
Naphthalene-containing polyimides: Synthesis, characterization and photovoltaic properties of novel donor-acceptor dyes used in solar cell
NIU Hai-jun(牛海军)1, 2, MU Jing-shan(穆景山)2, ZHANG Mi-lin(张密林)1, LUO Jun(罗 俊)2,
LUO Pei-hui(罗培辉)2, BAI Xu-duo(白续铎)2, WANG Wen(王 文)3
1. College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China;
2. Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education,
Heilongjiang University, Harbin 150080, China;
3. School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150080, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
Novel linear and star branched triphenylamine-containing aromatic imides were designed and synthesized by the condensation between amine-substituted triphenylamine and naphthalene-1, 4, 5, 8-tetra-carboxylic dianhydride in order to investigate the influence of topological structure on the photophysical performance. The polyimides were characterized by elementary analysis and FTIR measurements. Thermogravimetric analysis (TGA) shows that the onset temperatures of decomposition (Td) for 5% mass loss range from 371 to 445 ℃. UV-Vis spectrum, quantum chemical calculation and cyclic voltammetry (CV) were used to obtain the dependence of energy levels on the function of structures. The introduction of triphenylamine group changes the energy level of the entire dye system. The dye-sensitized solar cells (DSSCs) prepared by using the dyes have a strong photoelectric current response in visible light region of 400-600 nm.
Key words:
naphthalene diimide; triphenylamine; sensitized solar cell; electrochemical properties;
1 Introduction
DSSCs (Dye-sensitized solar cells, Gr?tzel cells) have attracted a great deal of interest, as they offer high energy-conversion efficiencies at low cost. Ru dyes are used in DSSCs containing titania nanocrystals, exhibit good performance and good stability with record solar energy-to-electricity conversion efficiency of 11% under AM 1.5 irradiation[1]. Due to the use of rare metals and the difficulty of purification, the organic and conducting polymers offer the prospect of very low cost fabrication and present attractive features that facilitate market entry.
Recently, organic dyes have received more and more attention for their structure varieties, high molar extinction coefficients, and simple preparation process of low cost in comparison to Ru complexes. Metal-free dyes such as perylene dyes[2], cyanine dyes[3], merocyanine dyes[4], coumarin dyes[5], porphyrin dyes[6], and indoline dyes[7] have been investigated as sensitizers for DSSCs, yielding respectable conversion efficiencies between 5% and 9% with the traditional iodide/triiodide redox.
Naphthalene dianhydrides are important electron acceptors and electron-transporting materials used in high-tech applications, spanning from electronic to biological fields, such as optical switches, lasers and DNA/RNA probes. Sensitizers derived from naphthalene imides have also received much attention due to their outstandingly chemical, thermal and photochemical stability. But a few researches of application on DSSCs have been reported[8-10].
Recently, it has been found that by incorporating electron donor triarylamine group into the dye molecules, the physical separation of the dye cation from the electrode surface will be increased, which facilitates to achieve high rates of charge separation and collection compared with interfacial charge-recombination processes. And many higher efficient DSSCs based on the concept have been built[11]. Based on the above consideration, in order to improve the solubility of the imide and to enlarge intramolecular charge separation,we designed, synthesized the novel organic and polymeric sensitizers: PⅠ, PⅡ, and PⅢ that consist of the triarylamine moiety acting as electron donor (D) and naphthalene acid moiety acting as acceptor (A) and investigated the influence of D-A-D2, D-A-D, D-A types of structures on the optical, electrochemical, energy levels, photovoltaic properties, and morphologies.
2 Experimental
2.1 Materials
1, 4, 5, 8-naphthalene-tertracarboxylic danhydride acid (NTDA) and isoquinoline were purchased from Aldrich, USA. Ferrocene and tetrabutylammonium perchlorate were purchased from Acros, Belgium, and used for the electrochemical characterization of polymers. N-methyl-2-pyrrolidinone (NMP), dimethyl sulfoxide (DMSO), m-cresol and other reagents were reagent grade and used as received unless otherwise stated. Cis-di(thiocyanato)-N,N′-bis(2, 2′-bipyridyl-4-carboxylic acid-4′-tetrabutyl ammonium carboxylate) ruthenium (Ⅱ) was the commercial product by the trade name of N719 obtained from Solaronix S.A., Aubonne, Switerland.
2.2 Typical procedure for monomer synthesis
The monomers with triphenylamine were synthesized according to Refs.[12-14].
2.3 Typical procedure for synthesis of imides and polyimides
In a three-necked, round-bottomed flask fitted with an Ar inlet/outlet and a Dean-Stark trap, NTDA (0.268 2 g, 1 mmol), amine I (0.520 6 g, 2 mmol), 2 mL of isoquinoline were dissolved in 60 mL of m-cresol. The reaction solution was heated to 200 ℃ for 24 h, and in the course of the reaction, about 15 mL of m-cresol was distilled off. The mixture was then cooled to 100 ℃ and poured into 300 mL of methanol. The precipitated polymer was collected by filtration and washed with methanol several times. Unreacted NTDA was removed by treatment with 10% sodium hydroxide several times until the fluorescent color in basic solution faded away. The crude product was treated with acetone in a Soxhlet apparatus for 24 h to remove high boiling point solvents such as m-cresol and isoquinoline. The dark brown precipitate was collected and dried at 80 ℃ under vacuum (0.564 6 g, 75% yield). Other polyimides were prepared by an analogous procedure according to Fig.1.
Preparation of PⅠ: Yield: 78%; IR (KBr): 1 716, 1 666 cm-1 (imide stretch); 1H NMR(DMSO-d6, δ, chemical shift): 7.13 (ArH), 7.33 (ArH), 8.71 (NTDA-H).
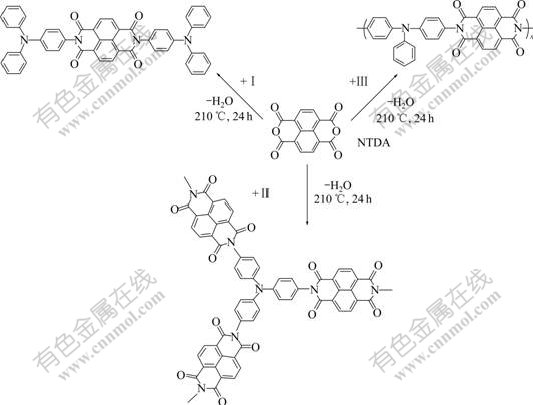
Fig.1 Synthesis route to polyimides ( PⅠ, PⅡ and PⅢ) containing naphthalene
Preparation of PⅡ: Yield: 81%; IR (KBr): 1 716, 1 666 cm-1 (imide stretch); 1H NMR(DMSO-d6, δ, chemical shift): 8.43 (ArH), 7.55-8.00 (ArH), 8.43 (NTDA-H).
Preparation of PⅢ: Yield: 80%; IR (KBr): 1 716, 1 666 cm-1 (imide stretch).
2.4 Fabrication of DSSCs
DSSCs were fabricated using fluorine-doped tin oxide (FTO) glass (20-25 W/square, Nippon Glass Co.) as substrates for both photoelectode and counterelectrode. For the preparation of photoeletrode, a mesoporous film of anatase TiO2 coated on the FTO glass substrate was fabricated by sol-gel process according to Ref.[15]. TiO2 was immersed in anhydrous m-cresol containing naphthalene dyes at room temperature for 48 h; excess dye was removed by rinsing with m-cresol, and dried under vacuum at 80 ℃. The thickness of TiO2 was about 16 mm determined by profiler. The FTO, counter electrode was thermally platinized by depositing 15 mL of 5 mmol/L H2PtCl6 in 2-propanol and heated to 380 ℃ for 15 min. The assembly of DSSCs was done by pressing the counter-electrode against the coated dye- sensitized electrode spaced with a 60 mm Surlyn-1760 frame, and then the electrolyte was introduced from the edge of the two glass substrates just before measurements. The surface area of the TiO2 film electrode under illumination was 0.25 cm2. The electrolyte was 0.6 mol/L 1-butyl-3-methylimidazolium iodide (BMII), 0.1 mol/L LiI, 0.05 mol/L I2 solution in acetonitrile with 0.5 mol/L 4-tertbutyl-pyridene (4-TBP).
2.5 Instruments and characterization
1H NMR spectra were recorded on a Bruker-300 instrument using tetramethylsilane as an internal reference. Infrared measurements were performed on a PE FTIR spectrometer. UV-Vis absorption spectra of the dyes in solution and adsorbed on TiO2 were measured on a Shimadzu UV-1700 spectrophotometer. Photo- luminescence spectra were measured on a Jasco FP-6200 spectrophotometer. The emission spectra of the series of dyes were recorded at λexc=375 nm.
The stabilities and glass-transition temperatures (Tg) of the polymer samples were determined using a NETGSCH49C at a heating rate of 10 ℃/min under nitrogen flow (50 mL/min). X-ray diffraction patterns were recorded using powder samples on a wide-angle D/max-gB diffractometer working in typical Bragg geometry with Cu Kα radiation. The morphology observation of the samples was carried out on a field- emission scanning electron microscopy (Mx2600fe).
Electrochemical measurements were performed on a CHI660 electrochemical analyzer to obtain all ![]() (half wave potential) via cyclic voltammogram (CV) calibrated to Fe(Ⅱ)/Fe(Ⅲ) oxidation couple. CV experiments were carried out in three-electrode cell consisting of a platinum working electrode, a platinum counter electrode and a Ag/Ag+ reference electrode. Solutions of the polyimides and compounds were prepared in reagent grade NMP dried over 4A molecular sieves. The supporting electrolyte was 0.1 mol/L tetra-n-butylammonium perchlorate dried overnight at 100 ℃. The solution was deoxygenated by sparging with Ar for 15 min prior to scanning and was blanketed with Ar during scans. Due to gelation of PⅢ during polymerization, CV was performed with microelectrode technique according to Ref.[16].
(half wave potential) via cyclic voltammogram (CV) calibrated to Fe(Ⅱ)/Fe(Ⅲ) oxidation couple. CV experiments were carried out in three-electrode cell consisting of a platinum working electrode, a platinum counter electrode and a Ag/Ag+ reference electrode. Solutions of the polyimides and compounds were prepared in reagent grade NMP dried over 4A molecular sieves. The supporting electrolyte was 0.1 mol/L tetra-n-butylammonium perchlorate dried overnight at 100 ℃. The solution was deoxygenated by sparging with Ar for 15 min prior to scanning and was blanketed with Ar during scans. Due to gelation of PⅢ during polymerization, CV was performed with microelectrode technique according to Ref.[16].
Photocurrent—voltage characteristics of solar cells and photocurrent action spectra (incident photon-to- photocurrent efficiency (IPCE)) for the polymer- modified electrodes were obtained with CHI instrument under AM1.5 simulated light source using a 500 W high-pressure Xe lamp as the excitation light source, with the collimated light beam passing through a grating monochromator (Tianjin Lanlik, China) to select the excitation wavelength when the steady state photocurrent was recorded. The incident light intensities at different wavelengths were measured with a light radiometer (Beijing Normal University Co, China).
Density-functional theory (DFT) calculations were performed on a computer. Geometry optimizations were carried out using the B3LYP functional and STO basis set, as implemented in Gaussian 98 program[17].
3 Results and discussion
3.1 Polymer synthesis
4-Aminotriphenylamine(Ⅰ), 4, 4′-diaminotriphenyl- amine (Ⅱ), 4, 4′, 4′′-triamino triphenyl- amine(Ⅲ) were prepared by the condensation of diphenylamine, aniline, 4-nitroaniline with 1-fluoro-4- nitrobenzene, followed by hydrazine Pd/C catalytic reduction according to the synthetic route[12-14]. The monomers synthesized were identified by elemental analysis, FTIR, and 1H NMR spectroscopic techniques that were in agreement with Refs.[12-14].
Polymerizations of diamine(Ⅱ) and triamine(Ⅲ) with dianhydrides (NTDA) at the reaction ratios of 1?1 and 2?3 were carried out at 200 ℃ in refluxing m-cresol containing a small amount of isoquinoline by one-step method. Model compound Ⅰ was synthesized with the reaction ratio of 2?1 in a similar way according to Fig.1. But for PⅢ, because of intensive cross linking reaction, the product was precipitated during the procedure. The generated water was removed through a gentle argon flow, and hence the reaction equilibrium moved to the formation of polyimide. Because the polymers were partly soluble in common organic solvents, their molecular weights could not be obtained.
The reaction mixture slowly dissolved upon imidization to give a deep red solution and the product precipitated upon cooling to 170 ℃. The 1H NMR spectra of naphthalene imides show multiplet resonances at about 8.7 (chemical shift), representing the aromatic protons from naphthalene moiety, and other resonances at about 7.2 (chemical shift) representing the aromatic protons from triphenylamine. Complete imidization is confirmed by FTIR spectroscopy, which indicates the loss of 6-membered anhydride peaks at 1 770 and 1 743 cm-1 and the appearance of the characteristic imide peaks at 1 717 and 1 675 cm-1(Fig.2). The reaction procedure can also be monitored by FTIR.
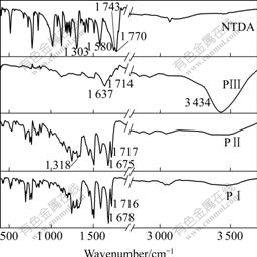
Fig.2 FTIR spectra of NTDA and polymers PⅠ, PⅡ and PⅢ
3.2 Thermal properties and crystalline
As indicated by the onset temperatures of decomposition (Td) for 5% mass loss ranging from 371 to 445 ℃ (Fig.3), polyimides are quite thermally stable in nitrogen. The content of carbonized residue (char yield) of NDTA-containing polymers is higher than 37%. Tg of all the polymers is observed in the range of 248-294 ℃. Only compound PⅠ shows clear melting endotherm peak near decomposition temperature on the DSC thermogram.
In order to investigate the aggregation state in the compounds, the XRD patterns of model compounds and polyimides powders were obtained and shown in X-ray diffraction patterns. The pristine polyimides and model compounds except PⅠ are amorphous as determined by the XRD patterns due to the kinked bulky triphenylamine structure. The steric repulsion of the bulky triphenylamine unit twists the rings dramatically out of the plane. The twisted conformation inhibits chain packing and crystallization. Broad diffraction peaks of diffusion type centered at 23? (2θ) are observed in plots
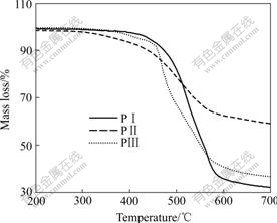
Fig.3 TG curves of polyimides at heating rate of 10 ℃/min
presented in X-ray diffraction patterns. There is a sharp peak at 12.5? (2θ), representing some crystallinity nature in PⅠ. The results are in line with those of DSC determination. PⅠ and PⅡ are soluble in polar aprotic solvents like NMP, DMSO, and concentrated sulfuric acid, m-cresol and stable at room temperature. The polyimides exhibit solubility because of the increased flexibility and free volume caused by the introduction of a bulky pendent triphenylamine group in the repeat unit.
3.3 Optical properties
Seen from Fig.4, in DMSO, the dye spectrum shows there are two typical peaks at 362 and 382 nm. The peaks dyes are in the similar place and shift to low energy region by 6 or 13 nm compared with monomer. This implies that the star and linear structures play a minor role in the UV-Vis spectrum compared with the model compound. The absorption and fluorescence spectra of naphthalene series of compounds showed consistent mirror-image behavior[18].
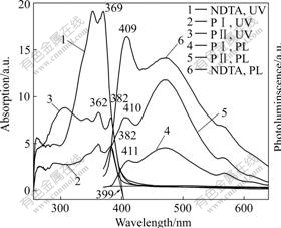
Fig.4 UV-Vis spectra and photoluminescence of NTDA, PⅠ and PⅡ in DMSO
The result does not indicate that H- or J- aggregation formed in concentrated solution or in films that quench the photo-generated electron, induces to recombination of electrons and holes, thus decreases the photocurrent density. This can be explained by the introduction of non-planar triphenylamine moiety that gives the hindrance of aggregation.
3.4 Electrochemical properties
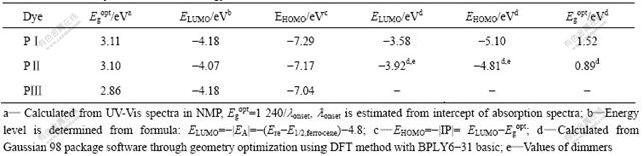
CV was used to determine the redox potentials of the dyes in order to estimate the driving force of the photoinduced electron injection process. The CV curves of PⅠ, PⅡ and PⅢ are shown in Fig.5. And Table 1 summarizes ELUMO calculated from the redox half-wave potentials in CV and band gap (Egopt) estimated from the onset absorption wavelength of the maximum absorption wavelength (lmax), assuming that the HOMO energy level for Fc/Fc+ standard is 4.80 eV with respect to the zero vacuum level. The measured ELUMO and the calculated EHOMO are listed in Table 1, which suggests that the three types of triphenylamine derivatives exhibit analogous energy levels.
In spite of different chemical structures, it is mainly the conjugated planar structure that determines the energy levels as well as the band gaps other than alkyl group[19]. The results from CV measurements illustrated that a series of compounds could be easily reduced (n-doped), which implies that the polymers with the capability for accepting electron are n-type semiconductors. The energy levels of the HOMO are lower for polymers compared to the redox potential of I-3/I- (-5.03 eV, referring to vacuum). EHOMO of PⅠ and PⅡ is low, which means that PⅠ and PⅡ have more driving force to be reduced by the I-3/I- redox system in thermodynamics due to the shift in ionization potential. The excited-state levels (ELUMO) calculated from CV are similar for the both dyes (shown in Table 1) and sufficiently high for electron injection into TiO2 (energy level of conducting band is about -4.40 eV). All the present dyes satisfy the energy gap rule. This means that, in thermodynamics, the designed dyes molecules can be employed as sensitizers for DSSCs application, in which
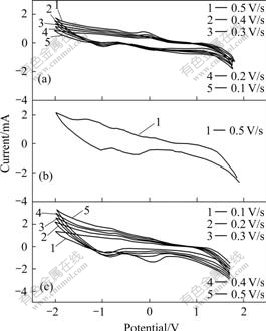
Fig.5 CV curves of PⅠ(a), PⅡ(b) and PⅢ(c)
BII has a stronger electron injection driving force. The kinetics of the naphthalene-based electrodes can be discussed by considering the effect of the scan rate on the peak intensity and potential values. It can be seen that the higher the sweep rate values, the higher the peak currents for the film electrodes. At the same time, increasing the scan rate promotes peak broadening and separation, indicating kinetic limitations in the compounds films. This data points to a diffusion- controlled reaction[20].
3.5 Photovoltaic performance of DSSCs based on dyes
The coatings are homogenous and porous with nanocrystalline particle size of about 40 nm, suitable for applications in photovolatic devices. The nanocrystalline TiO2 can strongly adhere to FTO, which can not only lower the electron resistance, but also adsorb enough dyes to produce photo-generated electrons. According to Ref.[21], the IPCEs are obtained by the following equation:
Table 1 CV data of dyes and calculated energy levels
![]() (1)
(1)
where Isc is the short-circuit photocurrent density for monochromatic irradiation, A/cm2; l is the wavelength, nm; and Iinc is the incident radioative flux, W/cm2.
The IPCE action spectra of the solar cells based on imides and polyimides are shown in Fig.6. A series of compounds yield IPCE up to 35% at 395 nm. The plots of IPCE are similar to the UV-Vis spectra of polyimides, which indicate that the current originates from the absorption of photon.
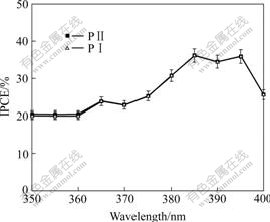
Fig.6 Spectra of monochromatic IPCE for DSSC based on dyes
The photovoltaic test of DSSCs was carried out by measuring the J—V characteristic curves under simulated AM 1.5 solar illumination (90 mW/cm2) in ambient atmosphere (Fig.7); the fill factor (FF) and overall light-to-electrical energy conversion efficiency (h) of DSSCs were calculated according to the following equations:
![]() (2)
(2)
![]() (3)
(3)
where Jsc (mA/cm2) is the short-circuit current density; Voc (V) is the open-circuit voltage; Pin is the incident light power; Jp (mA/cm2) and Vp (V) are the current density and voltage at the point of maximum power output on J—V curves, respectively.
From Fig.7, η values under different conditions are 0.108% and 0.214% which are lower than N719 (![]() was obtained in our experiment). But these values were of the same order of magnitude as some dyes with carboxylic groups reported[19]. It will be full of promise of the dyes after chemical modification.
was obtained in our experiment). But these values were of the same order of magnitude as some dyes with carboxylic groups reported[19]. It will be full of promise of the dyes after chemical modification.
A series of compounds show high efficiency. This may be attributed to the fact that EHOMO of a series of
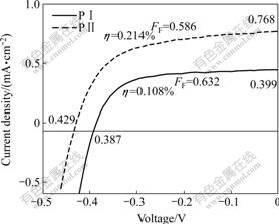
Fig.7 J—V characteristics of DSSCs with polyimide and model compounds dyes as sensitizers under illumination of AM 1.5 (90 mW/cm2) at effective area of 0.25 cm2
dyes (referring to vacuum) is low, so the electrons are easier to transfer from I- to dye+, facilitating the regeneration of dye easily. ELUMO (calculated from CV) of PⅡ is high, which means that PⅡ has stronger electron injection driving force. The above factors mentioned play an important role in improving efficiency of sensitized solar cell.
It has to be noted that for these comparative studies no complete optimization is performed, such as modulation of thickness of TiO2 and adding scattering layer. If the optimization of solar cell is carried out, the efficiency will be improved.
3.6 Electronic structures
The frontier molecular orbital energies are summarized in Table 1. With the degree of polymerization increasing and the D-A (donor-acceptor) structure forming, ELUMO decreases and EHOMO increases, resulting in narrow gap between LUMO and HOMO. The comparison of the electron distribution in the frontier molecular orbits reveals that photoinduced electron is transferred from the triphenylamine moiety to naphthalene moiety. Consequently, electrons can be injected into TiO2 via the naphthalene moiety. Assuming similar molecular orbital geometry when molecule anchors to TiO2, the position of the LUMO close to the anchoring group will enhance the orbital overlap with the titanium 3d orbits and favor electron injection.
4 Conclusions
1) Naphthalene-containing dyes are synthesized and the structures are confirmed by proton NMR spectra and FTIR. The optical and photophysical properties are characterized by UV-Vis absorption, CV, and quantum chemistry calculation. Five dyes can be dissolved in polar aproton solvents such as NMP, DMSO and concentrated H2SO4.
2) As observed with sensitizers adsorbed on nanoporous TiO2 films, the photosensitization takes place by a mechanism involving photo-induced electron injection from the dyes to TiO2, and subsequent electron hopping to the conductive FTO electrode surface.
3) The results demonstrate the viability of polymeric photosensitizers for porous nanoparticle metal oxide electrodes and reveal the way of design of molecules to provide the bright future prospect of developing more efficient DSSCs.
References
[1] NAZEERUDDIN M K, de ANGELIS F, FANTACCI S, SELLONI A, VISCARDI G, LISKA S P, TAKERU B, GR?TZEL M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers [J]. Journal of American Chemistry Society, 2005, 127(48): 16835-16847.
[2] GREGG B A. Evolution of photophysical and photovoltaic properties of perylene bis(phenethylimide) films upon solvent vapor annealing [J]. Journal of Physical Chemistry, 1996, 100(2): 852-859.
[3] HARA K, SATO T, KATOH R, FURUBE A, OHGA Y, SHINPO A, SUGA S, SAYAMA K, SUGIHARA H, ARAKAWA H. Molecular design of coumarin dyes for efficient dye-sensitized solar cells [J]. Journal of Physical Chemistry B, 2003, 107(2): 597-606.
[4] SAYAMA K, TSUKAGOSHI S, HARA K, OHGA Y, SHINPOU A, ABE Y. Photoelectrochemical properties of J aggregates of benzothiazole merocyanine dyes on a nanostructured TiO2 film [J]. Journal of Physical Chemistry B, 2002, 106(6): 1363-1371.
[5] HARA K, KURASHIGE M, DAN Y, KASADA C, SHINPO A, SAGA S. Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells [J]. New Journal of Chemistry, 2003, 27(5): 783-785.
[6] TOKUHISA H, HAMMOND P T. Solid-state photovoltaic thin films using TiO2, organic dyes, and layer-by-layer polyelectrolyte nanocomposites [J]. Advanced Functional Materials, 2003, 13: 831-839.
[7] HORIUCHI T, MIURA H, UCHIDA S. High efficiency of dye- sensitized solar cells based on metal-free indoline dyes [J]. Journal of American Chemistry Society, 2004, 126(39): 12218-12219.
[8] ERTEN S, ALP S, ICLI S. Photooxidation quantum yield efficiencies of naphthalene diimides under concentrated sun light in comparisons with perylene diimides [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2005, 175: 214-220.
[9] MACKINNON S M, WANG Z Y. Synthesis and characterization of poly (aryl ether imides)s containing electroactive perylene diimide and naphthalene diimide units [J]. Journal of polymer Science A: Polymer Chemistry, 2000, 38: 3467-3475.
[10] CHEN Z, ZHENG Y, YAN H, FACCHETTI A. Naphthalene- dicarboximide- vs perylenedicarboximide-based copoly- mers. synthesis and semiconducting properties in bottom-gate n-channel organictransistors [J]. Journal of American Chemistry of Society, 2009, 131: 8-9.
[11] LI G, JIANG K, LI Y, LI S, YANG L. Efficient structural modification of triphenylamine-based organic dyes for dye-sensitized solar cells [J]. Journal of Physical Chemistry C, 2008, 112: 11591-11599.
[12] CHIU K Y, SU T X, LI J H, LIN T, LIOU G S, CHENG S H. Novel trends of electrochemical oxidation of amino-substituted triphenylamine derivatives [J]. Journal of Electroanalytical Chemistry, 2005, 575: 95-101.
[13] MENG Xiang-li, HUANG Yu-dong, NIU Hai-jun, LEI Zuo-tao. Synthesis and purification of 4,4′-diamino triphenylamine [J]. Chinese Journal of Organic Chemistry, 2007, 27(5): 682-684. (in Chinese)
[14] FANG J, KITA H, OKAMOTO K. Hyperbranced polyimides for gas separation applications. 1. Synthesis and characterization [J]. Macromolecules, 2000, 33: 4639-4646.
[15] LIANG M, XU W, CAI F, CHEN P, PENG B, CHEN J, LI Z. New triphenylamine-based organic dyes for efficient dye-sensitized solar cells [J]. Journal of Physical Chemistry C, 2007, 111: 4465-4472.
[16] CHA C S, YANG H X. Recent advances in experimental methods applied to lithium battery researches [J]. Journal of Power Sources, 1993, (43): 145-155.
[17] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PEDERSON M R, SINGH D J, FIOLHIS C. Atoms, molecules, solids, and surfaces: Application of the generalized gradient approximation for exchange and correlation [J]. Phys Rev B, 1992, 46: 6671-6687.
[18] SCHOUWINK P, SCHAFER A H, SEIDEL C, FUCHS H. The influence of molecular aggregation on the device properties of organic light emiting diodes [J]. Thin Solid Films, 2000, 372: 163-168.
[19] ZAFER C, KUS M, TURKMEN G, DINCALP H, DEMIC S, KUBAN B, TEOMAN Y, ICLI S. New perylene derivative dyes for dye-sensitized solar cells [J]. Solar Energy Materials and Solar Cells, 2007, 91: 427-431.
[20] PARRA V, DEL CA?O T, RODR?GUEZ-M?NDEZ M L, de SAJA J A, AROCA R F. Electrochemicalcharacterization of two perylenetracarboxylic diimides: Langmuir-blodgett films and carbon paste electrodes [J]. Chemistry Materials, 2004, 16: 358-364.
[21] KAMAT P V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion [J]. Journal of Physical Chemistry C, 2007, 111: 2834-2860.
(Edited by CHEN Wei-ping)
Foundation item: Projects(50502013, 562869) supported by the National Natural Science Foundation of China; Project(2005AFQXJ062) supported by Harbin Youth Foundation, China; Project(20070410892) supported by China Postdoctoral Science Foundation
Corresponding author: NIU Hai-jun; Tel: +86-13684501571; E-mail: niuhaijun@hlju.edu.cn; haijunwangwen@yahoo.com.cn


