Trans. Nonferrous Met. Soc. China 26(2016) 1396-1402
Influences of transition metal on structural and electrochemical properties of Li[NixCoyMnz]O2 (0.6≤x≤0.8) cathode materials for lithium-ion batteries
Cheng-chi PAN, Yi-rong ZHU, Ying-chang YANG, Hong-shuai HOU,
Ming-jun JING, Wei-xin SONG, Xu-ming YANG, Xiao-bo JI
Cheng-chi PAN, Yi-rong ZHU, Ying-chang YANG, Hong-shuai HOU,
Ming-jun JING, Wei-xin SONG, Xu-ming YANG, Xiao-bo JI
Key Laboratory of Resources Chemistry of Nonferrous Metals, Ministry of Education,
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 21 April 2015; accepted 12 October 2015
Abstract:
p; Abstract: Li[NixCoyMnz]O2 (0.6≤x≤0.8) cathode materials with a typical hexagonal α-NaFeO2 structure were prepared utilizing a co-precipitation method. It is found that the ratio of peak intensities of (003) to (104) observed from X-ray diffraction (XRD) increases with decreasing the Ni content or increasing the Co content. The scanning electron microscopy (SEM) images reveal that the small primary particles are agglomerated to form the secondary ones. As the Mn content increases, the primary and secondary particles become larger and the resulted particle size for the Li[Ni0.6Co0.2Mn0.2]O2 is uniformly distributed in the range of 100-300 nm. Although the initial discharge capacity of the Li/Li[NixCoyMnz]O2 cells reduces with decreasing the Ni content, the cyclic performance and rate capability are improved with higher Mn or Co content. The Li[Ni0.6Co0.2Mn0.2]O2 can deliver excellent cyclability with a capacity retention of 97.1% after 50 cycles.
Key words:
Li[NixCoyMnz]O2; electrochemical performance; cathode material; lithium-ion battery ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;
1 Introduction
Lithium-ion batteries (LIBs) possess numerous outstanding features including high energy density, excellent conversion efficiency, improved safety and long cycle life [1,2]. It is important to research the promotion of cathode materials in the application potential of LIBs. Note that layered LiCoO2 has been limited by the relatively low specific capacity and high cost of cobalt [3], and olivine LiFePO4 and spinel LiMn2O4 also do not exhibit high enough gravimetric and volumetric energy densities [4-6]. In comparison, lithium nickel cobalt manganese oxides have been investigated as advanced positive electrode materials for lithium-ion batteries, in which Ni-rich material Li[NixCoyMnz]O2 (NCM) (x≥0.6) [7-9] has attracted significant interest as a promising cathode material for application in plug-in hybrid vehicles (PHEVs) and electric vehicles (EVs), mainly coming from its high capacity of LiNiO2, good cycle performances and excellent rate capability of LiCoO2, and low price and safety of LiMnO2 [10-16].
Since OHZUKU and MAKIMURA [17] first proposed that Li[Ni1/3Co1/3Mn1/3]O2 material owned excellent cyclability in 2001, many researchers [18-21] have extensively focused on the Li[NixCoyMnz]O2 material with various as-tuned values of x, y and z. Even though Li[Ni0.8Co0.1Mn0.1]O2 has shown poor cycle life and safety issues, it can provide high capacity of approximately 200 mA·h/g, still remaining attractive for high-energy batteries [22]. In consideration of maintaining its high capacity, it is indispensable for substitutions of Ni with Co and/or Mn to stabilize its structural stability and further improve its electrochemical performances. Conversely, Ni is beneficial to increasing the lattice cell and the specific capacity, but the over-high content of Ni can lead to more cation mixing and exhibits irreversible initial capacities [23,24]. Though Co incorporation is effective to suppress the migration of transition metal ions into the Li sites, the increase of Co content can result in a decrease of the lattice cell and a reduction in specific capacity [25,26]. It should be noted that Mn is electrochemically inactive to maintain the structural stability of the α-NaFeO2 phase, while superfluous Mn might suffer from a phase transition from a layered structure to a spinel one [27-29].
So far, the optimum compositions of transition metal ions in Li[NixCoyMnz]O2 (x≥0.6) for high capacity and acceptable thermal stability have not been clearly understood. Additionally, the core-shell-structured and concentration-gradient cathode materials based on lithium nickel cobalt manganese oxides [30-32], showing outstanding cycling performances and thermal stability as well as high reversible capacity, have been considered as urgent areas of research focus. Hence, it is necessary to systematically study the Li[NixCoyMnz]O2 (x≥0.6) in efforts to produce desirable cathode materials. To our knowledge, there are few studies reported for interchangeable Co and Mn replacement for Ni in type of Li[NixCoyMnz]O2 (x≥0.6) cathode materials.
In this work, the effects of the transition metal composition on the structural and electrochemical properties of Li[NixCoyMnz]O2 (0.6≤x≤0.8) applied in LIBs were reported, and the variation of Co and Mn interchangeable substitution for Ni in Li[Ni0.8Co0.1Mn0.1]O2 was investigated in details. The optimal composition of the lithium transition metal oxide Li[Ni0.6Co0.2Mn0.2]O2 with uniform size, excellent cycle performance and rate capability was obtained, which established the foundation for full concentration gradient cathode materials in Li[NixCoyMnz]O2 (0.6≤x≤0.8). We will use the following abbreviations, for x=0.8, y=0.1, z=0.1, 811; x=0.7, y=0.2, z=0.1, 721; x=0.7, y=0.1, z=0.2, 712; x=0.6, y=0.2, z=0.2, 622, respectively.
2 Experimental
Li[NixCoyMnz]O2 (811, 721, 712, 622) powders with different kinds of metal compositions were prepared by the co-precipitation method followed by solid-state reaction. The appropriate amounts of NiSO4·6H2O, CoSO4·7H2O, and MnSO4·5H2O were undertaken as the starting materials and dissolved in the distilled water. The transition metal sulfate solution with a concentration of 2.0 mol/L was slowly dripped into the beaker (150 mL) under a N2 atmosphere. Concurrently, a 2.0 mol/L NaOH solution (aqueous) as a coprecipitating agent was used to adjust the pH value and the desired amount of NH4OH solution (aqueous) as a chelating agent was separately pumped into the continuously stirred beaker at a constant rate. The reactor temperature was maintained at 60 °C for 4 h with closely controlling the concentration, pH value, temperature, and stirring speed of the mixture. The obtained precipitate was filtered and washed several times to ensure that the residual ions (Na+, SO42-, or others) were almost removed. The precipitate was then dried in a vacuum oven at 40 °C for 12 h to remove the adsorbed water, and the [NixCoyMnz](OH)2 (811, 721, 712, 622) precursor with a homogeneous distribution was obtained. LiOH and [NixCoyMnz](OH)2 (811, 721, 712, 622) with the molar ratio of 1.02 were thoroughly mixed in ethanol using a mortar and pestle, which were preheated to 480 °C for 4 h with the heating rate of 4 °C/min in the flowing oxygen atmosphere, and then calcined for 15 h at various temperatures [18,19,31,33,34]: 780 °C for 811, 800 °C for 721, 820 °C for 712 and 850 °C for 622. The main reason is that the materials 811, 721,712 and 622, which have different Ni contents, need different calcine temperatures.
Powder X-ray diffraction (XRD, Rigaku, Rint-2000) using Cu Kα radiation was employed to identify the crystalline phases of the prepared powders at each stage. The XRD data were obtained in the 2θ range of 10°-80° in a continuous scan mode with a step size of 0.02°. The lattice parameters of Li[NixCoyMnz]O2 (811, 721, 712, 622) were calculated from the XRD patterns using the least-squares method based on the space group R3m. The morphologies of the prepared powders were observed using scanning electron microscopy (SEM, JSM-6510, JEOL, Japan). Chemical compositions (lithium and metals) of the obtained powders were determined by atomic absorption analysis (Vario 6, Analyticjena, Germany) and are summarized in Table 1.
The cell consisted of a positive cathode and a lithium metal anode separated by a porous polypropylene film. Positive electrodes were fabricated using a mixture of Li[NixCoyMnz]O2 active materials (80%), carbon black (10%), and polyvinylidene fluoride (10%) (mass fraction) in N-methyl-pyrrolidone. The slurry was cast onto aluminum foil and dried at 110 °C for 12 h in a vacuum oven. The electrochemical characterizations were tested using 2016 coin-type cells equipped with a Li metal negative electrode and a counter electrode. The electrolyte solution was 1 mol/L LiPF6 in a mixture of ethylene carbonate, dimethyl carbonate and ethyl methyl carbonate (volume ratio of 1:1:1, PANAX ETEC Co. Ltd., Korea). The cells were assembled in an argon filled glove box (MB-Labstar (1200/780), M.Braun Inertgas- Systeme GmbH, Germany) where both the moisture and oxygen contents were less than 1×10-6. Cycling tests were performed at a 0.2C rate (0.1C=20 mA/g) in the voltage range of 2.8-4.3 V (vs Li+/Li) at room temperature using a BT2000 Battery testing system (BT2000-10V10A8CH, ARBIN Corporation, USA).
3 Results and discussion
The results of chemical analysis for LiNixCoyMnzO2 (811, 721, 712, 622) are shown in Table 1, confirming that the samples prepared by the mixture of hydroxide method possess a composition similar to the nominal ratio in the mixtures.
Table 1 Chemical compositions of as-prepared samples Li[NixCoyMnz]O2 ( 811, 721, 712, 622 )

Figure 1 shows the XRD patterns of cathode material LiNixCoyMnzO2 (811, 721, 712, 622) powders. A layer structure based on a hexagonal α-NaFeO2 structure (space group: R3m) is observed for all of the powders without any obvious impurities and secondary phases. Note that for x=0.8 in 811 (Fig. 1(a)), the split peaks of (006)/(102) and (108)/(110) are hard to distinguish from each other, which become better defined by decreasing the amount of Ni in Figs. 1(b)-(d), suggesting more developed crystalline layer materials. It means that higher Ni content can bring less-ordered layer structure from split peaks. In fact, these are also evidenced from the ratio of relative intensity of (003) and (104) peaks. It is well known that the intensity ratio of I(003)/I(104) is utilized as the major indication of cation mixing [29], where a small amount of nickel ions (rNi2+=0.69 may take place of lithium ions (rLi+=0.76
may take place of lithium ions (rLi+=0.76 ) in the Li layer. It is shown that the Ni2+ in the Li layer cannot only decrease the discharge capacity but also slow down the diffusivity of Li+, while such structural disorder is considered as a main cause for this kind of poor electrochemical performance [23,24].
) in the Li layer. It is shown that the Ni2+ in the Li layer cannot only decrease the discharge capacity but also slow down the diffusivity of Li+, while such structural disorder is considered as a main cause for this kind of poor electrochemical performance [23,24].
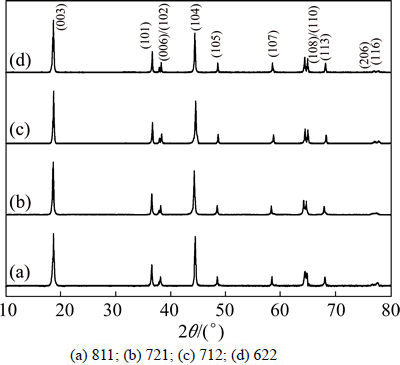
Fig. 1 XRD patterns of as-prepared Li[NixCoyMnz]O2
Table 2 shows the structural parameters of LiNixCoyMnzO2 (811, 721, 712, 622) and the intensity ratio of the I(003)/I(104). The intensity ratio of the I(003)/I(104) increases from 1.085 in 811 to 1.336 in 622 as the Ni content decreases, which is clearly resulted from the similar ionic radius of Ni2+ and Li+.
However, the intensity ratio of 721 exceeds that of 712, which may come from the increase of the content of Co, since higher ratio of I(003)/I(104) can be found for LiCoO2 which shows clear split peaks of (006)/(102) and (108)/(110) pairs as well [19,27]. In consideration of the ionic radius of Ni2+ (0.69 ), Ni3+ (0.60
), Ni3+ (0.60  ), Co3+ (0.54
), Co3+ (0.54  ), and Mn4+ (0.53
), and Mn4+ (0.53  ), with the decrease of Ni content in LiNixCoyMnzO2 (811, 721, 712, 622), the values of lattice parameter of c and unit cell volume increase, which can be attributed to the Mn or/and Co substitution for Ni, leading to the increased Ni2+ concentration from partial reduction of Ni3+ caused by balancing the cation and anion in LiNixCoyMnzO2 (811, 721, 712, 622) [27]. In addition, no apparent trend in the lattice parameter of a is found as the Mn content decreases. As discussed above, it should be noted that the change of the Mn content is not a simple substitution reaction, and it is believed that its structure can also be influenced by other transition metal ions and sintering temperature [29].
), with the decrease of Ni content in LiNixCoyMnzO2 (811, 721, 712, 622), the values of lattice parameter of c and unit cell volume increase, which can be attributed to the Mn or/and Co substitution for Ni, leading to the increased Ni2+ concentration from partial reduction of Ni3+ caused by balancing the cation and anion in LiNixCoyMnzO2 (811, 721, 712, 622) [27]. In addition, no apparent trend in the lattice parameter of a is found as the Mn content decreases. As discussed above, it should be noted that the change of the Mn content is not a simple substitution reaction, and it is believed that its structure can also be influenced by other transition metal ions and sintering temperature [29].
Figures 2(a)-(d) illustrate the SEM images of these four as-prepared [NixCoyMnz](OH)2 (811, 721, 712, 622) powders, respectively. The morphologies of the samples show large agglomerates composed of rather small layered particles with the size in the range of 100-300 nm. The formation of the hydroxide precipitate can be attributed to the primary chemical precipitation from cations and hydroxyl groups, which grew and agglomerated in the violent stirring solution.
As the Mn content increases and the Ni content decreases, the shape of primary and secondary particles changes from a thin thread type to a thick one and exhibits uniform particle size distribution. Therefore, it is proposed that the Mn content has much influence on the morphological characteristics of the [NixCoyMnz](OH)2 particles [27,35].
Figure 3 shows the morphologies of primary particles on the surfaces of he Li[NixCoyMnz]O2 (811, 721, 712, 622) powders calcined at different temperatures. It is clear that smoothly edged polyhedron and round particles are observed instead of layered ones on the surface of hydroxides after the heat treatment.
Table 2 Structural parameters and intensity ratio of I(003)/I(104) of Li[NixCoyMnz]O2 (811, 721, 712, 622 )
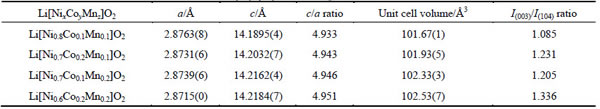

Fig. 2 SEM images of as-coprecipitated [NixCoyMnz](OH)2 hydroxides
Fig. 3 SEM images of Li[NixCoyMnz]O2
Figure 4 illustrates the initial voltage versus capacity curves on charge and discharge between 2.8 and 4.3 V for cells with Li metal anodes and cathodes of the following materials: Li[Ni0.8Co0.1Mn0.1]O2 (811), Li[Ni0.7Co0.2Mn0.1]O2(721), Li[Ni0.7Co0.1Mn0.2]O2 (712), and Li[Ni0.6Co0.2Mn0.2]O2 (622) at a constant current density of 20 mA/g (0.1C). The initial discharge capacity of Li/Li[NixCoyMnz]O2 cells reduces with decreasing the Ni content with the values of 202.2 mA·h/g for 811, 186.8 mA·h/g for 721, 182.0 mA·h/g for 712 and 173.4 mA·h/g for 622, which indicates that Ni is a main redox species in the host structure during the tested range.
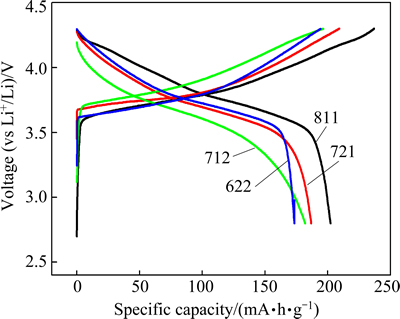
Fig. 4 Initial charge-discharge capacities of Li/Li[NixCoyMnz]O2 cells at current density of 20 mA/g between 2.8 and 4.3 V at 25 °C
It is also found that the irreversible capacities of Li[NixCoyMnz]O2 (811, 721, 712, 622) vary considerably with the formation of the solid electrolyte interphase (SEI) layer and the loss of lithium [37,38]. Meanwhile, the initial discharge capacity of 721 is greater than that of 712, showing that the increased Co content in the Li[NixCoyMnz]O2 can reduce the resistance of the diffusion of lithium ions [36].
Figure 5 shows the discharge capacity versus the cycle number of the Li/ Li[NixCoyMnz]O2 (811, 721, 712, 622) cells between 2.8 and 4.3 V at a constant current density of 40 mA/g (0.2C). As is well known, Ni-rich Li[NixCoyMnz]O2 (x≥0.6) typically suffers from poor Li+ intercalation stability, which results from the formation of a more stable spinel phase or stable LixNi1-xO phase [29,39,40], leading to an increase of the interfacial resistance upon cycling.

Fig. 5 Discharge capacity vs number of cycles of Li/Li[NixCoyMnz]O2 cells in voltage range of 2.8-4.3 V at 25 °C
Although the Li/Li[Ni0.8Co0.1Mn0.1]O2 cell deliveres the highest initial discharge capacity of 197.7 mA·h/g, it shows gradual capacity fade during cycling and reaches 87.8% capacity retention after 50 cycles, which can be primarily ascribed to the structural transformation near the surface region that increases the charge-transfer resistance between the positive electrode and the electrolyte upon electrochemical cycling [27,41]. Meanwhile, though the Li/Li[NixCoyMnz]O2 (721, 712, 622) cells with decreased Ni content have lower initial capacities, they show much better capacity retention over the same cycling period, voltage range and temperature: 95.1% for 721, 91.7% for 712, and 97.1% for 622 after 50 cycles. It is believed that the enhanced capacity retention may be partly attributed to the enhanced structural stability coming from the decrease of cation mixing and the increase of stable tetravalent Mn ions [27,42].
The rate capabilities of the Li/Li[NixCoyMnz]O2 (811, 721, 712, 622) cells are shown in Fig. 6. Each cell was firstly charged galvanostatically at 0.1C (20 mA/g) until the voltage reached 4.3 V and then discharged at 0.2C (40 mA/g), 0.5C (100 mA/g), 1C (200 mA/g), 2C (400 mA/g), 5C (1000 mA/g) and 0.2C (40 mA/g) for every five cycles, respectively. As can be seen from Fig. 6, the rate capability of Li[NixCoyMnz]O2 is gradually improved with decreasing the Ni content, indicating that 721 and 622 may have slightly better rate performance, confidently that the rate capability can be improved with higher content of Co [36]. For example, the capacity of 811 at 5C is 70.4% of that at 0.2C, while 622 exhibits a much enhanced capacity retention of 82.2%. These results reveal that the enhanced rate capability with decreasing the Ni content can be mainly attributed to the decrease of cation mixing and polarization. It is equally important to consider the effects of the electronic conduction, lithium diffusion and charge transfer reaction (kinetics) on the performance of the materials [18,43].
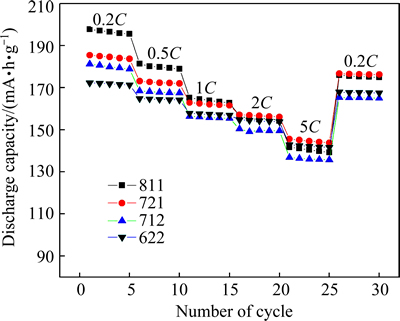
Fig. 6 Comparison of rate capabilities of Li/Li[NixCoyMnz]O2 cells at different rates between 2.8 and 4.3 V at 25 °C
4 Conclusions
1) Layer-structured Li[NixCoyMnz]O2 (811, 721, 712, 622) materials were obtained utilizing a co-precipitation method. The morphologies of the samples show that large agglomerates are composed of small and rock-shaped particles, and the material 622 exhibits uniform particle size distribution. The XRD patterns show that Li[NixCoyMnz]O2 (811, 721, 712, 622) with higher Ni content has less-ordered layer structure.
2) Though the initial discharge capacity of the Li/Li[NixCoyMnz]O2 (811, 721, 712, 622) cells reduces with decreasing the Ni content, the capacity retentions of 622, 721, 712 are superior to that of 811. Excellent cyclability is delivered for 622 with a capacity retention of 97.1% after 50 cycles.
References
[1] HUANG H, YIN S C, NAZAR L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J]. Electrochem Solid-State Lett A, 2001, 4: 170-172.
[2] HE Ping, YU Hai-jun, ZHOU Hao-shen. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries [J]. J Mater Chem, 2012, 22: 3680-3695.
[3] KOENIG G M, BELHAROUAK I, DENG H X, SUN Y K, AMINE K. Composition-tailored synthesis of gradient transition metal precursor particles for lithium-ion battery cathode materials [J]. Chem Mater, 2011, 23: 1954-1963.
[4] BELHAROUAK I, SUN Y K, LIU J, AMINE K. Li[Ni1/3Co1/3Mn1/3]O2 as a suitable cathode for high power applications [J]. J Power Sources, 2003, 123: 247-252.
[5] PROSINI P P, ZANE D, PASQUALI M. Improved electrochemical performance of a LiFePO4-based composite cathode [J]. Electrochim Acta, 2001, 46: 3517-3523.
[6] THACKERAY M M, DAVID W I F, BRUCE P G, GOODENOUGH J B. Lithium insertion into manganese spinels [J]. Mater Res Bull, 1983, 18: 461-472.
[7] GU Yi-jie, CHEN Yun-bo, LIU Hong-quan, WANG Yan-ming, WANG Cui-ling, WU Hang-hui. Structural characterization of layered LiNi0.85-xMnxCo0.15O2 with x=0, 0.1, 0.2 and 0.4 oxide electrodes for Li batteries [J]. J Alloy Compd, 2011, 509: 7915-7921.
[8] WANG L, LI J G, CHI N, YU H Y, DONG T. Comparison of electrochemical performance of LiNi0.7Co0.15Mn0.15O2 with different surface composition [J]. Adv Mater Res, 2012, 554: 445-449.
[9] GU Yi-jie, JIAN Fang-fang. Hollow LiNi0.8Co0.1Mn0.1O2-MgO coaxial fibers: Sol-gel method combined: with co-electrospun preparation and electrochemical properties [J]. J Phys Chem C, 2008, 112: 20176-20180.
[10] NEUDECKER B J, ZUHR R A, KWAK B S, BATES J B, ROBERTSON J D. Lithium manganese nickel oxides Lix[MnyNi1-y]2-xO2—Part 1. Synthesis and characterization of thin films and bulk phases [J]. J Electrochem Soc, 1998, 145: 4148-4159.
[11] CUSHING B L, WILEY J B. Topotactic routes to layered calcium cobalt oxides [J]. J Solid State Chem, 1998, 141: 385-391.
[12] BROUSSELY M, BIENSAN P, SIMON B. Lithium insertion into host materials: The key to success for Li ion batteries [J]. Electrochim Acta, 1999, 45: 3-22.
[13] TUKAMOTO H, WEST A R. Electronic conductivity of LiCoO2 and its enhancement by magnesium doping [J]. J Electrochem Soc, 1997, 144: 3164-3168.
[14] ARMSTRONG A R, BRUCE P G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries [J]. Nature, 1996, 381: 499-500.
[15] BRUCE P G, AROBERT ARMSTRONG A, GITZENDANNER R. New intercalation compounds for lithium batteries: Layered LiMnO2 [J]. J Mater Chem, 1999, 9: 193-198.
[16] HWANG S J, PARK H S, CHOY J H, CAMPET G. Evolution of local structure around manganese in layered LiMnO2 upon chemical and electrochemical delithiation/relithiation [J]. Chem Mater, 2000, 12: 1818-1826.
[17] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries [J]. Chem Lett, 2001, 30: 642-643.
[18] NOH H J, YOUN S E, YOON C S, SUN Y K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries [J]. J Power Sources, 2013, 233: 121-130.
[19] LEE K S, MYUNG S T, AMINE K, YASHIRO H, SUN Y K. Structural and electrochemical properties of layered Li[N1-2xCoxMnx]O2 (x=0.1-0.3) positive electrode materials for Li-ion batteries [J]. J Electrochem Soc A, 2007, 154: 971-977.
[20] LI Jiang-gang, WANG Li, ZHANG Qian, HE Xiang-ming. Synthesis and characterization of LiNi0.6Mn0.4-xCoxO2 as cathode materials for Li-ion batteries [J]. J Power Sources, 2009, 189: 28-33.
[21] LI Z, CHERNOVA N A, ROPPOLO M, UPRETI S, PETERSBURG C, ALAMGIR F M, WHITTINGHAM M S. Comparative study of the capacity and rate capability of LiNiyMnyCo1-2yO2 (y=0.5, 0.45, 0.4, 0.33) [J]. J Electrochem Soc A, 2011, 158: 516-522.
[22] KIM M H, SHIN H S, SHIN D W, SUN Y K. Synthesis and electrochemical properties of Li[Ni0.8Co0.1Mn0.1]O2 and Li[Ni0.8Co0.2]O2 via co-precipitation [J]. J Power Sources, 2006, 159: 1328-1333.
[23] KIM J M, CHUNG H T. Role of transition metals in layered Li[Ni,Co,Mn]O2 under electrochemical operation [J]. Electrochim Acta, 2004, 49: 3573-3580.
[24] TSAI Y W, LEE J F, LIU D G, HWANG B J. In-situ X-ray absorption spectroscopy investigations of a layered LiNi0.65Co0.25Mn0.1O2 cathode material for rechargeable lithium batteries [J]. J Mater Chem, 2004, 14: 958-965.
[25] NGALA J K, CHERNOVA N A, MA M, MAMAK M, ZAVALIJ P Y, WHITTINGHAM M S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound [J]. J Mater Chem, 2004, 14: 214-220.
[26] PARK B C, BANG H J, YOON C S, MYUNG S T, PRAKASH J, SUN Y K. Structural transformation of Li[Ni0.5-xCo2xMn0.5-x]O2 (2x≤0.1) charged in high-voltage range (4.5 V) [J]. J Electrochem Soc A, 2007, 154: 520-526.
[27] SUN Y K, KANG H B, MYUNG S T, PRAKASH J. Effect of manganese content on the electrochemical and thermal stabilities of Li[Ni0.58Co0.28-xMn0.14+x]O2 cathode materials for lithium-ion batteries [J]. J Electrochem Soc A, 2010, 157: 1335-1340.
[28] DAHBI M, SAADOUNE I, GUSTAFSSON T, EDSTROM K. Effect of manganese on the structural and thermal stability of Li0.3Ni0.7-yCo0.3-yMn2yO2 electrode materials (y=0 and 0.05) [J]. Solid State Ionics, 2011, 16: 37-41.
[29] HWANG B J, TSAI Y W, CHEN C H, SANTHANAM R. Influence of Mn content on the morphology and electrochemical performance of LiNi1-x-yCoxMnyO2 cathode materials [J]. J Mater Chem, 2003, 13: 1962-1968.
[30] SUN Y K, MYUNG S T, PARK B C, PRAKASH J, BELHAROUAK I, AMINE K. High-energy cathode material for long-life and safe lithium batteries [J]. Nat Mater, 2009, 8: 320-324.
[31] SUN Y K, CHEN Z H, NOH H J, LEE D J, JUNG H G, REN Y, WANG S, YOON C S, MYUNG S T, AMINE K. Nanostructured high-energy cathode materials for advanced lithium batteries [J]. Nat Mater, 2012, 11: 942-947.
[32] SUN Y K, KIM D H, YOON C S, MYUNG S T, PRAKASH J, AMINE K. A novel cathode material with a concentration-gradient for high-energy and safe lithium-ion batteries [J]. Adv Funct Mater, 2010, 20: 485-491.
[33] LIU Z L, YU A S, LEE J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries [J]. J Power Sources, 1999, 81: 416-419.
[34] CAO Hui, ZHANG Yao, ZHANG Jian, XIA Bao-jia. Synthesis and electrochemical characteristics of layered LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries [J]. Solid State Ionics, 2005, 176: 1207-1211.
[35] LIAO P Y, DUH J G, SHEEN S R. Effect of Mn content on the microstructure and electrochemical performance of LiNi0.75-xCo0.25MnxO2 cathode materials [J]. J Electrochem Soc A, 2005, 152: 1695-1700.
[36] SUN Yu-cheng, OUYANG Chu-ying, WANG Zhao-xiang, HUANG Xue-jie, CHEN Li-quan. Effect of Co content on rate performance of LiMn0.5-xCo2xNi0.5-xO2 cathode materials for lithium-ion batteries [J]. J Electrochem Soc A, 2004, 151: 504-508.
[37] WANG Zhao-xiang, SUN Yu-cheng, CHEN Li-quan, HUANG Xue-jie. Electrochemical characterization of positive electrode material LiNi1/3Co1/3Mn1/3O2 and compatibility with electrolyte for lithium-ion batteries [J]. J Electrochem Soc A, 2004, 151: 914-921.
[38] ROUGIER A, SAADOUNE I, GRAVEREAU P, WILLMANN P, DELMASA C. Effect of cobalt substitution on cationic distribution in LiNi1-yCoyO2 electrode materials [J]. Solid State Ionics, 1996, 90: 83-90.
[39] WOO S U, YOON C S, AMINE K, BELHAROUAK I, SUN Y K. Significant improvement of electrochemical performance of AlF3-coated Li[Ni0.8Co0.1Mn0.1]O2 cathode materials [J]. J Electrochem Soc A, 2007, 154: 1005-1009.
[40] ABRAHAM D P, TWESTEN R D, BALASUBRAMANIAN M, PETROV I, MCBREEN J, AMINE K. Surface changes on LiNi0.8Co0.2O2 particles during testing of high-power lithium-ion cells [J]. Electrochem Commun, 2002, 4: 620-625.
[41] WOO S U, PARK B C, YOON C S, MYUNG S T, PRAKASH J, SUN Y K. Improvement of electrochemical performances of Li[Ni0.8Co0.1Mn0.1]O2 cathode materials by fluorine substitution [J]. J Electrochem Soc A, 2007, 154: 649-655.
[42] BANG H J, KIM D H, BAE Y C, PRAKASH J, SUN Y K. Effects of metal ions on the structural and thermal stabilities of Li[Ni1-x-yCox- Mny]O2 (x+y≤0.5) studied by in situ high temperature XRD [J]. J Electrochem Soc A, 2008, 155: 952-958.
[43] HU S K, CHOU T C, HWANG B J, CEDER G. Effect of Co content on performance of LiAl1/3-xCoxNi1/3Mn1/3O2 compounds for lithium-ion batteries [J]. J Power Sources, 2006, 160: 1287-1293.
过渡金属对锂离子电池正极材料Li[NixCoyMnz]O2 (0.6≤x≤ 0.8)结构和电化学性能的影响
潘成迟,朱裔荣,杨应昌,侯红帅,景明俊,宋维鑫,杨旭明,纪效波
中南大学 化学化工学院 有色金属资源化学教育部重点实验室,长沙 410083
摘 要:利用共沉淀法制备具有典型六边形α-NaFeO2结构的正极材料Li[NixCoyMnz]O2 (0.6≤x≤0.8)。XRD结果表明:(003)峰与(104)峰的强度比随镍含量的减少而增加,随钴含量的增加而增加。SEM结果表明:材料是由微小的初级颗粒聚集而成的二次颗粒,并且随锰含量的增加,初级颗粒和二次颗粒变大,同时Li[Ni0.6Co0.2Mn0.2]O2颗粒分布比较均匀,颗粒大小为100~300 nm。尽管锂离子电池Li/Li[NixCoyMnz]O2的首次放电容量随镍含量的减少而减小,但是循环和倍率性能却随锰或钴含量的增加而得到改善。Li[Ni0.6Co0.2Mn0.2]O2 具有良好的循环性能,在循环50次后还能保持97.1%的容量保持率。
关键词:Li[NixCoyMnz]O2;电化学性能;正极材料;锂离子电池
(Edited by Mu-lan QIN)
Foundation item: Project (21473258) supported by the National Natural Science Foundation of China; Project (13JJ1004) supported by the Distinguished Young Scientists of Hunan Province, China; Project (NCET-11-0513) supported by the New Century Excellent Talents in University, China
Corresponding author: Xiao-bo JI; Tel: +86-731-88877237; E-mail: xji@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64244-9
Abstract: Li[NixCoyMnz]O2 (0.6≤x≤0.8) cathode materials with a typical hexagonal α-NaFeO2 structure were prepared utilizing a co-precipitation method. It is found that the ratio of peak intensities of (003) to (104) observed from X-ray diffraction (XRD) increases with decreasing the Ni content or increasing the Co content. The scanning electron microscopy (SEM) images reveal that the small primary particles are agglomerated to form the secondary ones. As the Mn content increases, the primary and secondary particles become larger and the resulted particle size for the Li[Ni0.6Co0.2Mn0.2]O2 is uniformly distributed in the range of 100-300 nm. Although the initial discharge capacity of the Li/Li[NixCoyMnz]O2 cells reduces with decreasing the Ni content, the cyclic performance and rate capability are improved with higher Mn or Co content. The Li[Ni0.6Co0.2Mn0.2]O2 can deliver excellent cyclability with a capacity retention of 97.1% after 50 cycles.


