Article ID: 1003-6326(2005)03-0536-06
Microstructure and corrosion property of AZ61 magnesium alloy by electromagnetic stirring
FANG Can-feng(房灿峰), ZHANG Xing-guo(张兴国), JI Shou-hua(季首华),
JIN Jun-ze(金俊泽), CHANG Yu-bao(常玉宝)
(Research Center of Foundry Engineering, Dalian University of Technology,
Dalian 116024, China)
Abstract:
The influence of permanent-magnet-driven stirring during solidification on the microstructure and corrosion property of AZ61 magnesium alloy was investigated. The corrosion behaviour of AZ61 was studied in 3.5mol/L NaCl by measuring electrochemical polarization. The results show that the permanent-magnet stirring refines the microstructure of AZ61 magnesium alloy, which improves the precipitation amount and distribution uniformity of β phase and decreases the content of hydrogen, but it has less influence on the distribution uniformity of Zn. The change of precipitation amount of β phase influences the corrosive nature of the matrix, and it has no direct proportion with the corrosion resistance of the matrix.
Key words:
magnesium alloy; permanent magnet; microstructure; corrosion CLC;
number: TG146.2 Document code: A
1 INTRODUCTION
The ever-increasing demands for light mass alloys in the aerospace and automobile industries have led to the development of new materials and advanced processing techniques. The electro-magnetic processing is an important processing technique and it has been frequently applied to improve the properties and performance of metals[1-3], such as making use of interaction between magnetic field and induced current to produce driving, stirring, purifying, transmitting or shape-control[4-6]. On the electromagnetic stirring, Vives[7, 8] firstly brought forward the method of permanent-magnet(PM) rotation. The mechanical rotation of permanent magnet produces rotatory magnetic field and induced current in the liquid metal, thus generates non-touch stir. At the present time, the more increased magnetic flux density and energy product of ferromagnetic permanent-magnet are, the more intensified stir effectiveness is.
Magnesium is one of the most activated and lightest metals. In particular, their high strength and stiffness to mass ratio make magnesium alloys extremely attractive for applications requiring light mass, such as transport, aerospace. The development of magnesium alloys in transportation applications were first used extensively during World WarⅠ[9]. In this paper, AZ61 magnesium alloys were cast by the method of PM rotation to study their microstructure and properties.
2 BASIC MECHANISM
Stirring of permanent magnet generates the changing magnetic field at the domain of the molten metal pool. According to the theory of the electromagnetic field, the Faradays law of electromagnetic induction can be given as follows:
![]()
where E is the electric-field intensity, B is the density of magnetic field. The changing magnetic field produces induced current. Moreover, the Ohms law can be expressed in the flowing metal as follows:
![]()
where σ is the electric conductivity of metal, J is the induced current density in the molten metal, v is the rate of flow of the molten metal. As a result, the interaction of induced current and magnetic field would produce Lorentz force, i.e. electromagnetic volume force F:
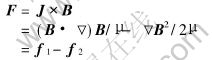
where μ is the permeability of metal. The Lorentz force consists of two parts: f1 is the rotational part which results in stirring of the liquid metal and f2 is the irrotational part or magnetic pressure which can support a static head. As the frequency of the changing magnetic field is decreased, rotational forces dominate gradually[10]. In this experiment, f1 was used for stirring of magnesium alloys, and effects of permanent magnet stirring on the microstructure and corrosion performance were studied.
3 APPARATUS AND METHOD
The sketch of the experimental apparatus is shown in Fig.1. The working substance was the ferromagnetic magnetic material, NbFeB permanent-magnet, which was driven by electric motor. The rotation velocity of permanent magnet was controlled by frequency-converter which ranges between 0r/s and 50r/s. The height of the permanent magnet was 2cm. The inner container was made of stainless steel, which had been preheated to 400℃. Commercial AZ61 magnesium alloys were used and their chemical composition is shown in Table 1. The alloy was melted in electric resistance crucible furnace under fusing agent, and cast at 730-740 ℃. At the same time, electric motor was started, and the rotation velocity of permanent magnet was controlled at 15, 10 and 0r/s, respectively. The revolution of permanent magnet produced a changing magnetic field, and stirred the liquid magnesium alloy. The ingot was air solidified, and its size was d30mm×100mm.
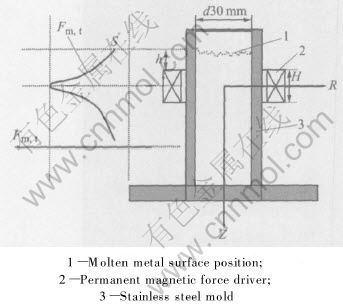
Fig.1 Schematic of permanent magnetic force driving apparatus and principle
Table 1 Chemical composition of AZ61 alloy(mass fraction, %)

Microstructure and corrosion property of AZ61 magnesium alloy The obtained samples were selected at the height of the position of permanent magnet. The metallographic specimens were prepared by cutting, grinding, polishing and etching. The etchant was the solution of 4% glacial acetic acid, 4% ethylene alcohol, 8% concentrated hydrogen nitrate and 84% de-ionized water. The microstructure was observed by optical microscope and electron probe. The crystal structure features were examined by X-ray diffraction(XRD) technique. Electron probe analysis was done on a Shimadzu EPMA-1600 and XRD analysis was conducted on a Shimadzu XRD-6000 diffractometer using CuKα radiation.
Potentiodynamic polarization curves were measured using an electrochemical measurement system, Potentiostat & Galvanostat Model CP6, in which Pt was used as a counter electrode, and saturated calomel electrode(SCE) was used as a reference electrode. The magnesium electrodes of each material were cut from the metallographic specimens, polished to 800 grade silicon carbide and cleaned in acetone. Polarization curves were measured at room temperature in an electrolytic cell containing about 200mL of Mg(OH)2 saturated 3.5%NaCl solution, which was exposed to the air. The scan rate was 60mV/min. In order to avoid influence of some transient processes which may occur after a polarization applying to the working electrode, the polarization curve measured in the first few minutes should be discarded better. Hence, the cathodic polarization for study started from about -200mV relative to corrosion potential, and the part beyond 200mV below the corrosion potential was discarded.
4 RESULTS AND DISCUSSION
4.1 Phase constitution
The XRD patterns of the AZ61 magnesium alloys by PM stirring are shown in Fig.2, the rotation speed correspondingly being 15r/s(a), 10r/s(b) and 0r/s(c). All the diffraction patterns contain peaks due to α-Mg, β-Mg17Al12 and Mg2C3, respectively. The application of PM stirring makes the α-Mg peaks shift little to lower angular position. In binary Mg-Al alloys, the shift of α-Mg peaks to higher angular positions indicates that the α-Mg solid solution phase has relatively smaller lattice constants. The solid solubility of Al in α-Mg affects the lattice constant. The lattice constant decreases with the increase of the solid solubility of Al[11]. Therefore, based on the analysis given above, a conclusion can be drawn that the PM stirring reduces the quantities of solid solubility of Al in α-Mg, which results in the increase of β phase. Mg2C3 is formed by the reaction of Mg and C entrained into molten magnesium alloys during melting and pouring. The patterns of Mg2C3 are lamina and grow along the skeleton-shape β phase,
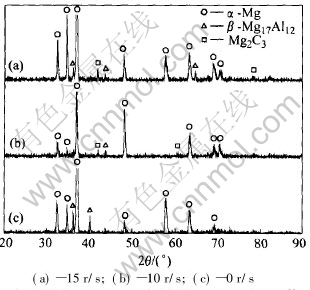
Fig.2 XRD patterns of AZ61 magnesium alloys stirred by permanent magnetic force at different rotation speeds
4.2 Microstructure
The microstructures of magnesium alloys stirred using PM are shown in Fig.4. It shows the as-cast microstructures when the rotation rate is 15, 10 and 0r/s, respectively. Figs.4(a), (b) and (c) represent the side of ingot, and Figs.4(a′), (b′) and (c′) represent the center. The as-cast microstructure of magnesium alloy is mainly composed of two parts, of which the matrix is primary α-Mg, surrounded by the mixture of eutectic α-Mg and β-Mg17Al12 phase. Under the three conditions, the grain sizes of the side are smaller than those of the center, and more β-Mg17Al12 phases precipitate relatively. The PM stirring results in the grain size reducing, the β phase increasing and distributing more uniformly and continuously, but the amount of β phase precipitate decreases with the increasing speed of PM rotation. The flow of molten magnesium alloys increases the temperature gradient and shearing force near the solid-liquid interface, which
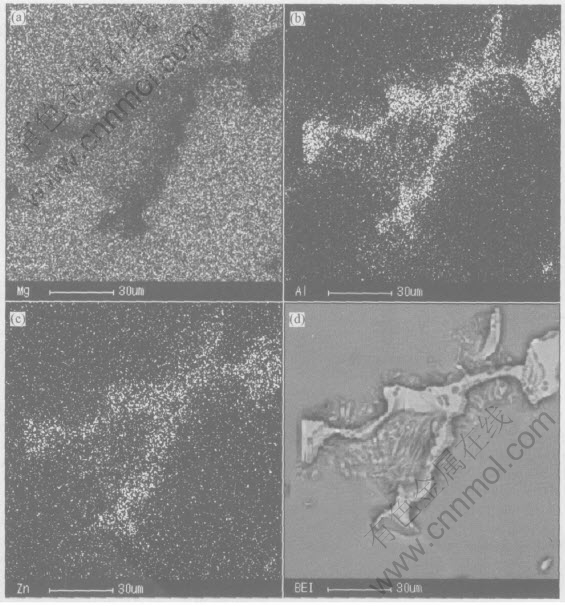
Fig.3 Backscattered electron images of β-Mg17Al12 phase in sample a
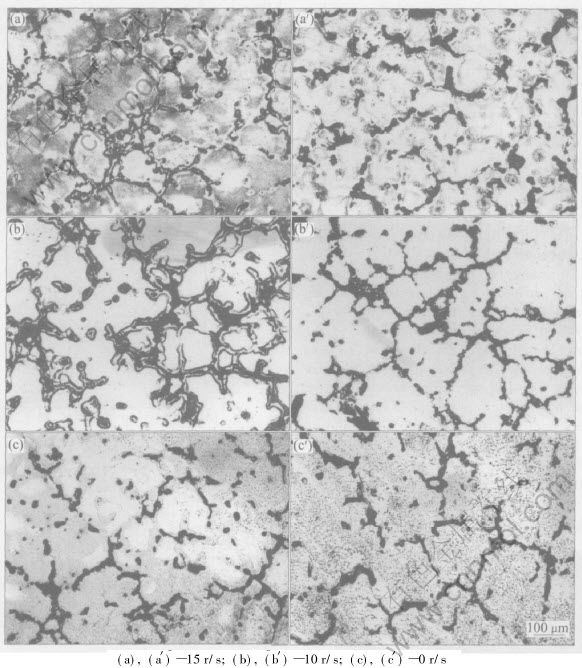
Fig.4 Optical micrographs of AZ61 magnesium alloys stirred by permanent magnetic rotation rate
makes the solidified microstructure refined and the precipitation of β phase uniform. This conclusion of observation is accordant to the analysis of phase structure. The equilibrium phase diagram of Mg-Al indicates that non-equilibrium, metastable, eutectic normally forms during the equilibrium solidification of AZ61 magnesium alloys. Because of magnesium having higher diffusion activation energy and lower crystal latent heat, the cooling rates in commercial casting processes will cause eutectic to be formed during solidification of magnesium alloys containing more than 2%Al, and it increases with the rise of the cooling rate[12], which indicates that the stirring improves the solidifying rate of the melt. The uniformity and more precipitation of β phase have great effects on the properties of magnesium alloys. In Mg-Al alloy, β-Mg17Al12 phase serves a dual purpose in corrosion: the β phase can act either as a barrier or as a galvanic cathode. If it is present as a small fraction, it serves mainly as a galvanic cathode, and accelerates the overall corrosion of the matrix. However if its fraction is high, then it may act mainly as an anodic barrier against the overall corrosion of the alloy[13]. Therefore, it should be possible to use the properties of β-Mg17Al12 to develop corrosion-resistant Mg alloys. Moreover, β phase pins up grain boundary and inhibits rotation of high-temperature grain boundary. The facial component distribution of β-Mg17Al12 phase is similar in three cases. The facial component distribution of β phase of sample a is shown in Fig.3, which indicates that the distribution of Al and Zn is mainly in the domain of β phase, and has little fraction in the α-Mg matrix. Moreover, Zn lays in matrix, acting as the solid solution form[14]. Electromagnetic stirring is propitious to the distribution uniformity of the melt component[15]. The distribution of Zn isnt uniformly after PM stirring, as the effects of stirring on distribution uniformity are relative to flow pattern and cooling rate. Under the condition of low cooling rate or isothermal stirring, the uniformity of component distribution is obvious. In this experiment, the ingot has small size and solidifies quickly, therefore, the distribution of Zn in β phase doesnt chang greatly.
The microporosities shown in Fig.5 are observed in samples b(b′) and c(c′), but not found in samples a(a′). There are two reasons of shrinkage porosity present in Mg-Al alloy[12]: 1) the solidification shrinkage is responsible for porosity formation, the accumulative feeding capacity of Mg alloy is low because of its low heat conductivity; 2) the dissolved hydrogen does contribute to the incidence of microporosity. The above two reasons occur in unison and act collaboratively to form microporosity. The PM stirring reduces the extent of microporosity. With the increase of rotation rate of PM, less microporosity appears in samples b(b′) than in samples c(c′), and it disappears in samples a(a′). This investigation of microporosity suggests that the PM stirring enhances the interdendritic feeding capacity of Mg alloys, and decreases the solubility of the gas simultaneously.

Fig.5 Microporosity in sample b
4.3 Electrochemical behavior
Fig.6 shows the electrochemical behavior of samples a, b and c, stirred using PM rotation rates 15, 10 and 0r/s, respectively. The corrosion potential increases in the order Ecorr(a)〈Ecorr(c)〈Ecorr(b). The magnitude of the cathodic current density around its corrosion potential also increases in the above order. Fig.6 shows that the cathodic hydrogen evolution rates under the three condition alloys increases in the following order b〈 c〈a. From Fig.4, the β phase precipitate increases in the order c〈a〈b. This phenomenon can be explained that a little increase of β phase will accelerate the corrosion of the matrix by a galvanic effect. Because of the existence of large amount of β phase in sample b, the β phase acts as a barrier influence, and improves the corrosion resistance of the matrix. In other words, the precipitation amount of β phase does not increase with the increasing speed of PM rotation, and the increase of the content of β phase does not imply that the matrix has a better corrosion resistance. These are accordant to the opinion of Song et al[13].
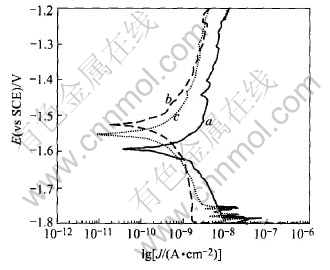
Fig.6 Polarization curves of samples a, b, c in 3.5mol/L NaCl
5 CONCLUSIONS
1) The PM stirring refines the microstructures of AZ61 magnesium alloys and improves the precipitation amount and distribution uniformity of β phase.
2) The PM stirring enhances interdendritic feeding capacity of Mg alloys, and simultaneously reduces the solubility of the gas.
3) A little increase in the content of β phase does not improve the corrosion resistance of the matrix by a galvanic effect. When the content of β phase is considerable, it will serve as a barrier to protect the matrix.
REFERENCES
[1]Cho Y W, Chung S H, Shim J D, et al. Fluid flow and heat transfer in molten metal stirred by a circular inductor [J]. International Journal of Heat and Mass Transfer, 1999(42): 1317-1326.
[2]Vanags J, Viesturs U, Fort I. Mixing intensity studies in a pilot plant stirred bioreactor with an electromagnetic drive [J]. Biochemical Engineering Journal, 1999(3): 25-33.
[3]Spitzer K H, Dubke M, Schwerdtfeger K. Rotational electromagnetic stirring in continuous casting of round strands [J]. Metall Trans, 1986, B17: 119-131.
[4]CAO Zhi-qiang, ZHANG Xing-guo, JIA Fei. Microstructures and mechanical characteristics of EMC and DCC 2024 aluminum alloys[J]. Materials Science and Engineering A, 2002, A327(2): 133-137.
[5]ZHANG Xing-guo, HE Wen-qing, QU Ruo-jia, et al. Technology of hot-top electromagnetic casting for Al alloy slab[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(7): 1084-1088.(in Chinese)
[6]WU Jia-xiong, REN Zhong-ming, ZHANG Bang-wen, et al. Electromagnetic purification of aluminum alloy melt only by alternating current[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(3): 354-358.(in Chinese)
[7]Vives C. Elaboration of semisolid alloys by means of new electromagnetic rheocasting processes [J]. Metall Trans, 1992, 23B(3): 189-205.
[8]Vives C. Elaboration of metal matrix composites from thixotropic alloy slurries using magnetohydrodynamic caster [J]. Metall Trans, 1993, 24B(6): 493-510.
[9]Maker G L, Kruger J. Corrosion of magnesium [J]. International Materials Reviews, 1993, 38(3): 138-153.
[10]Asai S. The trend of electromagnetic processing of materials [J]. Iron and Steel, 1989, 75(1): 32-41.(in Japanese)
[11]Cho S S, Chun B S, Won C W, et al. Structure and properties of rapidly solidified Mg-Al alloys [J]. Journal of Materials Science, 1999, 34(17): 4311-4320.
[12]Dahle A K, Lee Y C, Paul M D, et al. Development of the as-cast microsturcture in magnesium-aluminium alloys [J]. Journal of Light Metals, 2001, 1(1): 61-72.
[13]Song G L, Atrens A, Matthew D. Influence of microstructure on the corrosion of diecast AZ91D [J]. Corrosion Science, 1999, 41(2): 249-273.
[14]LIU Zheng, ZHANG Kui, ZENG Xiao-qin, et al. Theoretical Basis and Application of Magnesium-Base Alloy [M]. Beijing: Mechanical Industry Press, 2002.
[15]YUAN Xiao-guang, LIU Zheng, XU Yi. Effect of electromagnetic cast on microstructure and mechanical properties of AZ91D alloy [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(4): 784-790. (in Chinese)
(Edited by LI Xiang-qun)
Foundation item: Projects(50234020; 50475157) supported by the National Natural Science Foundation of China; Project(105052) supported by the Ministry of Education of China
Received date: 2004-09-22; Accepted date: 2004-12-19
Correspondence: FANG Can-feng, PhD; Tel: +86-411-84706183; E-mail: fcf@student.dlut.edu.cn


