件下形成[6],上述化合物中Mn2+的氧化能力弱,因此,MnCO3、MnSO4对砷的稳定性促进作用最差;KMnO4易溶于水且具有强氧化能力,会导致二次污染,综合考虑,选取Fe-MnO2为最佳的稳定剂体系。
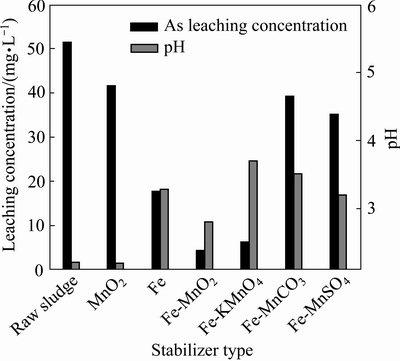
图3 稳定剂体系对模拟砷酸铁渣稳定效果的影响
Fig. 3 Effect of stabilizer system on stability of simulated arsenic bearing solid waste
2.2 铁锰比
将铁粉与MnO2以不同摩尔比组合,控制球料比为10:1、球磨时间1 h、添加量为2%条件下对含砷废渣进行稳定处理,结果如图4所示。以不同铁锰比稳定剂稳定后与原渣浸出液相比,pH值先升高后降低再上升,但在2.2~3.3范围内变化不大。稳定剂中铁锰比由0:1至1:0过程中,随着铁含量的增多,砷的浸出毒性有一定的波动,当铁锰比为0.4:0.6时降至最低,为6.92 mg/L。结果表明,铁锰比决定体系氧化性和铁离子浓度,进而影响砷的稳定性能。当铁锰比为0.4:0.6时对砷的稳定效果最佳,铁和MnO2过多或过少,砷的浸出毒性都会升高。因此,取nFe:nMn=0.4:0.6为稳定剂的最佳铁锰比。
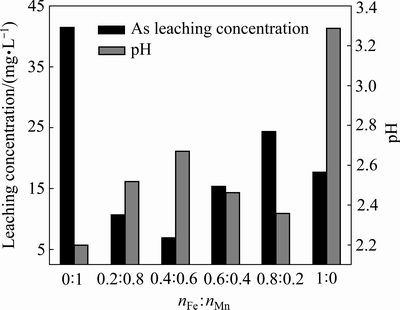
图4 铁锰摩尔比对砷酸铁渣稳定效果的影响
Fig. 4 Effect of mole ratio of Fe and Mn on stability of simulated arsenic bearing solid waste
2.3 稳定剂的添加量
采用稳定剂Fe-MnO2、铁锰比为0.4:0.6、球磨时间1 h,不同稳定剂添加量对球磨稳定效果的影响如图5所示。原渣浸出液pH值为2.44,而随着稳定剂用量的增加,pH值递增。随着稳定剂添加量的增加,砷的浸出毒性先降低,当添加量为10%时降至最低,为2.826 mg/L。当添加量增至12%时,砷的浸出毒性又上升到3.27 mg/L。添加量为6%时砷的浸出毒性下降最明显,此后下降幅度较小。因此,确定8%为最佳稳定剂添加量。
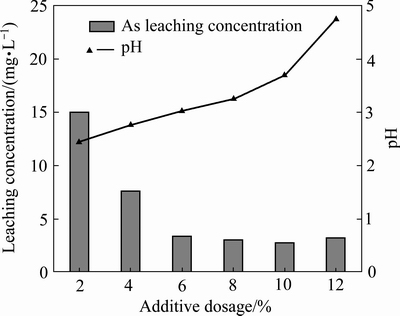
图5 稳定剂用量对砷酸铁渣稳定效果的影响
Fig. 5 Effect of stabilizer dosage on stability of simulated arsenic bearing solid waste
2.4 球磨时间
稳定剂为Fe-MnO2、添加量8%,铁锰比为0.4:0.6。不同球磨时间对含砷废渣的稳定效果如图6所示。球磨时间对浸出液pH值的影响不大,在2.35~3.6间波动。球磨时间为0~240 min时,砷的浸出毒性先降低,至60 min时降至最低,为2.364 mg/L。60 min后,砷的浸出毒性出现波动,并随着球磨时间延长,砷毒性逐渐增大。当球磨时间延长至240 min时其浸出毒性为6.71 mg/L,已经超过砷的浸出毒性限值。适宜范围内,球磨时间越长,反应效果越好。球磨时间过长,稳定剂粉末成团粘结,影响反应效果。此外,零价铁的固砷机制主要是其腐蚀产生的二价铁类化合物,而在MnO2作用下,过长的机械球磨时间可能会降低二价铁类化合物的活性。因此,最佳球磨时间为60 min。
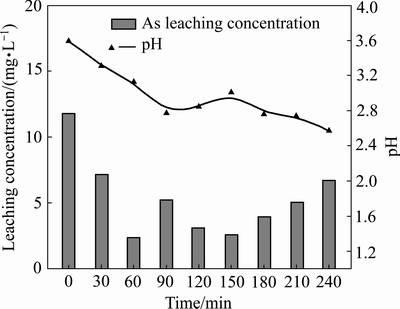
图6 球磨时间对砷酸铁渣稳定效果的影响
Fig. 6 Effect of ball-milling time on stability of simulated arsenic bearing solid waste
3 铁锰复合稳定剂稳定含砷废渣机理
3.1 球磨过程中的反应机制
3.1.1 球磨过程中发生的反应
球磨物料在球磨过程中发生的反应十分复杂。为了研究含砷废渣球磨稳定过程中的稳定机制,将不同球磨时间所得的稳定剂进行XRD分析,其结果如图7所示。由图7可看出,球磨0~4 h时,XRD谱上并没有出现新物质的衍射峰,说明在球磨4 h内,铁与MnO2不会发生明显的反应。相关研究表明,球磨后铁与MnO2表面会发生一定的反应,但由于反应放热过小,因此相关反应不能延续至反应内核。此外,球磨过程中铁与MnO2的特征峰均出现了一定的宽化现象,说明长时间机械球磨会对反应物的晶体结构产生一定的破坏作用。
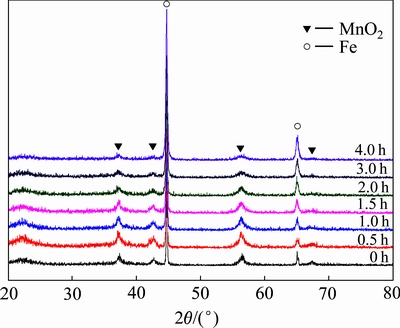
图7 球磨时间对稳定剂物相的影响
Fig. 7 Effect of ball-milling time on stabilizer of phase
3.1.2 球磨前后稳定剂粒度及比表面积变化
球磨过程能极大地改变零价铁粉的表面性质和颗粒大小。铁粉粒径越小,其活性越高,在酸性溶液中形成腐蚀层的速度越快、数量越多。XRD分析表明球磨过程中铁与MnO2并未发生明显的化学反应。故推测Fe-MnO2稳定砷是由于其在球磨过程中发生了物理变化,即表面性质和颗粒大小发生了改变。但是发生的变化仍在一个数量级,比表面及粒度有一定程度的增大,增强了反应的效果,但并非决定反应效果的主要因素。球磨前后稳定剂比表面积及粒度分布如表1、表2和图8所示。
球磨后零价铁粉累积粒径分布从球磨前的105 μm 降低到球磨后的40 μm。结合球磨铁粉SEM分析(见图9)可知,铁粉的形状由球磨前的颗粒状变成球磨后的片状,而片状铁粉对砷的腐蚀和稳定具有重要意义。因此,球磨过程仅仅改变了零价铁粉的粒径和表面形貌。此外,在球磨过程中,MnO2比表面积也有一定的增加,对零价铁稳定砷有促进作用。
表1 不同球磨时间下铁粉的比表面积
Table 1 Specific surface of Fe powder at different ball- milling time

表2 不同球磨时间下MnO2的比表面积
Table 2 Specific surface of MnO2 at different ball-milling time
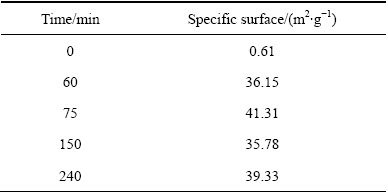
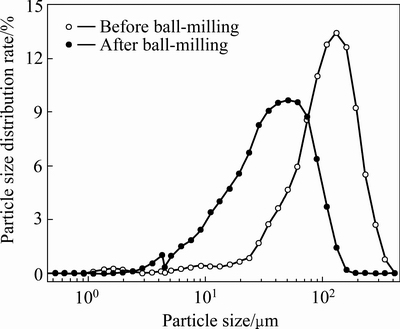
图8 球磨前后铁粉颗粒粒径分布
Fig. 8 Particle size distribution of Fe powder before and after ball-milling
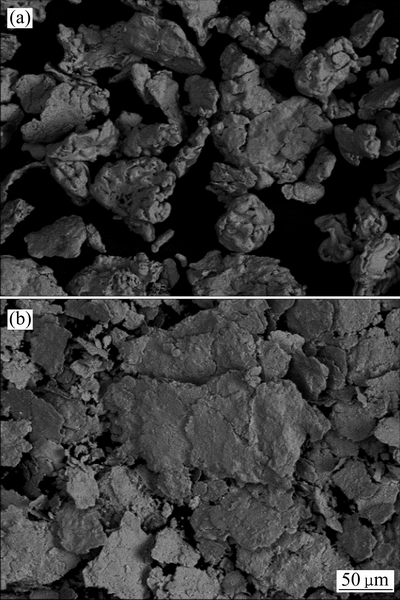
图9 铁粉球磨前后SEM像
Fig. 9 SEM images of Fe powder before(a) and after(b) ball-milling
3.2 腐蚀过程的反应机制
为了模拟球磨铁粉在酸性环境下物相和形貌的变化,将球磨铁粉浸泡于pH值为5.0的酸性溶液中并翻转振荡18 h,之后将铁粉取出干燥后进行XRD检测,结果见图10。
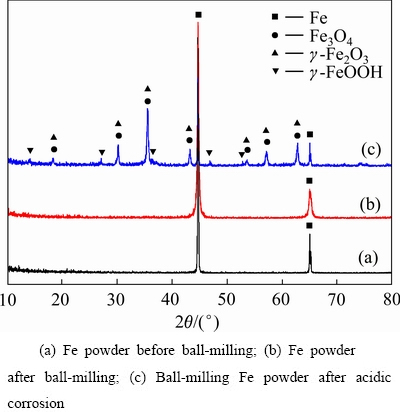
图10 球磨铁粉酸腐蚀前后的XRD谱
Fig. 10 XRD patterns of ball-milling Fe powder after acidic corrosion
球磨前后两者的特征峰没有明显的变化,球磨铁粉的特征峰比原铁粉的特征峰略宽。但是醋酸浸泡后的球磨铁粉XRD谱上明显出现了Fe3O4、γ-Fe2O3和γ-FeOOH的特征峰。说明球磨铁粉在醋酸溶液中表面被腐蚀生成了一层铁氧化物和水合氧化铁的腐蚀层。这一层腐蚀层对砷具有较强的吸附作用[30],发生的反应如下[31-33]:
2Fe0+2H2O+O2=2Fe2++4OH- (1)
4Fe2++2H2O+O2=4Fe3++4OH- (2)
Fe3++2H2O=FeOOH+3H+ (3)
2Fe0+2H2O+O2=2Fe(OH)2 (4)
4Fe0+6H2O+3O2=4Fe(OH)3 (5)
6Fe(OH)2+O2=2Fe3O4+6H2O (6)
2Fe(OH)3=Fe2O3+3H2O (7)
再将球磨铁粉和MnO2稳定剂浸泡于pH值为5.0的酸性溶液中,翻转振荡18 h,将铁粉取出干燥后进行XRD检测,结果如图11所示。Fe-MnO2吸附剂与零价铁粉产生了可与砷反应的铁氧化物腐蚀层,对砷有稳定作用。但Fe-MnO2稳定剂还明显出现了γ- FeOOH的特征峰。产生的MnO2、Mn3O4等锰类物质并未对砷有强烈的稳定作用。因此,Fe-MnO2稳定剂对砷的稳定作用主要是由于零价铁的作用,同时,加入MnO2促进了铁对砷的稳定作用。因此,球磨Fe-MnO2颗粒在酸性溶液中被腐蚀,其表面产生一层腐蚀层,而这层水铁矿类物质的腐蚀层对砷的吸附是废渣中砷的浸出毒性降低的主要原因。
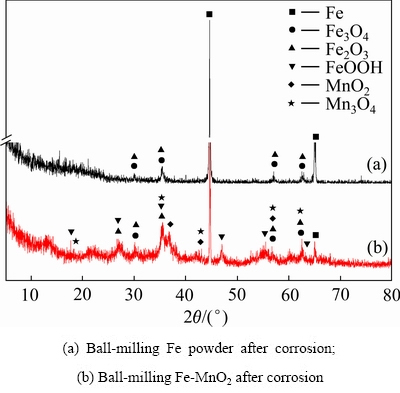
图11 球磨铁粉和MnO2稳定剂酸腐蚀后的XRD谱
Fig. 11 XRD patterns of ball-milling Fe-MnO2 stabilizer after acidic corrosion
3.3 机械力活化Fe-MnO2稳定砷的机制
3.3.1 球磨的作用
为了模拟Fe-MnO2稳定剂对砷的作用过程,将未球磨稳定剂和球磨后稳定剂分别置于砷浓度为1 g/L、pH值为5.0的砷酸钠溶液中翻转振荡18 h。过滤并收集残渣,对残渣进行SEM-EDS检测,其结果如图12所示。对比图12(a)与(b)可以看出,稳定剂球磨后表面絮状物质显著增多,并呈现球状规则絮状,与对砷有较强吸附作用的水铁矿物质近似。对比图12(a)与(c)、图12(b)与(d)可知,吸附砷后稳定剂表面絮状物质结成块状。
3.3.2 MnO2的作用
为分析MnO2在砷稳定机制中的作用,将球磨铁粉稳定剂和球磨Fe-MnO2稳定剂分别置于浓度为1 g/L、pH值为5.0的砷酸钠溶液中翻转振荡18 h。过滤并收集残渣,对残渣进行SEM检测,其结果如图13所示。由图13(a)可看出,在未添加MnO2的情况下,零价铁粉球磨腐蚀产物为杆状晶体,推断其为赤铁矿(γ-Fe2O3),并非对砷有较强吸附作用的水铁矿物质。而Fe-MnO2球磨后的腐蚀产物为絮状物(见图13(b)),根据水铁矿类物质大多呈无定型絮团状的结构特点,推断其以水铁矿类物质为主。自然条件下,水铁矿不稳定,很容易晶化为赤铁矿;然而锰原子可与水铁矿物质结合,促使其向更稳定的针铁矿或赤铁矿转化[34],使其保持对砷的高捕集特性。水铁矿与砷接触后,表面絮状物变成了稳定的块状物质(见图13(c)),说明对砷进行有效的吸附。由此可知,MnO2可以促进稳定剂表面水铁矿类物质的产生,进而促进了零价铁对砷的吸附,增强了体系对砷的稳定效果。
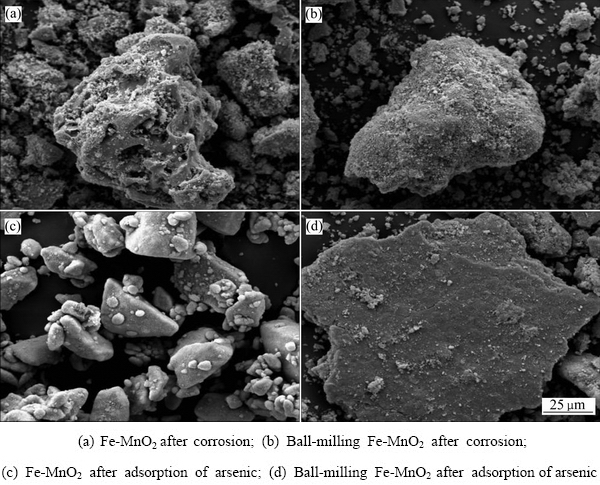
图12 球磨前后Fe-MnO2腐蚀及吸附砷后的SEM像
Fig. 12 SEM images of Fe-MnO2 and ball-milling Fe-MnO2 after acidic corrosion and adsorption of arsenic
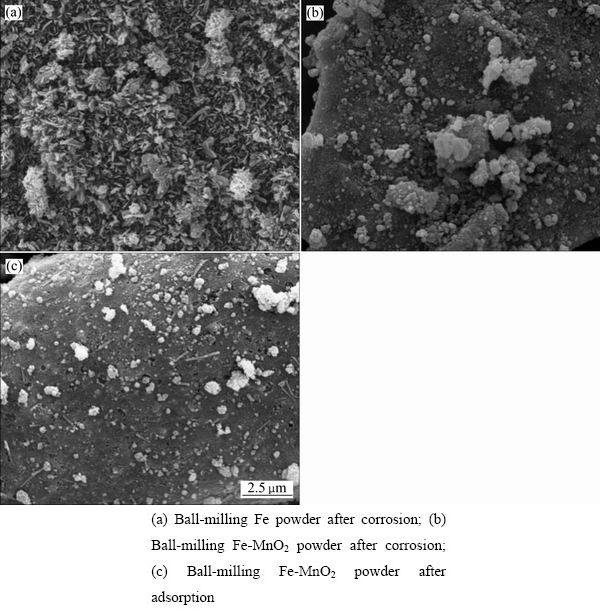
图13 不同球磨稳定剂腐蚀和吸附后的 SEM像
Fig. 13 SEM images of different ball-milling stabilizers after corrosion and adsorption
4 结论
1) 采用机械力球磨活化Fe-MnO2稳定剂可实现含砷废渣的稳定化。含砷废渣球磨稳定最佳工艺参数为稳定剂种类Fe-MnO2、稳定剂铁锰比为0.4:0.6、稳定剂用量8%、球磨时间1 h,此条件下砷的浸出浓度由51.45 mg/L 降至2.36 mg/L,浸出毒性低于国家危险废物鉴别标准浸出毒性鉴别(GB 5085—2007)。
2) 球磨铁粉在酸性环境下其表面会被腐蚀生成一层铁氧化物和水合氧化铁的腐蚀层,其对砷的吸附作用是废渣中砷浸出毒性降低的主要原因。故球磨Fe-MnO2稳定剂对砷的稳定作用主要是由于零价铁的作用,加入MnO2更大地促进了铁对砷的稳定能力。
REFERENCES
[1]  L, SUN Gui-fan, BERG M, ZHANG Qiang, XUE Han-bin, ZHENG Quan-mei, JOHNSON C A. Groundwater arsenic contamination throughout China[J]. Science, 2013, 341(6148): 866-868.
L, SUN Gui-fan, BERG M, ZHANG Qiang, XUE Han-bin, ZHENG Quan-mei, JOHNSON C A. Groundwater arsenic contamination throughout China[J]. Science, 2013, 341(6148): 866-868.
[2] 吴万富, 徐 艳, 史德强, 杨项军, 王世雄. 我国河流湖泊砷污染现状及除砷技术研究进展[J]. 环境科学与技术, 2015, 38(S1): 190-197.
WU Wan-fu, XU Yan, SHI De-qiang, YANG Xiang-jun, WANG Shi-xiong. The arsenic pollution status of the rivers and lakes in China and the research progress on arsenic removal techniques[J]. Environmental Science & Technology, 2015, 38(S1): 190-197.
[3] 王 萍, 王世亮, 刘少卿, 李艳霞, 何孟尝, 林春野. 砷的发生, 形态, 污染源及地球化学循环[J]. 环境科学与技术, 2010, 33(7): 90-97.
WANG Ping, WANG Shi-liang, LIU Shao-qing, LI Yan-xia, HE Meng-chang, LIN Chun-ye. Occurrence, speciation, source and geochemical cycle of arsenic[J]. Environmental Science & Technology, 2010, 33(7): 90-97.
[4] 贾 海. 高砷冶金废料的回收与综合利用[D]. 长沙: 中南大学, 2013.
JIA Hai. Recycling and comprehensive utilization of metallurgical waste with high arsenic content[D]. Changsha: Central South University, 2013.
[5] 马承荣. 含砷废渣资源化利用技术现状[J]. 广东化工, 2013, 40(6): 119-120.
MA Cheng-rong. Current situation of resource utilization technologies of arsenic waste residue[J]. Guangdong Chemical Industry, 2013, 40(6): 119-120.
[6] MIN Xiao-bo, LIAO Ying-ping, CHAI Li-yuan, YANG Zhi-hui, XIONG Shan, LIU Lin, LI Qing-zhu. Removal and stabilization of arsenic from anode slime by forming crystal scorodite[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1298-1306.
[7] 水志良, 陈起超, 水浩东. 砷化学与工艺学[M]. 北京: 化学工业出版社, 2014.
SHUI Zhi-liang, CHEN Qi-chao, SHUI Hao-dong. Arsenic chemistry and technology[M]. Beijing: Chemical Industry Press, 2014.
[8] 刘树根, 田学达. 含砷固体废物的处理现状与展望[J]. 湿法冶金, 2005, 24(4): 183-186.
LIU Shu-gen, TIAN Xue-de. Situation and prospect on treating of arsenic-containing solid waste[J]. Hydrometallurgy of China, 2005, 24(4): 183-186.
[9] BARTH E F. An overview of the history, present status and future direction of solidification/stabilization technologies for hazardous waste treatment[J]. Journal of Hazardous Material, 1990, 24(2): 103-109.
[10] 方兆珩, 石 伟, 韩宝玲, 夏光祥. 高砷溶液中和脱砷过程[J]. 化工冶金, 2000, 21(4): 359-362.
FANG Zhao-hen, SHI Wei, HAN Bao-ling, XIA Guang-xiang. Removal of arsenic from high arsenic solutions by scorodite precipitation[J]. Engineering Chemistry & Metallurgy, 2000, 21(4): 359-362.
[11] WANG Qian-kun, DENOPOULOS G P. A novel hydrometallurgical process for the immobilization of arsenic[C]// YANG Xian-wan. ICHM’ 98: Proceedings of the Third International Conference on Hydrometallurgy. Kunming: International Academic Publishers, 1998: 543-553.
[12] WU Su-mei, XUE Yu-zhi, ZHOU Li-mei, LIU Xiang, XU Ding-yong. Structure and morphology evolution in mechanochemical processed CuInS2 powder[J]. Journal of Alloys and Compounds, 2014, 600(25): 96-100.
[13] LU Sheng-yong, HUANG Jian-xin, PENG Zheng, LI Xiao-dong, YAN Jian-hua. Ball milling 2,4,6-trichlorophenol with calcium oxide: Dechlorination experiment and mechanism considerations[J]. Chemical Engineering Journal, 2012, 195/196: 62-68.
[14] STELLACCI P, LIBERTI L, NOTARNICOLA M, BISHOP P L. Valorization of coal fly ash by mechano-chemical activation: Part I. Enhancing adsorption capacity[J]. Chemical Engineering Journal, 2009, 149(1): 11-18.
[15] NASERI E, REYHANITABAR A, OUSTAN S, HEYDARI A A, ALIDOKHT L. Optimization arsenic immobilization in a sandy loam soil using iron-based amendments by response surface methodology[J]. Geoderma, 2014, 232/234: 547-555.
[16] SETOUDEH N, WELHAM N J. Ball milling induced reduction of SrSO4 by Al[J]. International Journal of Mineral Processing, 2011, 98(3/4): 214-218.
[17] TAKACS L. Self-sustaining reactions induced by ball milling[J]. Progress in Materials Science, 2002, 47(4): 355-414.
[18] CALOS N J, FORRESTER J S, SCHAFFER G B. A crystallographic contribution to the mechanism of a mechanically induced solid state reaction[J]. Journal of Solid State Chemistry, 1996, 122(2): 273-280.
[19] ZHANG Wang, HUANG Jun, YU Gang, DENG Shu-bo, ZHU Wan-ping. Mechanochemical destruction of dechlorane plus with calcium oxide[J]. Chemosphere, 2010, 81(3): 345-350.
[20] NOMURA Y, FUJIWARA K, TERADA A,NAKAI S, HOSOMI M. Mechanochemical degradation of γ-hexachlorocyclohexane by a planetary ball mill in the presence of CaO[J]. Chemosphere, 2012, 86(3): 228-234.
[21] INOUE T, MIYAZAKI M, KAMITANI M, KANO J, SAITO F. Dechlorination of polyvinyl chloride by its grinding with KOH and NaOH[J]. Advanced Powder Technology, 2005, 16(1): 27-34.
[22] CHAI Li-yuan, LIANG Yan-jie, KE Yong, MIN Xiao-bo, TANG Chong-jian, ZHANG Hai-jing, XIE Xian-de, YUAN Cui-yu. Mechano-chemical sulfidization of zinc oxide by grinding with sulfur and reductive additives[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(4): 1129-1138.
[23] KE Yong, CHAI Li-yuan, LIANG Yan-jie, MIN Xiao-bo, YANG Zhi-hui, CHEN Jie, YUAN Sheng. Sulfidation of heavy-metal- containing metallurgical residue in wet-milling processing[J]. Minerals Engineering, 2013, 53(6): 136-143.
[24] MONTINARO S, CONCAS A, PISU M, CAO G. Remediation of heavy metals contaminated soils by ball milling[J]. Chemosphere, 2007, 67(4): 631-639.
[25] MONTINARO S, CONCAS A, PISU M, CAO G. Immobilization of heavy metals in contaminated soils through ball milling with and without additives[J]. Chemical Engineering Journal, 2008, 142(3): 271-284.
[26] STELLACCI P, LIBERTI L, NOTARNICOLA M, BISHOP P L. Valorization of coal fly ash by mechano-chemical activation: Part Ⅱ. Enhancing pozzolanic reactivity[J]. Chemical Engineering Journal, 2009, 149(1/3): 19-24.
[27] SONG S, LOPEZ-VALDIVIESO A, HERNANDEZ-CAMPOS D J, PENG C, MONROY-FERNANDEZ M G,RAZO-SOTO I. Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite[J]. Water Research, 2006, 40(2): 364-372.
[28] LIU Rui-ping, SUN Li-hua, QU Jiu-hui, LI Gui-bai. Arsenic removal through adsorption, sand filtration and ultrafiltration: In situ precipitated ferric and manganese binary oxides as adsorbents[J]. Desalination, 2009, 249(3): 1233-1237.
[29] SUN F, OSSEO-ASARE K A, CHEN Yong-sheng, DEMPSEY B A. Reduction of As(Ⅴ) to As(Ⅲ) by commercial ZVI or As(0) with acid-treated ZVI[J]. Journal of Hazardous Materials, 2011, 196: 311-317.
[30] STREAT M, HELLGARDT K, NEWTON N L R. Hydrous ferric oxide as an adsorbent in water treatment: Part 3: Batch and mini-column adsorption of arsenic, phosphorus, fluorine and cadmium ions[J]. Process Safety and Environmental Protection, 2008, 86(1): 21-30.
[31] CHANG Dong-yin, CHEN Tian-hu, LIU Hai-bo, XI Yun-fei, QING Cheng-song, XIE Qiao-qin, FROST R L.A new approach to prepare ZVI and its application in removal of Cr(Ⅵ) from aqueous solution[J]. Chemical Engineering Journal, 2014, 244: 264-272.
[32] TRISZCZ J M, PORTA A, EINSCHLAG F S G. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal[J]. Chemical Engineering Journal, 2009, 150(2/3): 431-439.
[33] YE Meng, HUANG Jin, CHEN Rui, HE Qi-zhuang. Removal of arsenic (Ⅲ) from water by using a new class of zero-valent iron modified mesoporous silica molecular sieves SBA-15[J]. Advanced Materials Research, 2012, 356/360: 423-429.
[34] OUVRARD S, DEDONATO P H, SIMONNOT M O. 2005. Natural manganese oxide: Combined analytical approach for solid characterization and arsenic retention[J]. Geochimica et Cosmochimica Acta, 2005, 69(11): 2715-2724.
Stabilization of arsenic bearing solid waste with Fe-MnO2 activated by mechanochemical process
XU Hui1, MIN Xiao-bo1, 2, LIANG Yan-jie1, 2, WANG Yun-yan1, 2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China)
Abstract: The simulated arsenic bearing solid waste was prepared, and the parameters of stabilizer, mole ratio of Fe and Mn, dosage of stabilizer and ball-milling time for the stabilization effect were optimized. The results show that the extraction toxicity of arsenic decreases from 51.45 mg/L to 2.36 mg/L under the optimized conditions of stabilizer of Fe-MnO2, mole ratio of Fe and Mn of 0.4:0.6, stabilizer dosage of 8% and ball-milling of 1 h, which is lower than that of identification standards for hazardous wastes-identification for extraction toxicity (GB 5085—2007), and the stabilization of arsenic is accomplished. Stabilization mechanism of arsenic bearing solid waste with Fe-MnO2 activated by mechanochemical process was investigated with XRD, SEM-EDS, particle size and specific surface analysis. Because of the synergistic action of mechanochemical process and MnO2, the surface corrosion process of zero-valent iron powder is accelerated and large amount of ferrihydrite material absorbing arsenic effectively forms. Therefore, highly effective stabilization of arsenic is achieved.
Key words: arsenic bearing solid waste; stabilization; mechanochemical process; extraction toxicity
Foundation item: Project(51474247) supported by the National Natural Science Foundation of China; Project (201509050) supported by the National Environmental Protection Public Welfare Industry Research; Project(2014FJ1011) supported by the Key Projects of Science and Technology of Hunan Province, China
Received date: 2016-09-27; Accepted date: 2017-03-07
Corresponding author: WANG Yun-yan; Tel: +86-731-88830511; E-mail: wyy@csu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金面上资助项目(51474247);国家环境保护公益性行业科研专项(201509050);湖南省科技重大专项(2014FJ1011)
收稿日期:2016-09-27;修订日期:2017-03-07
通信作者:王云燕,教授,博士;电话:0731-88830511;E-mail: wyy@csu.edu.cn
摘 要:以模拟含砷废渣为研究对象,考察机械活化铁锰稳定剂对砷稳定效果的影响,确定了最优参数:稳定剂种类为Fe-MnO2、稳定剂铁锰摩尔比为0.4:0.6、稳定剂用量为8%、球磨时间为1 h。在此最佳条件下,可将砷酸铁渣中As的浸出毒性由51.45 mg/L降至2.36 mg/L,优于国家危险废物鉴别标准浸出毒性鉴别(GB 5085—2007)。借助X射线衍射、粒度和比表面分析、扫描电镜等,从球磨过程中铁锰反应机制、铁锰复合稳定剂腐蚀过程特征以及砷吸附后的铁锰复合稳定剂形貌结构变化等方面,研究铁锰复合稳定剂稳定含砷废渣的机理。在机械力化学和MnO2复合作用下,促进零价铁粉表面的腐蚀过程,生成了大量高效吸附砷的水铁矿类物质,实现砷的高效稳定。
[2] 吴万富, 徐 艳, 史德强, 杨项军, 王世雄. 我国河流湖泊砷污染现状及除砷技术研究进展[J]. 环境科学与技术, 2015, 38(S1): 190-197.
[3] 王 萍, 王世亮, 刘少卿, 李艳霞, 何孟尝, 林春野. 砷的发生, 形态, 污染源及地球化学循环[J]. 环境科学与技术, 2010, 33(7): 90-97.
[4] 贾 海. 高砷冶金废料的回收与综合利用[D]. 长沙: 中南大学, 2013.
[5] 马承荣. 含砷废渣资源化利用技术现状[J]. 广东化工, 2013, 40(6): 119-120.
[7] 水志良, 陈起超, 水浩东. 砷化学与工艺学[M]. 北京: 化学工业出版社, 2014.
[8] 刘树根, 田学达. 含砷固体废物的处理现状与展望[J]. 湿法冶金, 2005, 24(4): 183-186.
[10] 方兆珩, 石 伟, 韩宝玲, 夏光祥. 高砷溶液中和脱砷过程[J]. 化工冶金, 2000, 21(4): 359-362.


