文章编号:1004-0609(2011)06-1314-06
热处理对Mg-Al-Pb阳极电化学性能及腐蚀行为的影响
王乃光1,王日初1,彭超群1,冯 艳1,张翔宇2
(1. 中南大学 材料科学与工程学院,长沙 410083;
2. 长沙矿冶研究院,长沙 410012)
摘 要:
采用感应熔炼法制备Mg-6%Al-5%Pb(质量分数)阳极材料,采用电化学法和化学浸泡法研究热处理对其电化学性能及腐蚀行为的影响,采用扫描电镜对其不同热处理状态下阳极材料的显微组织及腐蚀表面形貌进行观察。结果表明:Mg-6%Al-5%Pb阳极试样经100 ℃时效8 h处理后Al和Pb弥散分布在镁基体中,其稳定电位为-1.455 V(相对于标准氢电极),腐蚀电流密度为0.063 mA/cm2,平均析氢速率为0.8 mL/(cm2·h),表现出较好的综合性能;Mg-6%Al-5%Pb阳极的析氢在达到稳态之前存在孕育期,在孕育期内析氢速率较小,达到稳态以后析氢速率增大,直至趋于恒定。
关键词:
中图分类号:TG 113;TG 146.1+1 文献标志码:A
Influences of heat treatment on electrochemical performances and corrosion behaviors of Mg-Al-Pb anodes
WANG Nai-guang1, WANG Ri-chu1, PENG Chao-qun1, FENG Yan1, ZHANG Xiang-yu2
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy, Changsha 410012, China)
Abstract: Mg-6%Al-5%Pb (mass fraction) anode material was prepared by the induction melting protected by argon. The electrochemical performances and corrosion behaviors of the anode specimens under different heat treatment conditions were studied by electrochemical measurements and immersion tests. The microstructures and the corroded surfaces of the anode specimens were examined by SEM. The results show that Mg-6%Al-5%Pb anode specimen aged at 100 ℃ for 8 h has the optimal performance with the stable potential of -1.455 V (vs SHE), corrosion current density of 0.063 mA/cm2 and average rate of hydrogen evolution of 0.8 mL/(cm2·h). There is an incubation period to the corrosion onset of Mg-6%Al-5%Pb anode, during which the hydrogen evolution rate is small. For long time immersion, the hydrogen evolution rate becomes linear and the process of corrosion achieves steady state.
Key words: magnesium anode; heat treatment; electrochemical performances; hydrogen evolution behavior
镁合金阳极材料具有较高的电化学活性、较高的能量密度、较负的稳定电位和较长的存储时间等性 能[1-4],广泛应用于海水激活动力电池、海底声音测试装置、潜水艇、警告浮标、紧急救生设备、空间飞行器和阴极保护等领域[5-8]。镁合金阳极可以利用海水作为电解液,在运输过程中绝对安全[9-10]。但镁合金阳极材料存在加工难、自腐蚀速率大且电流效率低等缺点[10-12],目前,解决此类问题的方法是改善热处理制度和添加适量的合金元素。AP65是镁合金阳极中的一种,其名义成分为Mg-6%Al-5%Pb(质量分数)。Al可以剥离镁阳极放电过程中的腐蚀产物并起到去极化的作用,同时影响镁阳极的耐蚀性,其效果取决于Al的含量和第二相的分布[13-16]。适量的Pb可以增强镁阳极的耐蚀性[17],尽管Pb对环境存在污染。目前,国内外关于热处理工艺对AP65阳极电化学性能影响的报道较少。 本文作者研究不同的热处理工艺对AP65镁阳极电化学性能的影响,优化出最佳的热处理制度。
1 实验
采用感应熔炼法制备Mg-6%Al-5%Pb(质量分数)阳极材料,将Mg、Al和Pb等以纯金属(纯度99.99%)放入高纯石墨坩埚,750 ℃时熔炼,充氩气保护。金属熔体在氩气保护下于水冷铁模浇铸。所得试样于400 ℃固溶24 h,水淬,在100 ℃或200 ℃分别时效 8 h,空冷至室温。采用原子吸收光谱分析Mg-6%Al-5%Pb阳极材料的化学成分,其结果如表1所列。采用Quanta-200扫描电镜观察经打磨、抛光后各试样的显微组织及恒电流测试后的腐蚀表面形貌。
表1 Mg-6%Al-5%Pb阳极的化学成分
Table 1 Chemical compositions of Mg-6%Al-5%Pb anode (mass fraction, %)

采用电化学方法测定试样的电化学性能。将试样用同型号SiC砂纸打磨去除表面氧化层,保留其经打磨的工作面,并使其呈10 mm×10 mm的矩形。然后用铜导线捆绑样品,非工作面用环氧树脂密封。电化学仪器为IM6ex,采用三电极体系进行测量。工作电极为Mg-6%Al-5%Pb阳极,辅助电极为铂电极,参比电极为标准氢电极。实验温度为25 ℃,电解液为3.5%(质量分数)的NaCl中性溶液。采用恒电流法(电流密度为180 mA/cm2,持续时间为1 000 s)和动电位极化扫描法(扫描速度为2 mV/s,电压范围为开路电位±0.5 V)分别测定试样的稳定电位、腐蚀电位和腐蚀电流密度。
采用化学浸泡法研究试样的腐蚀行为,用排水法收集试样浸泡过程中析出的氢气。将试样用同型号SiC砂纸打磨去除表面氧化层,保留其经打磨的工作面,并使其呈10 mm×10 mm的矩形,非工作面用环氧树脂密封。实验温度为25 ℃,电解液为3.5%的 NaCl中性溶液,浸泡时间为13 h,测定析出氢气的体积随时间的变化规律,计算出试样的平均析氢速率。
2 结果与讨论
图1所示为Mg-6%Al-5%Pb阳极在不同热处理状态下的恒电流极化曲线和动电位极化扫描曲线。从图1(a)可以看出:除铸态试样外,各试样均无极化现 象,且放电较平稳,去极化效果较好;铸态和固溶态试样激活时间很短,各时效态试样激活时间相对较长。100 ℃时效态试样稳定电位相对其它试样较负,达到-1.455 V,表现出较好的电化学活性。各试样的稳定电位列于表2。由表2可以看出:各试样的稳定电位由正到负的顺序为铸态、200 ℃时效态、固溶态、100 ℃时效态。一般来说,镁合金阳极材料依靠合金元素及第二相起到去极化作用[2, 4-5, 10],合金元素Al及含Al的第二相化合物在电化学反应过程中能剥落镁阳极表面的腐蚀产物(膜),使反应能维持下去,其分布对去极化效果有较大的影响。根据图2(a)所示的铸态试样显微组织及其对应的Al(见图3(a))和Pb(见图 3(b))元素的面分布可以看出:铸态试样在晶界处存在不连续分布的第二相,且Al在晶界处偏聚,表明第二相与基体相比富含Al、Pb与Al相比分布较均匀,偏聚情况不明显,因此,铸态试样的去极化效果相对较差。根据图2(b)所示的100 ℃时效态试样显微组织及其对应的Al(见图3(c))和Pb(见图3(d))元素面分布可以看出:时效态试样的组织均匀,Al和Pb均匀地分布在镁基体中。固溶态和200 ℃时效态试样具有类似于100 ℃时效态试样的均匀组织,因此,其去极化效果相对较好。

图1 不同热处理状态下Mg-6%Al-5%Pb阳极的恒电流曲线和动电位极化扫描曲线
Fig.1 Galvanostatic(a) and potentiodynamic polarization(b) curves of Mg-6%Al-5%Pb anodes in 3.5%NaCl solution under different heat treatment conditions
表2 不同热处理状态下Mg-6%Al-5%Pb阳极的电化学数据
Table 2 Data of electrochemical measurements of Mg-6%Al- 5%Pb anodes under different heat treatment conditions


图2 铸态和100 ℃时效态Mg-6%Al-5%Pb阳极试样的SEM像
Fig.2 SEM images of as-cast (a) and 100℃-aged (b) specimens of Mg-6%Al-5%Pb anodes
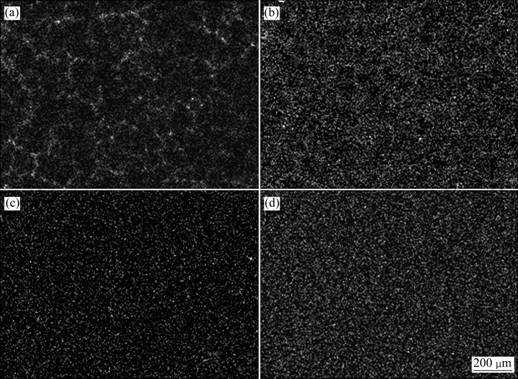
图3 铸态和100 ℃时效态Mg-6%Al-5%Pb阳极中Al元素和Pb元素的面分布图
Fig.3 Surface distribution of Al and Pb of as-cast and 100 ℃-aged Mg-6%Al-5%Pb; (a) Al, as-cast; (b) Pb, as-cast; (c) Al, 100 ℃-aged; (d) Pb, 100 ℃-aged
图4所示为100 ℃时效态试样在180 mA/cm2的电流密度下放电1 000 s后的腐蚀表面形貌。从图4可以看出:腐蚀产物疏松多孔,对基体起不到保护作用,在放电过程中不断从试样表面剥落,从而维持阳极材料的活化状态。
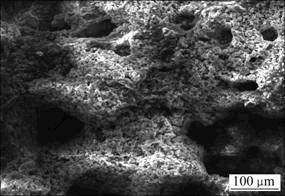
图4 Mg-6%Al-5%Pb阳极100 ℃时效态试样经恒电流放电1 000 s后的腐蚀表面形貌
Fig.4 Surface corrosion morphology of Mg-6%Al-5%Pb anode specimen aged at 100 ℃ after galvanostatic test for 1 000 s
从图1(b)所示的动电位极化扫描曲线可以看出:各试样的阳极和阴极支不对称,阳极支电流密度随电位增加的速率高于阴极支的。一般来说,阴极支的电流主要受析氢控制,阳极支的电流则主要受金属的阳极溶解控制[18]。根据动电位极化扫描曲线可以得出各试样的腐蚀电位及腐蚀电流密度列于表2。从表2可以看出:各试样的腐蚀电流密度都不高,与其它试样相比,各时效态试样的腐蚀电流密度均较低,表现出较好的耐蚀性。各试样腐蚀电流密度由大到小的顺序为铸态、固溶态、200 ℃时效态、100 ℃时效态。一般来说,镁合金阳极材料中电偶腐蚀为主要的腐蚀形 式[2, 4-5, 10],合金元素Al和Pb以及含Al的第二相比镁基体具有更正的电极电位充当阴极,其分布对电化学腐蚀行为有较大的影响。铸态试样中Al在晶界偏聚严重,Pb的分布也不均匀,导致腐蚀驱动力增大,腐蚀电流密度相对较高。淬火后,固溶态试样处于热力学不稳定状态,其腐蚀电流密度相对较大。各时效态试样中Al和Pb分布较为弥散均匀,腐蚀电流密度较 低。腐蚀电流密度可以采用以下公式换算成腐蚀速 率[13, 19]:
![]() (1)
(1)
式中:PJ-Mg为腐蚀速率,mm/a;Jcorr为腐蚀电流密度,mA/cm2。根据式(1)计算出Mg-6%Al-5%Pb阳极各试样的腐蚀速率,如表3所列。
表3 不同热处理状态下Mg-6%Al-5%Pb阳极的平均析氢速率(![]() )及腐蚀速率
)及腐蚀速率
Table 3 Average hydrogen evolution rate ![]() and corrosion rate of Mg-6%Al-5% Pb anodes under different heat treatment conditions immersed in 3.5% NaCl solution
and corrosion rate of Mg-6%Al-5% Pb anodes under different heat treatment conditions immersed in 3.5% NaCl solution
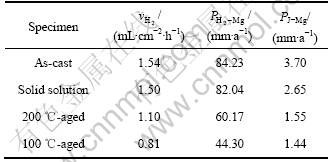
图5所示为不同热处理状态下Mg-6%Al-5%Pb阳极在3.5%NaCl溶液中析出氢气的体积随时间的关系。从图5可以看出:各试样析氢的开始阶段均存在一个孕育期,在该孕育期内,析氢速率很小;且随着时间的延长,析氢速率逐渐增大,即曲线的斜率越来越大,直至趋于直线,此时析氢进入稳态。这是因为在浸泡的开始试样表面只有少数地方被腐蚀,有氢气析出,随着浸泡时间的延长,被腐蚀的区域增大,逐渐蔓延至试样的整个表面,导致析氢速率增大,腐蚀过程进入稳态。在整个浸泡过程中,氢气在被腐蚀的区域析出,表明腐蚀区域与未腐蚀区域相比具有较高的电极电位,两区域之间形成电位差,成为腐蚀蔓延的驱动力。
各试样的平均析氢速率可以通过以下公式计算:
![]() (2)
(2)
式中:![]() 为平均析氢速率,mL/(cm2·h);
为平均析氢速率,mL/(cm2·h);![]() 为析氢总体积,mL,t为浸泡时间,h;S为试样表面积,cm2。根据式(2)计算出各试样的平均析氢速率,结果列于表3。从表3可以看出:各试样的析氢速率都不高,这是因为合金元素Pb具有较小的析氢反应交换电流密度,氢气难以在Pb上析出[19],且Pb在镁基体中分布相对均匀,能有效抑制氢气的析出。各试样的平均析氢速率由大到小的顺序为铸态、固溶态、200 ℃时效态、100 ℃时效态,与腐蚀电流密度的大小顺序一致。平均析氢速率可以采用以下公式换算成腐蚀速率[13, 20],
为析氢总体积,mL,t为浸泡时间,h;S为试样表面积,cm2。根据式(2)计算出各试样的平均析氢速率,结果列于表3。从表3可以看出:各试样的析氢速率都不高,这是因为合金元素Pb具有较小的析氢反应交换电流密度,氢气难以在Pb上析出[19],且Pb在镁基体中分布相对均匀,能有效抑制氢气的析出。各试样的平均析氢速率由大到小的顺序为铸态、固溶态、200 ℃时效态、100 ℃时效态,与腐蚀电流密度的大小顺序一致。平均析氢速率可以采用以下公式换算成腐蚀速率[13, 20],
![]() (3)
(3)
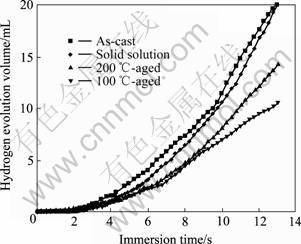
图5 不同热处理状态下Mg-6%Al-5%Pb阳极在3.5%NaCl溶液中浸泡13 h后析氢体积随时间的变化曲线
Fig.5 Changing curves of hydrogen evolution volume with immersion time for Mg-6%Al-5%Pb anodes immersed in 3.5% NaCl solution for 13 h under different heat treatment conditions
根据式(3)计算出各试样的腐蚀速率![]() (见表3)。从表3可以看出,该腐蚀速率大于根据腐蚀电流密度换算成的腐蚀速率。原因如下:实验中将各试样放入NaCl溶液后立即进行动电位极化扫描测试,此时试样处于腐蚀孕育期,腐蚀速率较小;而整个析氢过程长达13 h,试样已经进入稳态,因而具有较大的腐蚀速率。
(见表3)。从表3可以看出,该腐蚀速率大于根据腐蚀电流密度换算成的腐蚀速率。原因如下:实验中将各试样放入NaCl溶液后立即进行动电位极化扫描测试,此时试样处于腐蚀孕育期,腐蚀速率较小;而整个析氢过程长达13 h,试样已经进入稳态,因而具有较大的腐蚀速率。
3 结论
1) Mg-6%Al-5%Pb阳极材料的电化学性能取决于Al和Pb在镁基体中分布的弥散均匀程度。100 ℃时效的Mg-6%Al-5%Pb阳极试样中Al和Pb弥散均匀地分布在镁基体中,其稳定电位为-1.455 V(vs SHE),腐蚀电流密度为0.063 mA/cm2,平均析氢速率为0.81 mL/(cm2·h),表现出较好的综合性能。
2) Mg-6%Al-5%Pb阳极的析氢主要发生在被腐蚀区域,且析氢过程在达到稳态之前存在孕育期,在孕育期内析氢速率较小,达到稳态以后析氢速率增大,直至趋于恒定。
REFERENCES
[1] RENUKA R. Influence of allotropic modifications of surphur on the cell voltage in Mg-CuI(S) seawater activated batter[J]. Materials Chemistry and Physics, 1999, 59(1): 42-48.
[2] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, LI Wen-xian. Influence of Ga and Hg on microstructure and electrochemical corrosion behavior of Mg alloy anode materials[J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 1363-1366.
[3] RENUK A R. AgCl and Ag2S as additives to CuI in Mg-CuI seawater activated batteries[J]. Journal of Applied Electrochemistry, 1997, 27(12): 1394-1397.
[4] CAO Dian-xue, WU Lin, WANG Gui-ling, LU Yan-zhou. Electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn in sodium chloride solution[J]. Journal of Power Sources, 2008, 183: 799-804.
[5] FENG Yan, WANG Ri-chu, PENG Chao-qun, WANG Nai-guang. Influence of Mg21Ga5Hg3 compound on electrochemical properties of Mg-5%Hg-5%Ga alloy[J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 154-159.
[6] FIRA S S, KIBL L, LIW L W. Water-activated disposable and long shelf-life microbatteries[J]. Sensors and Actuators A, 2004, 111: 79-86.
[7] VENKATESARA R K. Performance evaluation of Mg-AgCl batteries for under water propulsion[J]. Detense Science Journal, 2001, 5(2): 161-170.
[8] FIDEL G M, JUAN M F, RUBEN D R, GENESCA J. Electrochemical study on magnesium anodes in NaCl and CaSO4-Mg(OH)2 aqueous solutions[J]. Electrochimic Acta, 2006, 51: 1820-1830.
[9] KIM J G, JOO J H, KOO S J. Development of high-driving potential and high-deficiency Mg-based sacrificial anodes for cathodic protection[J]. Journal of Materials Science Letters, 2000, 19(6): 477-479.
[10] 王乃光, 王日初, 余 琨, 冯 艳, 彭超群. 合金化和热处理对镁合金阳极材料组织及性能的影响[J]. 中国有色金属学报, 2009, 19(1): 38-43.
WANG Nai-guang, WANG Ri-chu, YU Kun, FENG Yan, PENG Chao-qun. Effect of alloying and heat treatment on electrochemical behavior of Mg anode[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(1): 38-43.
[11] 马正青, 曹军纪. 海水介质中高活性镁合金负极的电化学性能[J]. 材料保护, 2002, 35(12): 16-18.
MA Zheng-qing, CAO Jun-Ji. Electrochemical performance of magnesium alloys anode materials in seawater medium[J]. Materials Protection, 2002, 35(12): 16-18.
[12] FLAMINI D O, SAIDMAN S B, BESSONE J B. Aluminium activation produced by gallium[J]. Corrosion Science, 2006, 48(6): 1413-1425.
[13] ZHAO Ming-chun, SCHMUTZ P, BRUNNER S, LIU Ming, SONG Guang-ling, ATRENS A. An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing[J]. Corrosion Science, 2009, 51: 1277-1292.
[14] GU Xue-nan, ZHENG Yu-feng, CHENG Yan, ZHONG Sheng-ping, XI Ting-fei. In vitro corrosion and biocompatibility of binary magnesium alloys[J]. Biomaterials, 2009, 30: 484-498.
[15] PARDO A, MERION M C, COY A E, VIEJO F, ARRABAL R, FELIUJR S. Influence of microstructure and composition on the corrosion behaviour of Mg/Al alloys in chloride media[J]. Electrochimica Acta, 2008, 53: 7890-7902.
[16] ZHAO Ming-chun, LIU Ming, SONG Guang-ling, ATRENS A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91[J]. Corrosion Science, 2008, 50: 1939-1953.
[17] CANDAN S, UNAL M, TURKMEN M, KOC E, TUREN Y, CANDAN E. Improvement of mechanical and corrosion properties of magnesium alloy by lead addition[J]. Material Science and Engineering A, 2009, 501: 115-118.
[18] TAMAR Y, MANDLER D. Corrosion inhibition of magnesium by combined zirconia silica sol-gel films[J]. Electrochimica Acta, 2008, 53: 5118-5127.
[19] 曹楚南. 腐蚀电化学原理[M]. 三版. 北京: 化学工业出版社, 2008: 76-77.
CAO Chu-nan. Principles of electrochemistry of corrosion[M]. 3rd ed. Beijing: Chemical Industry Press, 2008: 76-77.
[20] ZHAO Ming-chun, LIU Ming, SONG Guang-ling, ATRENS A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41[J]. Corrosion Science, 2008, 50: 3168-3178.
(编辑 龙怀中)
基金项目:民口配套国家攻关资助项目(MKPT-02-181)
收稿日期:2010-06-28;修订日期:2010-09-28
通信作者:王日初,教授,博士;电话:0731-8836638;E-mail: wrc@mail.csu.edu.cn


