
Structural defects in L10 FePt by modified analytic embedded-atom method
SHU Xiao-lin(舒小林)1, CHEN Qiang(陈 强)1 , CHEN Zi-yu(陈子瑜)1, HU Wang-yu(胡望宇)2
1. School of Science, Beijing University of Aeronautics and Astronautics, Beijing 100083, China;
2. Department of Applied Physics, Hunan University, Changsha 410082, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The structural defects of L10 FePt are investigated by the molecular dynamics (MD) with a modified analytic embedded-atom method (MAEAM). The L10 ordered structure of FePt is relaxed from a trial fcc structure. The defect formation energies are calculated. The vacancy formation energies of Fe and Pt are 1.89 eV and 2.11 eV respectively. The antisite formation energy of Fe in Pt sublattice is 0.35 eV. The antisite formation energy of Pt in Fe sublattice is 0.09 eV. The tendency of the vacancy formation energy is in agreement with other calculation. The point defect structure types are Pt antisite in rich-Pt side and Fe antisite in rich-Fe side.
Key words:
intermetallics; FePt; point defects; molecular dynamics; embedded-atom method;
1 Introduction
FePt alloy of L10 ordered structure with large magnetocrystalline anisotropy energy has attracted growing interests in recent years. It has a potential application as ultrahigh density recording media. A lot of researches were reported for FePt alloy films obtained by conventional deposition techniques such as sputtering. It is well known, however, that the as-deposited FePt films are shown as a disordered fcc structure which has no strong magnetocrystalline anisotropy. The substrate temperature during deposition and/or the post annealing temperature are required to be high (about 600 °C) for FePt transforming from the disordered fcc to the ordered fct phase[1,2]. To obtain small FePt particle and to achieve a high signal-to-noise ratio, it is critical to reduce the growth temperature. Recently, several reports on lowering of the ordering temperature have been published. LAI et al[3] have reported that a highly ordered FePt L10 phase has been obtained by using 2 MeV He-ion irradiation induced without conventional postannealing at 230 ℃. RAVELOSONA et al[4] have reported that the long-range order parameter of sputtered FePt (001) films can be improved by using post growth He ion irradiation. SEKI et al[5] have reported the effect of extensive compositional change on the structure and magnetic properties of FePt sputtered films deposited at a substrate temperature of 300 ℃. L10 ordered FePt films have been successfully prepared by decreasing the Fe concentration from the stoichiometric composition. KAVITA et al [6] have reported that swift heavy ion irradiation in FePt system results in partial destruction of L10 ordering. MATSUMURA et al [7] have found that irradiation can suppress the coarsening of FePt nanoparticles at an elevated temperature and also have little assistance in promoting L10 ordering in the nanogranular films at a temperature as low as 573 K. The effect of ion-beam irradiation on reducing the ordering temperature of FePt nanoparticles at 220 ℃ is also reported[8]. The experimental researches indicate that ion irradiation introduces excess point defects in FePt films. The excess point defects can strongly increase the diffusibility of the Fe and Pt atoms. However, the behaviors of the point defects in FePt are less studied. Also, the FePt with a wide composition range has a L10 structure. There are some constitutional defects since the FePt structure exists in non-stoichiometric composition range. However, the detailed defect property is still incomplete for L10 FePt.
In the past few decades the methods for empirical and semi-empirical calculations of properties for metals and alloys have evolved rapidly. One of the most successful methods is the embedded atom method (EAM) originally presented by DAW and BASKES[9,10]. Recently, ZHANG et al[11] have provided a modified analytical EAM model (MAEAM) and it has been widely used to calculate the dilute-limit heats of solution, surface segregation of alloy and point defect formation energies of intermetallic compounds.
In this paper, the point defect properties of L10 FePt are determined by molecular dynamics with a simple modified analytic EAM model. On the basis of the calculated lattice constants, the formation energies of vacancy and antisite are calculated. The defect structures in L10 FePt are discussed.
2 Theory
The total energy of any atomic structure can be expressed as the sum of three terms, a many-body term, which depends on the local electron density, a two-body term, which depends on interatomic distances, and a modification term to correct the discrepancy from the assumption of the linear superposition of spherical atomic electron density in the original EAM [4]:
![]() (1)
(1)
where rm is the atomic distance of the m-th neighbour.
The Johnson’s analytic effective embedding function is used as
![]() (2)
(2)
where F0 is the model parameter and it can be determined from F0=Ec-E1v, ρe is the equilibrium electron density, n is an adjustable parameter and its specific value for each element is determined by fitting the empirical energy–volume relationship of ROSE et al[12].
The energy modification term is empirically taken as
![]() (3)
(3)
where the local electron density ![]() , its second order
, its second order ![]() for bcc and fcc. The atomic electron density for bcc and fcc is empirically taken as
for bcc and fcc. The atomic electron density for bcc and fcc is empirically taken as
![]() (4)
(4)
where fe was taken to be 0.468 5 for Fe and 0.386 7 for Pt, respectively. β is taken to be 4.5 for bcc and 4.7 for fcc metals. re is the equilibrium nearest neighbour atomic distance, f(r) is truncated at rce, for Fe, rce=r3+0.441 (r4-r3); for fcc, rce=r4+0.75(r5-r4), ri (i=3, 4, 5) are the ith neighbor distances for a perfect crystal, respectively.
The pair potential function for bcc metals is taken as
![]() (5)
(5)
The pair potential function for fcc metals is taken as
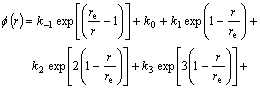
![]() (6)
(6)
The cutoff distances are rc=r2+0.75(r3-r2) for bcc and rc=r3+0.75(r4-r3) for fcc. At this point, the pair potential and its slope are 0.
The model parameters can be determined using the physical input parameters, such as the lattice constant (a0), the elastic constants (C11, C12 and C44), the cohesive energy Ec and vacancy formation energy E1v. The input physical parameters and the calculated model parameters for Fe, Pt and Cu are quoted from Ref.[4].
In the present paper, the alloy potential between different atomic species is taken as the formula proposed by Johnson:
![]() (7)
(7)
where A, B indicate different kinds of atoms, respectively.
In this work, the systems of the point defects are studied by molecular dynamics method (MD). The selected cells contain 4 000 atoms (including 2 000 Fe atoms and 2 000 Pt atoms) subjected to standard periodic boundary conditions. First, a perfect L10 FePt crystal lattice with a trail lattice constant is constructed. The crystal structure is relaxed to a stable structure by the MD program with the EAM potential discussed above. The stable crystal lattice constant can be gotten as an input parameter used in later calculations. The defect structures involving the various point defects are built and relaxed by MD. The energies of the defect structures are estimated. Finally the defect formation energies can be calculated.
3 Results and discussion
3.1 Phase structure of alloys
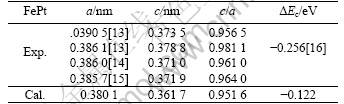
The primary crystal structure of FePt is chosen as fcc structure. The relaxed structure is L10 ordered structure. The relaxed lattice constant, formation energy of L10 FePt and the experimental value are shown in Table 1. The relaxed lattice constant is a=0.380 1 nm and c=0.361 7, respectively. The lattice constant a is little less than the experimental value 0.390 5 nm, 0.386 1nm[13], 0.386 nm[14] and 0.385 7 nm[15]. The lattice constant c is little less than the experimental value 0.373 5 nm, 0.378 8 nm[13], 0.371 0 nm[14] and 0.371 9 nm[15]. The c/a ratio is 0.95. It is in good agreement with the experimental value (0.956 5[13], 0.961 0[14], 0.964 0[15]) and less than the experimental value (0.981 1[13]). The formation energy of L10 FePt is -0.122 eV. The negative formation energy means that the L10 ordered structure is stable. This value is lower than that of the experimental value (-0.256 eV[16]).
Table 1 Lattice parameters and formation energy of L10 FePt
3.2 Defect energies
The calculations of the vacancy and antisite defect formation energies, as well as other calculated results, are shown in Table 2. The calculated formation energies of the Fe vacancy and Pt vacancy are found to be 1.89 eV and 2.11 eV, respectively. The antisite formation energies of the Fe antisite (Fe in Pt sublattice) and Pt antisite (Fe in Pt sublattice) are found to be 0.35 eV and 0.09 eV, respectively. The Fe and Pt vacancy formation energy is larger than that calculated by SHU X L[17] by Miedema theory (Fe vacancy 1.37 eV and Pt vacancy 1.75 eV), respectively.
Table 2 Defect formation energies of L10 FePt

3.3 Structure defect of alloys
In rich-Pt composition, the structure defects in L10 FePt are Fe vacancy and Pt antisite. The Pt antisite formation energy (0.09 eV) is less than the Fe vacancy formation energy (1.89 eV). The point defect type in rich-Pt side is Pt antisite.
In rich-Fe composition, the structure defects in L10 FePt are Pt vacancy (2.11 eV) and Fe antisite (0.35 eV). The Fe antisite formation energy is less than the Pt vacancy formation energy. So, the point defect type in rich-Fe side is Fe antisite. There is no structural vacancy formed in L10 FePt ordered alloy from our calculations.
4 Conclusions
The point defects of L10 ordered FePt are researched by the molecular dynamics method (MD) with a modified analytic embedded-atom method (MAEAM). The alloy potential is taken as Johnson’s formula. The L10 ordered structure of FePt is relaxed from a trial fcc structure by MD. The calculated lattice constants of L10 FePt are a=0.380 1 nm and 0.361 7 nm. The c/a ratio is 0.951 6. The formation energy of L10 FePt is -0.122 eV. The calculated results are in agreement with the experimental value. The calculated vacancy formation energies of Fe and Pt are 1.89 eV and 2.11 eV respectively, which is in agreement with the tendency of the vacancy formation energy calculated by others. The antisite formation energy (0.35 eV) of Fe in Pt site is less than the vacancy formation energy in Pt sublattice (2.11 eV). The antisite formation energy (0.09 eV) of Pt in Fe site is less than the vacancy formation energy in Fe sublattice (1.89 eV). The point defect structure types are Pt antisite in rich-Pt side and Fe antisite in rich-Fe side.
Acknowledgement
The authors are grateful to Prof. ZHU Feng-wu, Prof. ZHANG Bang-wei and Prof. WANG Ti-ming for their continuous encouragement and discussion.
References
[1] CHRISTODOULIDES J A, BONDER M J, HUANG Y, ZHANG Y, STOYANOY S, HADJIPANAYIS G C, SIMOPOULOS A, WELLER D. Intrinsic and hysteresis properties of FePt nanoparticles [J]. Phys Rev B, 2003, 68: 054428.
[2] TAKAHASHI Y K, HONO K. On low-temperature ordering of FePt films[J]. Scripta Materialia, 2005, 53: 403–409.
[3] LAI C H, YANG C H, CHIANG C C. Ion-irradiation- induced direct ordering of L10 FePt phase[J]. Appl Phys Lett, 2003, 83(22): 4550-4552.
[4] RAVELOSONA D, CHAPPERT C, MATHET V. Chemical order induced by ion irradiation in FePt (001) films[J]. Appl Phys Lett, 2000, 76: 236-238.
[5] SEKI T, SHIMA T, TAKANASHI K, TAKAHASHI Y E. L10 ordering of off-stoichiometric FePt (001) thin films at reduced temperature[J]. Appl Phys Lett, 2003, 82: 2461-2463.
[6] KAVITA S, REDDY V R, GUPTA A, AVSTHI D K. Effect of swift heavy ion irradiation in FePt system[J]. Nucl Instr and Meth in Phys Res B, 2006, 244: 19-22.
[7] MATSUMURA S, HORIUCHI T, YASUDAA K, KANEKO K, WATANABE M, MASUMOTO T. Morphological change in FePt nanogranular films induced by irradiation with 100 keV He ions[J]. Scripta Materialia, 2005, 53: 441–445.
[8] SEETALA N V, HARRELL J W, LAWSON J, NIKLES E D, WILLIAMS J R, TAMARA I S. Ion-irradiation induced chemical ordering of FePt and FePtAu nanoparticles[J]. Nucl Instr and Meth in Phys Res B, 2005 241: 583–588.
[9] DAW M S, BASKES M I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals[J]. Phys Rev B, 1984, 29(12): 6443-6453.
[10] DAW M S, FOILES S M, BASKES M I. The embedded-atom method: a review of theory and applications[J]. Mater Sci Rep, 1993, 9: 251-310.
[11] ZHANG B W, HU W Y, SHU X L. Theory of Embedded Atom Method and its Application to Materials Science—Atomic Scale Materials Design Theory[M]. Changsha: Hunan University Press, 2003.
[12] ROSE J H, SMITH J R, GUINEA F, FERANTE J. Universal features of the equation of state of metals[J]. Phys Rev B, 1984, 29 (6): 2963-2969.
[13] VILLARS P, CALVENT L D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases[M]. (2nd vol.3) Metals Park, OH: American Society for Metals. 1991.
[14] SATO K, HIROTSU Y. Magnetoanisotropy, long-range order parameter and thermal stability of isolated L10 FePt nanoparticles with mutual fixed oriention[J]. J Magn Magn Mater, 2004, 272-276: 1497-1499.
[15] WIERMAN K W, PLATT C L, HOWARD J K. Impact of stoichiometry on L10 ordering in FePt and FePtCu thin film[J]. J Magn Magn Mater, 2004, 278: 214-217.
[16] HULTGREN R, DESAI D D, HAWKIMS D T, GLEISER M, KELLEY K K. Selected Values of the Thermodynamic Properties of Binary Alloys[M]. Metals Park, Ohio: ASM, 44073, 1973.
(Edited by PENG Chao-qun)
Foundation item: Projects(50541036, 50371026) supported by the National Natural Science Foundation of China
Corresponding author: SHU Xiao-lin; Tel: +86-10-82317935; E-mail: shuxlin@buaa.edu.cn


