DOI: 10.11817/j.ysxb.1004.0609.2020-39500
基于硅藻土多孔陶瓷衬底制备SnO2纳米线及其低温H2S气敏性能
钟祥熙1,沈岩柏1,李停停1,赵思凯1,高淑玲1,魏德洲1,张云海2
(1. 东北大学 资源与土木工程学院,沈阳 110819;
2. 北京矿冶研究总院 矿物加工科学与技术国家重点实验室,北京102628)
摘 要:
采用热蒸发法在镀金硅藻土多孔陶瓷衬底表面合成出SnO2纳米线,通过XRD、SEM、TEM和XPS等检测手段对SnO2纳米线的微观形貌、晶体结构及元素组成进行分析,探讨硅藻土多孔陶瓷衬底对SnO2纳米线生长的影响。结果表明:该衬底上所获产物为具有四方相晶体结构的SnO2纳米线,直径为53~170 nm,长度为10 μm~ 1 mm。该硅藻土陶瓷衬底的多孔特性增加材料的成核位点,有利于Sn蒸气与Au液滴的高概率融合并快速饱合析出,从而促使SnO2纳米线具有高产率和高长径比。气敏检测结果表明,在较低工作温度条件下,基于所获SnO2纳米线的气体传感器对浓度为5×10-7~1×10-5(体积分数)的H2S具有优异的气敏特性,同时具有良好的稳定性及选择性。
关键词:
文章编号:1004-0609(2020)-10-2350-10 中图分类号:TN304.92 文献标志码:A
矿业作为国民经济的支柱产业,对我国经济的发展和人民生活水平的提高做出了巨大的贡献。然而,我国矿业安全生产状况却一直不容乐观,形势依然严峻。近年来,对矿业生产中有毒有害、易燃易爆气体的监测越来越受到人们的重视。其中,硫化氢(H2S)是一种易燃、无色、有剧毒的腐蚀性气体,吸入人体后会导致呼吸循环衰竭,肺水肿甚至致人死亡。此外,H2S易腐蚀金属并放出大量的热,当散热受到阻碍时,H2S会产生积聚,有可能引发自燃并导致爆炸,严重威胁井下工作人员的人身安全[1]。在我国,已有煤矿及含硫矿井发生过因H2S大量涌出造成的事故。因此,亟需研发具有高灵敏度、快响应恢复、低检测下限的H2S气体传感器,以进行及时、准确、高效的检测及预警,保障矿山工作人员的安全。
基于金属氧化物半导体气敏材料的气体传感器,以其灵敏度高、响应快速、稳定性高等优点,已成为世界上产量最大、使用最广的传感器之一。此外,因其低功耗、小型化及能够实时在线监测的特点,使其有望广泛应用于矿业安全生产领域。目前,用于检测H2S气体的金属氧化物半导体材料包括SnO2[2-3]、WO3[4-5]、ZnO[6-7]、Fe2O3[8-9]、CuO[10-11]等,为进一步提高其气敏性能,还对它们进行了贵金属修饰、多种金属氧化物复合、以及负载石墨烯或碳纳米管等新型功能材料的开发研究[12-14]。其中,SnO2作为一种宽禁带半导体材料,禁带宽度为 3.6 eV,具有材料成本低、气敏性能优异等特点,是应用最为广泛的气敏材料。然而,基于SnO2气敏材料的H2S气体传感器的工作温度一般都在200~400 ℃[15-18],过高的工作温度不仅增加了功耗,还存在引燃矿井易燃易爆气体的隐患[19]。而目前许多基于SnO2开发的新型复合气敏材料,虽然能够在室温或低温条件下对H2S产生响应,但仍存在响应/恢复时间较长、灵敏度过低等不足,其响应/恢复时间有的长达几分钟甚至几十分钟[20-21],加之过低的灵敏度,显然不利于对异常现象的及时预警,开发可在低温或室温条件下对H2S气体快速响应的气体传感器仍是研究的重点和难点,尤其是在矿业安全生产领域。
本研究采用热蒸发法在自制镀金硅藻土多孔陶瓷衬底表面合成SnO2纳米线,利用其高长径比、高比表面积以及不易发生团聚的特点,使气敏元件敏感层暴露出更多的反应活性位点,以实现在低温条件下对H2S的快速响应;讨论了基于多孔陶瓷衬底高效合成SnO2纳米线的生长机理,并分析了Au与SiO2对SnO2纳米线的无意掺杂对其气敏特性可能造成的影响。
1 实验
1.1 硅藻土多孔陶瓷衬底的制备
硅藻土多孔陶瓷衬底以硅藻土(AR,科密欧)为原料,经行星式球磨机在300 r/min的转速下球磨8 h,最终获得平均粒径为2 μm的硅藻土颗粒。以球型石墨作为造孔剂,其在原料中的质量分数为40%。经冷等静压机压制成板状,然后置于高温箱式炉中在1000 ℃条件下烧结成型[22],最后将所获得的硅藻土多孔陶瓷衬底依次置于丙酮、乙醇和去离子水中,分别超声清洗15 min后,烘干待用。
1.2 SnO2纳米线的制备
首先,采用直流溅射仪在清洗干净的多孔陶瓷衬底表面溅射一层约10 nm厚的Au膜,然后将衬底放置在装有0.25 g高纯Sn粒(纯度99.99%,国药集团)的Al2O3瓷舟上方,并与管式炉进气方向保持45°夹角放置。密封好法兰后,先以200 mL/min流速通入氩气(纯度99.99%)10 min,用来排除石英管内空气;然后,以10 ℃/min的速度升温至950 ℃并保温1 h,此时控制氩气流速为50 mL/min;当管式炉温度升至250 ℃时,开启控制氧气流速(纯度99.99%)的针阀引入微量的氧气,针阀开度为3圈。待管式炉自然冷却至室温后,在衬底表面可发现大量的白色絮状产物。
1.3 样品的结构表征方法
用S-4800型扫描电子显微镜(SEM,加速电压2 kV)分析样品形貌,用 X’Pert Pro X射线衍射仪(XRD,Cu Kα,λ=0.15406 nm)对样品的物相组成进行分析,用JEM-2010型透射电子显微镜(TEM)及 X射线能谱仪(EDS)分析样品的晶体结构及元素分布,用SMF EscaLab 250Xi型X光电子能谱仪(XPS)分析样品的化学组成,用ASAP 2020比表面测试仪测定产物的比表面积。
1.4 样品的气敏测试
将SnO2纳米线均匀分散在装有乙醇的2 mL离心管中,通过移液器将含有SnO2纳米线的悬浊液滴涂在镀有Au电极的Al2O3陶瓷片上(尺寸为1.5 mm×1 mm×0.2 mm),陶瓷片背面附有微型加热器,可通过调整电压改变该电极的工作温度;将制备好的气敏元件置于老化台上在300 ℃条件下老化48 h后,用WS-30A型气敏测试系统采用静态配气法测试气敏元件的性能。在本研究中,气体灵敏度S定义为气敏元件在洁净空气中的稳定电阻值Ra与在待测气体中的稳定电阻值Rg的比值,即S=Ra/Rg;响应时间(τ90%-resp)和恢复时间(τ90%-recov)分别为气敏元件在吸附和脱附H2S气体过程中阻值变化90%所需的时间。
2 结果与讨论
2.1 多孔陶瓷衬底形貌与结构
图1(a)所示为本试验中所用硅藻土原料的SEM像。可以看出,硅藻土颗粒为圆盘状,直径约为30 μm,圆盘表面分布着大量有序的微孔结构,直径约为100~500 nm。为了将其用作制备多孔陶瓷衬底的原料,需减小硅藻土颗粒的尺寸以增加表面能和提高烧结驱动力。图1(b)所示为经过球磨后硅藻土颗粒的SEM像,硅藻土颗粒的平均粒径约为2 μm。图1(c)所示为经过1000 ℃烧结成型的多孔陶瓷衬底表面的SEM像。可以看出多孔陶瓷衬底表面分布着大量的孔结构,其孔结构主要由两种类型所构成,其一是由球型石墨造孔剂在经过高温烧结挥发后留下的直径约20 μm的大孔,其二是由硅藻土颗粒本身固有的多孔结构。本试验中造孔剂的添加是为了进一步暴露硅藻土颗粒固有的多孔结构,进而充分提高多孔陶瓷衬底的表面积,并为后续纳米气敏材料的沉积提供丰富的成核位点。图1(d)所示为硅藻土原料及硅藻土多孔陶瓷衬底的XRD谱。可以看出,硅藻土原料及多孔陶瓷衬底均主要由α-方石英相所构成,其中硅藻土原料的结晶性良好,未出现无定型的特征峰,说明该原料是经过煅烧处理后的产物,但仍含有少量的α-石英相。在经过1000 ℃烧结成型后,多孔陶瓷衬底的α-方石英相特征峰的衍射强度更高,峰型更加尖锐,说明α-方石英相的结晶程度进一步改善,同时原料中少量的石英相特征峰消失,这是因为高温条件使石英相完全转化为α-方石英相,从而有利于提高多孔陶瓷衬底的结构强度。
2.2 SnO2纳米线的形貌与结构
为了考察在硅藻土多孔陶瓷衬底上合成SnO2纳米线的情况,采用SEM分析产物的形貌结构。图2(a)所示为SnO2纳米线(SNWs)正视角度的SEM像,所获纳米线的直径分布统计直方图如图2(b)所示,分析可知所获纳米线直径为53~170 nm,平均直径为109 nm;结合图2(a)右上角的实物照片,可以确定衬底表面所获白色絮状产物的长度为10 μm~1 mm,其高长径比确保其具有较大的比表面积,提高了与待测气体的反应活性位点。经过BET比表面积测试可知,所制备的SnO2纳米线的比表面积为63.2 m2/g。

图1 硅藻土原料及硅藻土多孔陶瓷衬底的SEM像和XRD谱
Fig. 1 SEM images of raw diatomite powders(a), ball-milled raw diatomite powders(b), diatomite-based porous ceramic substrate(c) and XRD patterns of raw diatomite powders and sintered substrate at 1000 ℃(d)

图2 在硅藻土多孔陶瓷衬底上合成SnO2纳米线的SEM像和直径分布统计直方图
Fig. 2 SEM images of SNWs on diatomite-based porous ceramic substrate
图2(c)所示为SnO2纳米线侧视角度的低倍率SEM像。可以看出SnO2纳米线沿着多孔陶瓷衬底表面的微孔内壁密集生长,表明衬底的多孔结构为SnO2纳米线的生长提供了丰富的成核位点,有助于提高产物的产量。图2(d)所示为SnO2纳米线侧视角度的高倍率SEM像。可以看出在SnO2纳米线的末端有球形的纳米金颗粒附着,由此可以证明SnO2纳米线的生长符合“气-液-固”生长机制[23]。图3所示为所获产物的XRD谱。可以看出除了来自于多孔陶瓷衬底的α-方石英相以及催化剂层的Au相之外,其余衍射峰均能够对应于SnO2标准卡片JCPDS No. 41-1445,表明在镀金硅藻土多孔陶瓷衬底上获得了结晶度良好且具有四方相金红石晶体结构的SnO2纳米线。所获产物的衍射三强峰分别为(110)、(101)和(211),且没有出现其他杂质峰,说明生成的产物具有单一的晶体结构。
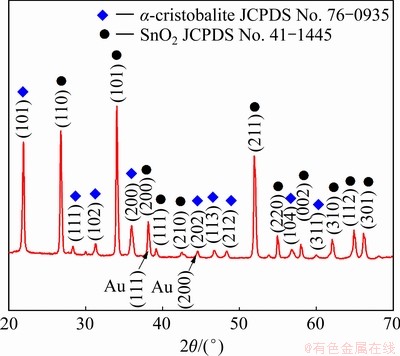
图3 在硅藻土多孔陶瓷衬底上合成SnO2纳米线的XRD谱
Fig. 3 XRD patterns of SNWs grown on diatomite-based porous ceramic substrate
为明确产物的化学组成及化学价态,对产物进行了XPS分析。图4(a)所示为产物的全谱图。可以看到产物中主要由Sn 3d和O 1s构成,其中C 1s被用于能量的标定,此外,还可以看到微弱的Si 2s和Si 2p特征峰。Sn 3d的高分辨XPS谱如图4(b)所示。图中位于486.15 eV和496.56 eV的峰分别对应于Sn元素的3d5/2和3d3/2自旋轨道峰,表明样品中的Sn为Sn4+。图4(c)所示为O 1s峰能谱分峰谱图。图中位于530.34 eV和531.26 eV的峰分别对应于SnO2纳米线的晶格氧(O 1s(1))和表面吸附氧(O 1s(2)),其中表面吸附氧的含量直接影响着材料的气敏性能。图4(d)所示为Si 2p的高分辨XPS谱图,其中位于102.18 eV处的特征峰可以表明该Si元素是以SiO2的形式分布在SnO2纳米线中,这可能是由于在热蒸发法的高温条件下,多孔陶瓷衬底的铝硅酸盐成分引入至产物中造成的。

图4 SnO2的XPS全谱图和Sn 3d、O 1s、Si 2p高分辨谱图
Fig. 4 XPS spectra of SNWs(a) and high-resolution XPS spectra of Sn 3d(b), O 1s(c), Si 2p(d)
为了进一步确认产物的元素组成及分布情况,对产物进行了TEM分析。图5(a)所示为单根SnO2纳米线的TEM像。可发现该纳米线直径为125 nm,与前述采用SEM观察的纳米线直径范围相一致。图5(b)所示为图5(a)中红圈处对应的EDS点扫描能谱图。可以看出,SnO2纳米线表面除了有Sn和O元素以外,还分布有Si及少量的Au元素,其中C和Cu元素来自于检测设备。由图5(c)~(f)的EDS面扫描能谱图中可以观察到各个元素的分布情况,除Sn和O元素外,还发现Si及少量Au元素也均匀分布在纳米线的表面,这与前述EDS点扫描能谱结果相符合,表明采用这种新型的硅藻土多孔陶瓷衬底用于热蒸发法制备SnO2纳米线时,会引入来自衬底的SiO2以及催化剂Au的引入,而这些杂质的引入有助于提升该材料的气敏性能,相关讨论在后续气敏特性分析部分进行探讨。
2.3 SnO2纳米线生长机理
根据以上的分析测试结果,结合“气-液-固”生长机制,对基于硅藻土多孔陶瓷衬底热蒸发法制备SnO2纳米线的生长机理进行了分析,图6所示为其生长机理示意图。首先,在多孔陶瓷衬底表面溅射一层Au膜,当温度升高时,组成金膜的Au纳米颗粒会融化成Au液滴,并发生相互聚集形成更大的Au纳米团簇(见图6(a))。需要说明的是,虽然Au的熔点是1064.18 ℃,但根据吉布斯汤姆森(Gibbs–Thomson)效应,纳米尺寸金属颗粒的熔点会随着尺寸的减少而降低[24]。与此同时,Sn源随温度的升高会变为Sn蒸气,并在载气输运下优先吸附在多孔陶瓷衬底表面的Au液滴上以降低化学能[25-26]。相对于平面型衬底(见图6(b),由于多孔结构会因空间限域的作用,一定程度上限制Sn蒸气的自由运动,进而提高了Sn蒸气与Au液滴碰撞融合的概率[27],而后续的Sn蒸气也会继续快速地吸附在已经成核的位点上,从而使Au/Sn合金液滴快速达到饱和,并析出生长成具有高长径比的SnO2纳米线。同时,多孔陶瓷衬底表面丰富的孔隙结构为产物的沉积提供了大量的成核位点,从而有效地提高了SnO2纳米线的产量。
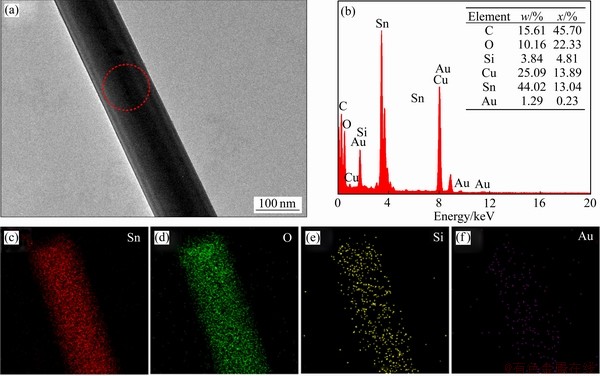
图5 SnO2纳米线的TEM像、EDS点扫描能谱图及EDS面扫描能谱图
Fig. 5 TEM image of single SNW(a), corresponding EDS spectrum of this SNW(b) and elemental mapping images of Sn(c), O(d), Si(e) and Au(f)

图6 在硅藻土多孔陶瓷衬底上合成SnO2纳米线的VLS生长机理示意图
Fig. 6 Schematic diagram of VLS growth mechanism of SNWs on diatomite-based porous ceramic substrate
此外,根据相关研究可知[28-29],α-方石英相在200~1470 ℃时处于亚稳态。而本试验中,构成多孔陶瓷衬底的α-方石英相在1000 ℃烧成温度下仍处于不稳定的状态。由此可以推断,当在950 ℃条件下制备SnO2纳米线时,可能会导致多孔陶瓷衬底表面Si元素的扩散,并融入到熔融的Au液滴中,同时,在微量的氧气环境中氧化生成SiO2,最终融入到了SnO2纳米线中。
2.4 气敏特性测试
为了探究硅藻土多孔陶瓷衬底对SnO2纳米线气敏性能的影响,考察了SnO2纳米线在不同工作温度对H2S气体的气敏性能。图7(a)所示为SnO2纳米线在不同工作温度对3×10-6 H2S气体的动态响应-恢复曲线。当通入H2S气体后,SnO2纳米线的电阻迅速下降,然后趋于稳定;当排出H2S气体后,SnO2纳米线可以恢复到初始值,说明所制备的气敏元件具有良好的响应-恢复特性。插图为SnO2纳米线对3×10-6 H2S气体的灵敏度与工作温度之间的关系,可以明显发现,该气敏元件的灵敏度先上升,然后在50 ℃时灵敏度值达到最高,继而在75 ℃时开始急剧下降,这是因为半导体气敏材料的最佳工作温度主要是由气敏材料表面气体吸附与解吸的平衡所决定的。在较低的工作温度下,气敏材料表面的活性较差,参与气敏反应的离子数量较少,导致灵敏度较低;当工作温度过高时,气体解吸速率的提高会降低参与气敏反应的离子数量,因此灵敏度也会降低。由试验结果可知,50 ℃是SnO2纳米线检测H2S气体的最佳工作温度,其最大灵敏度值为213.4。

图7 SnO2纳米线在不同工作温度条件下对3×10-6 H2S气体的动态响应-恢复曲线(插图为气体灵敏度与工作温度之间的关系图)及SnO2纳米线在不同工作温度条件下对3× 10-6 H2S气体的响应/恢复时间
Fig. 7 Dynamic response-recovery curves of SNWs to 3×10-6 H2S at various operating temperatures (Inset shows its corresponding sensor response curves)(a) and response and recovery times of SNWs to 3×10-6 H2S at various operating temperatures(b)
对于剧毒H2S气体的检测,快速的响应/恢复时间对其实际应用至关重要。图7(b)所示为不同工作温度时SnO2纳米线对3×10-6 H2S的响应/恢复时间。可以看出,响应/恢复时间随着工作温度的升高呈现减少的趋势,并在最佳工作温度50 ℃时的响应/恢复时间分别为4 s和118 s。快速的响应/恢复速度主要因为结晶度高、尺寸均一及高长径比的SnO2纳米线形成了不易团聚的网格状敏感层,可以暴露出更多吸附和反应位点,有利于吸附氧离子及待测气体迅速扩散并渗入至整个敏感层,从而促进了气敏反应的吸附与脱附过程。为了进一步测试SnO2纳米线的重复性与稳定性,在50 ℃的工作温度下对3×10-6 H2S气体进行了6次循环测试,结果如图8所示。可以看出,其动态响应-恢复曲线的特征基本相同,灵敏度值波动微小,表明该气敏元件具有良好的重复性以及稳定性。
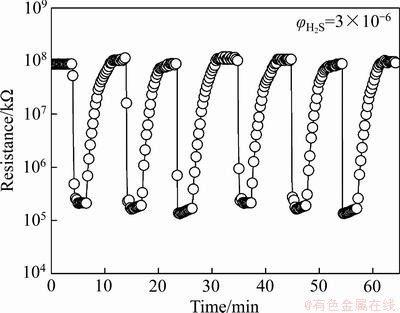
图8 SnO2纳米线在工作温度50 ℃时对3×10-6 H2S气体的重复性曲线
Fig. 8 Response-recovery curves of SNWs to 3×10-6 H2S at operating temperature of 50 ℃
图9(a)所示为SnO2纳米线在工作温度50 ℃时对不同浓度H2S气体的动态响应-恢复曲线,其相对应的灵敏度与H2S浓度之间的关系如图8(b)所示。可以看出,电阻变化或灵敏度随着H2S气体浓度的增加而显著增加,对5×10-7、1×10-6、3×10-6、5×10-6和1×10-5 H2S气体的灵敏度分别为5.5、25.3、213.4、402.9和565.4。此外,SnO2纳米线气敏元件对不同种类气体的选择性检测结果如图10所示。在工作温度50 ℃时还分别检测了SnO2纳米线对1×10-4 NH3、SO2、C2H5OH和CH2O的灵敏度,从图中可以看出,SnO2纳米线对3×10-6 H2S的灵敏度为213.4,远远高出浓度为1×10-4其他气体的灵敏度,表明SnO2纳米线对H2S气体优异的气体选择性。
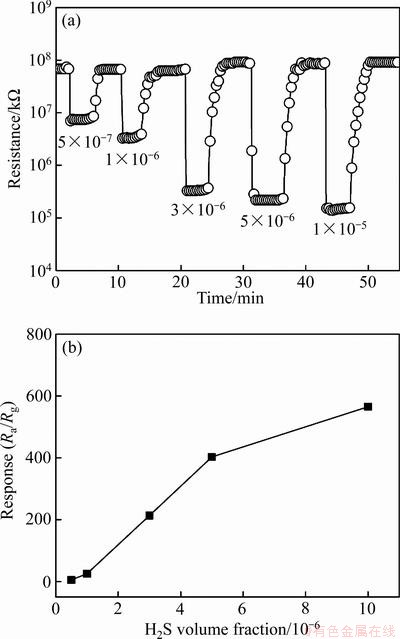
图9 SnO2纳米线在工作温度50 ℃时对不同浓度H2S气体的动态响应-恢复曲线及SnO2纳米线在工作温度50 ℃时的气体灵敏度与H2S气体浓度的关系
Fig. 9 Dynamic response curves of SNWs to various concentrations of H2S at 50 ℃(a) and relationships between sensor response and H2S concentration at 50 ℃(b)
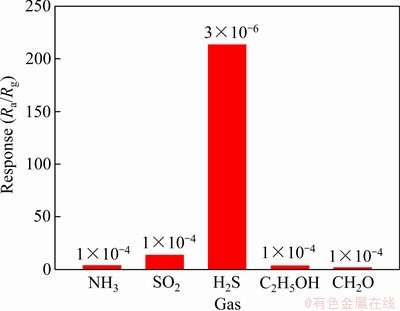
图10 SnO2纳米线在工作温度50 ℃时对不同种类气体的灵敏度
Fig. 10 Responses of SNWs to various kinds of detected gases at 50 ℃
由以上气敏试验结果可知,基于新型硅藻土多孔陶瓷衬底制备的SnO2纳米线对H2S气体具有良好的气敏特性,而且其气敏特性结果优于部分文献报道中的SnO2纳米线型H2S气体传感器[30-32]。可见,来自多孔陶瓷衬底中SiO2的渗入以及来自催化剂Au的引入并没有降低SnO2纳米线的气敏特性;相反地,贵金属Au的无意掺杂理论上则会增强其气敏特性。针对SiO2对SnO2纳米材料气敏特性的影响,KHODADADI等[33]在关于SiO2复合SnO2纳米颗粒及其气敏特性的研究中指出,SiO2纳米颗粒的修饰可以调节气敏材料的电阻,并因此提升其灵敏度,主要体现在当气敏材料置于空气中时,大气中的游离氧会吸附在气敏材料表面形成吸附氧,并捕获气敏材料中的电子,从而增加了材料的电阻。而在SnO2纳米颗粒之间的绝缘SiO2颗粒的存在则会进一步增加电子传输的势垒,从而抑制气敏材料中自由电子的快速输运,导致其在空气中的电阻进一步增加。当气敏元件置于还原性气氛时,待测气体与吸附氧负离子(O2-或O-相互作用,释放出大量电子并注入气敏材料的导带中,使电阻恢复到初始值,从而导致了显著的电阻差,更加有利于获得较高的灵敏度。
3 结论
1) 通过热蒸发法在硅藻土多孔陶瓷衬底成功制备出产量大、尺寸均一、长径比高的具有四方相晶体结构SnO2纳米线,直径为53~170 nm,长度为10 μm~1 mm,比表面积为63.2 m2/g。
2) SnO2纳米线在工作温度50 ℃时,对3×10-6 H2S气体灵敏度值为213.4,响应/恢复时间分别为4 s和188 s,且具有良好的稳定性及选择性。
3) 利用造孔剂成孔法使硅藻土陶瓷衬底具有更加丰富的多孔结构,为后续纳米材料的沉积提供了更多的成核位点,从而增加SnO2纳米线的产量。
4) 在纳米材料的生长过程中,衬底的孔结构可以增加Sn蒸气与Au催化液滴的融合几率,使Sn/Au合金液滴更快达到饱和,促使SnO2纳米线的高长径比 生长。
5) 衬底中SiO2对SnO2纳米线的均匀渗入以及催化剂Au在SnO2纳米线上的引入,显著增强了其H2S气体的气敏特性。
REFERENCES
[1] 张剑辉, 邓奇根. 煤矿H2S气体的危害、成因及防治[J]. 煤, 2015, 24(12): 19-22.
ZHANG Jian-hui, DENG Qi-gen. The hazards, causes and prevention of H2S gas in coal mines[J]. Coal, 2015, 24(12): 19-22.
[2] SONG Zhi-long, XU Song-man, LIU Jing-yao, HU Zhi-xiang, GAO Nai-bo, ZHANG Jian-bing, YI Fei, ZHANG Guang-zu, JIANG Sheng-lin, LIU Huan. Enhanced catalytic activity of SnO2 quantum dot films employing atomic ligand-exchange strategy for fast response H2S gas sensors[J]. Sensors and Actuators B (Chemical), 2018, 271: 147-156.
[3] STANOIU A, SIMION C E, SACKMANN A, BAIBARAC M, FLOREA G O, OSICEANU P, TEODORESCU V S, SOMACESCU S. Networked mesoporous SnO2 nanostructures templated by Brij 35 with enhanced H2S selective performance[J]. Microporous and Mesoporous Materials, 2018, 270: 93-101.
35 with enhanced H2S selective performance[J]. Microporous and Mesoporous Materials, 2018, 270: 93-101.
[4] LI Zhang-hong, XIE Ji, HU Xiao-bing, CHEN Cheng, XIE Li-li, ZHU Zhi-gang, ZHENG Liao-ying. Ultra-sensitive H2S gas sensor based on WO3 nanocubes with low operating temperature[J]. Materials Science Forum, Trans Tech Publ, 2018: 133-140.
[5] SHEN Yan-bai, ZHANG Bao-qing, CAO Xian-min, WEI Dei-zhou, MA Jia-wei, JIA Li-jun, GAO Shu-ling, CUI Bao-yu, JIN Yong-cheng. Microstructure and enhanced H2S sensing properties of Pt-loaded WO3 thin films[J]. Sensors and Actuators B (Chemical), 2014, 193: 273-279.
[6] NA Hui-bing, ZHANG Xian-fa, DENG Zhao-peng, XU Ying-ming, HUO Li-hua, GAO Shan. Large-scale synthesis of hierarchically porous ZnO hollow tubule for fast response to ppb-level H2S gas[J]. ACS Applied Materials & Interfaces, 2019, 11(12):11627-11635.
[7] GALSTYAN V, POLI N, COMINI E. Highly sensitive and selective H2S chemical sensor based on ZnO nanomaterial[J]. Applied Sciences, 2019, 9(6): 1167.
[8] WU Zhong-lin, LI Zhi-jie, LI Hao, SUN Meng-xuan, HAN Shao-bo, CAI Chao, SHEN Wen-zhong, FU Yong-qing. Ultrafast response/recovery and high selectivity of H2S gas sensor based on α-Fe2O3 nano-ellipsoids from one-step hydrothermal synthesis[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12761-12769.
[9] ZHANG Hai-jun, MENG Fan-na, LIU Li-zhu, CHEN Yu-jin. Convenient route for synthesis of alpha-Fe2O3 and sensors for H2S gas[J]. Journal of Alloys and Compounds, 2019, 774: 1181-1188.
[10] KIM H, JIN C, PARK S, KIM S, LEE C. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods[J]. Sensors and Actuators B (Chemical), 2012, 161(1): 594-599.
[11] LI Deng-ji, TANG Yong-liang, AO Dong-yi, XIANG Xia, WANG Shuang-yue, ZU Xiao-tao. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit[J]. International Journal of Hydrogen Energy, 2019, 44(7): 3985-3992.
[12] ALI F I, AWWAD F, GREISH Y E, MAHMOUD S T. Hydrogen sulfide (H2S) gas sensor: A review[J]. IEEE Sensors Journal, 2019, 19(7): 2394-2407.
[13] KHAN M A H, RAO M V, LI Qi-liang. Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S[J]. Sensors, 2019, 19 (4): 905.
[14] MIRZAEI A, KIM S S, KIM H W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances[J]. Journal of Hazardous Materials, 2018, 357: 314-331.
[15] SUN G J, CHOI S W, KATOCH A, WU Ping, KIM S S. Bi-functional mechanism of H2S detection using CuO-SnO2 nanowires[J]. Journal of Materials Chemistry C, 2013, 1(35): 5454-5462.
[16] DONG K Y, CHOI J K, HWANG I S, LEE L W, KANG B H, HAM D J, LEE J H, JU B K. Enhanced H2S sensing characteristics of Pt doped SnO2 nanofibers sensors with micro heater[J]. Sensors and Actuators B (Chemical), 2011, 157 (1): 154-161.
[17] HWANG I S, CHOI J K, KIM S J, DONG K Y, KWON J H, JU B K, LEE J H. Enhanced H2S sensing characteristics of SnO2 nanowires functionalized with CuO[J]. Sensors and Actuators B (Chemical), 2009, 142 (1): 105-110.
[18] CHOI S W, ZHANG J, AKASH K, KIM S S. H2S sensing performance of electrospun CuO-loaded SnO2 nanofibers[J]. Sensors and Actuators B (Chemical), 2012, 169: 54-60.
[19] JU Dian-xiang, XU Hong-yan, QIU Zhi-wen, ZHANG Zi-chao, XU Qi, ZHANG Jun, WANG Jie-qiang, CAO Bing-qiang. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with Au-loaded ZnO/SnO2 core-shell nanorods on flat alumina substrates[J]. ACS Applied Materials & Interfaces, 2015, 7 (34): 19163-19171.
[20] HOSSEINI Z S, MORTEZAALI A, ZAD A I, FARDINDOOST S. Sensitive and selective room temperature H2S gas sensor based on Au sensitized vertical ZnO nanorods with flower-like structures[J]. Journal of Alloys and Compounds, 2015, 628: 222-229.
[21] TIAN Jun-long, PAN Feng, XUE Rui-yang, ZHANG Wang, FANG Xiao-tian, LIU Qing-lei, WANG Yu-hua, ZHANG Zhi-jian, ZHANG Di. A highly sensitive room temperature H2S gas sensor based on SnO2 multi-tube arrays bio-templated from insect bristles[J]. Dalton Trans, 2015, 44(17): 7911-7916.
[22] ZHONG Xiang-xi, SHEN Yan-bai, ZHAO Si-kai, CHEN Xiang-xiang, HAN Cong, WEI De-zhou, FANG Ping, MENG Dan. SO2 sensing properties of SnO2 nanowires grown on a novel diatomite-based porous substrate[J]. Ceramics International, 2019, 45(2): 2556-2565.
[23] PARK S, HONG C, KANG J, CHO N, LEE C. Growth of SnO2 nanowires by thermal evaporation on Au-coated Si substrates[J]. Current Applied Physics, 2009, 9(3): S230-S233.
[24] MARUYAMA T, KONDO H, GHOSH R, KOZAWA A, NARITSUKA S, LIZUMI Y, OKAZAKI T LIJIMA S. Single-walled carbon nanotube synthesis using Pt catalysts under low ethanol pressure via cold-wall chemical vapor deposition in high vacuum[J]. Carbon, 2016, 96: 6-13.
[25] FILIPPO E, MANNO D, DE BARTOLOMEO A R, SERRA A. Single step synthesis of SnO2-SiO2 core-shell microcables[J]. Journal of Crystal Growth, 2011, 330 (1): 22-29.
[26] BARTH S, HERNANDEZ-RAMIREZ F, HOLMES J D, ROMANO-RODRIGUEZ A. Synthesis and applications of one-dimensional semiconductors[J]. Progress in Materials Science, 2010, 55 (6): 563-627.
[27] SHEKARI L, RAMIZY A, OMAR K, HASSAN A, HASSAN Z. High-quality GaN nanowires grown on Si and porous silicon by thermal evaporation[J]. Applied Surface Science, 2012, 263: 50-53.
[28] LAKSHTANOV D L, SINOGEIKIN S V, BASS J D, High-temperature phase transitions and elasticity of silica polymorphs[J]. Physics and Chemistry of Minerals, 2007, 34 (1): 11-22.
[29] TAGUCHI T, YAMAGUCHI K. Tailoring crystallinity and configuration of silica nanotubes by electron irradiation[J]. Nuclear Instruments and Methods in Physics Research Section B (Beam Interactions with Materials and Atom), 2015, 350: 1-5.
[30] SONG Zhi-long, XU Song-man, LI Min, ZHANG Wen-kai, YU Hao-xiong, WANG Yang, LIU Huan. Solution-processed SnO2 nanowires for sensitive and fast-response H2S detection[J]. Thin Solid Films, 2016, 618: 232-237.
[31] SONG Zhi-long, WEI Ze-ru, WANG Bao-cun, LUO Zhen, XU Song-man, ZHANG Wen-kai, YU Hao-xiong, LI Min, HUANG Zhao, ZHANG Jian-feng, YI Fei, LIU Huan. Sensitive room-temperature H2S gas sensors employing SnO2 quantum wire/reduced graphene oxide nanocomposites[J]. Chemistry of Materials, 2016, 28(4): 1205-1212.
[32] KAUR M, DADHICH B, SINGH R, GANAPATHI K, BAGWAIYA T, BHATTACHARYA S, DEBNATH A, MUTHE K, GADKARI S. RF sputtered SnO2: NiO thin films as sub-ppm H2S sensor operable at room temperature[J]. Sensors and Actuators B (Chemical), 2017, 242: 389-403.
[33] ASGARI M, SABOOR F H, MORTAZAVI Y, KHODADADI A. SnO2 decorated SiO2 chemical sensors: Enhanced sensing performance toward ethanol and acetone[J]. Materials Science in Semiconductor Processing, 2017, 68: 87-96.
Low-temperature H2S sensing properties of SnO2 nanowiresgrown on diatomite-based porous ceramic substrate
ZHONG Xiang-xi1, SHEN Yan-bai1, LI Ting-ting1, ZHAO Si-kai1,GAO Shu-ling1, WEI De-zhou1, ZHANG Yun-hai2
(1. School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
2. State Key Laboratory of Mineral Processing, Beijing General Research Institute of Mining and Metallurgy, Beijing 102628, China)
Abstract: SnO2 nanowires (SNWs) were grown on Au-coated diatomite-based porous substrate by a thermal evaporation method. The obtained SNWs were characterized by XRD, SEM, TEM and XPS to investigate their morphology, crystallization and composition. The effect of the diatomite-based porous substrate on the growth of SNWs was discussed. It demonstrates that the obtains SNWs with tetragonal rutile structure are 53-170 nm in diameter and 10 μm-1 mm in length. The porous ceramic substrate not only increases the nucleation sites of the SNWs, but also enhances the sticking probability between Sn vapors and Au droplets as well as rapid saturation precipitation, which raises the high yield and large length-to-diameter ratio of SNWs. The sensing measurements illustrate that the gas sensor based on SNWs shows excellent gas sensing properties to the volume fraction of 5×10-7-1×10-5 H2S at low operating temperatures. Moreover, the obtained SNWs show good stability and high selectivity.
Key words: diatomite; porous ceramic substrate; SnO2; nanowire; gas sensor
Foundation item: Projects(51674067, 51422402) supported by the National Natural Science Foundation of China; Projects(N180102032, N180106002, N180408018, N170106005) supported by Fundamental Research Funds for the Central Universities of China; Project(BGRIMM27 KJSKL-2019-12) supported by Open Foundation of State Key Laboratory of Mineral Processing, China
Received date: 2019-05-25; Accepted date: 2019-12-25
Corresponding author: SHEN Yan-bai; Tel: +86-24-83687381; E-mail: shenyanbai@mail.neu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51674067,51422402);中央高校基本科研业务费专项资助项目(N180102032,N180106002,N180408018,N170106005);矿物加工科学与技术国家重点实验室开放基金资助项目(BGRIMM27 KJSKL-2019-12)
收稿日期:2019-05-25;修订日期:2019-12-25
通信作者:沈岩柏,教授,博士;电话:024-83687381;E-mail:shenyanbai@mail.neu.edu.cn
摘 要:采用热蒸发法在镀金硅藻土多孔陶瓷衬底表面合成出SnO2纳米线,通过XRD、SEM、TEM和XPS等检测手段对SnO2纳米线的微观形貌、晶体结构及元素组成进行分析,探讨硅藻土多孔陶瓷衬底对SnO2纳米线生长的影响。结果表明:该衬底上所获产物为具有四方相晶体结构的SnO2纳米线,直径为53~170 nm,长度为10 μm~ 1 mm。该硅藻土陶瓷衬底的多孔特性增加材料的成核位点,有利于Sn蒸气与Au液滴的高概率融合并快速饱合析出,从而促使SnO2纳米线具有高产率和高长径比。气敏检测结果表明,在较低工作温度条件下,基于所获SnO2纳米线的气体传感器对浓度为5×10-7~1×10-5(体积分数)的H2S具有优异的气敏特性,同时具有良好的稳定性及选择性。


