Trans. Nonferrous Met. Soc. China 26(2016) 3232-3237
Facile synthesis of porous LiNiVO4 powder as high-voltage cathode material for lithium-ion batteries
Mu-lan QIN1, Wan-min LIU1, Shu-quan LIANG 2,3, An-qiang PAN2,3
1. School of Chemistry and Chemical Engineering, Hunan Institute of Engineering, Xiangtan 411104, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
3. Key Laboratory of Nonferrous Metals Materials Science and Engineering, Ministry of Education, Central South University, Changsha 410083, China
Received 13 June 2016; accepted 15 September 2016
Abstract:
Porous LiNiVO4 powder was synthesized via solution combustion synthesis method using lithium nitrate, nickel nitrate, ammonium metavanadate and citric acid as raw materials. Thermogravimetry (TG) and differential scanning calorimetry (DSC), X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectroscopy and transmission electron microscopy (TEM) were used to evaluate the structures and morphologies of samples. The results show that the calcination temperature has significant effect on the crystallinity and morphologies. Pure LiNiVO4 flaky nanoparticles with a mean particle size around 20 nm can be readily prepared by calcining the precursor in air at 500 °C for 2 h. As a cathode material for lithium-ion batteries, the porous LiNiVO4 powder exhibits a good structural reversibility.
Key words:
lithium-ion battery; LiNiVO4; cathode material; solution combustion synthesis; nanoparticle;
1 Introduction
Inverse spinel LiNiVO4 powder was proposed as a cathode material for lithium-ion batteries due to its high operation voltage of 4.8 V [1]. High voltage always possesses high energy density, thus the use of LiNiVO4 is expected to enhance the energy density of lithium-ion batteries [2]. However, the electrochemical performance is strongly related to the synthesis processes [3] which result in different degrees of crystallinity, particles sizes and morphologies.
LiNiVO4 prepared by the conventional solid-state reaction has the drawbacks of high temperature processing, long duration, large crystallite size and impurities [4-6]. These problems can be alleviated by soft chemistry reactions, such as sol-gel method [7,8], hydrothermal synthesis [9,10], solution precipitation method [11], rheological phase synthesis [12,13], polymerized complex method [14,15], Pechini method [16] and combustion synthesis [17-20]. Among these soft chemistry routes, the combustion synthesis is a versatile, simple and cost effective process for the synthesis of multi-component oxide powders.
In this work, a modified combustion method was used by directly transferring the mixture precursor solution into an electrical oven. The resulting LiNiVO4 powder was of highly porous and has good uniformity. Moreover, its electrochemical performance as a cathode material for lithium-ion battery was evaluated.
2 Experimental
2.1 Synthesis of LiNiVO4
LiNiVO4 nanoparticles were prepared by a facile solution combustion synthesis method. In a typical processing, lithium nitrate, nickel nitrate and ammonium metavanadate in a molar ratio of 1:1:1 (n(Li):n(Ni): n(V)=1:1:1) were dissolved in de-ionized water. Then, a citric acid solution was added into the obtained solution under constant stirring and the molar ratio of total metal ions to citric acid was kept constant as 1:1. The resulting solution was heated at 50 °C under constant stirring to evaporate excess water, yielding a high concentration solution which was then placed in a preheated muffle furnace at 400 °C. The solution boiled and then decomposed. The decomposition was accompanied by a mass of bubbles followed by the generation of gases such as NOx and ammonia. The entire reaction was completed within 5 min, giving rise to a pale brown precursor powder. The precursor was then calcined at 400-600 °C for 2 h to get the yellow product.
2.2 Characterization
The thermal decomposition process of the precursor was investigated by thermogravimetry (TG) and differential scanning calorimetry (DSC) using a simultaneous TG-DSC thermal analyzer (NETZSCH STA 449 C) at a heating rate of 10 °C/min under air atmosphere. The phase structures of the synthesized LiNiVO4 powders were determined by X-ray diffraction (XRD) (Rigaku D/max2500 XRD with Cu Kα radiation, λ=1.54178 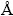 ). Infrared (IR) spectra were recorded with a WQF-410 Fourier transform infrared spectrometer (FT-IR, Nicolet). A field emission transmission electron microscope (FETEM, JEOL JEM-2100 F) was used to characterize the structural morphologies of the synthesized products.
). Infrared (IR) spectra were recorded with a WQF-410 Fourier transform infrared spectrometer (FT-IR, Nicolet). A field emission transmission electron microscope (FETEM, JEOL JEM-2100 F) was used to characterize the structural morphologies of the synthesized products.
The LiNiVO4 powders were assembled into coin-type cells (CR 2016) to evaluate their electro- chemical properties. LiNiVO4 powders, acetylene black and polyvinylidene fluoride (PVDF) binder in a mass ratio of 7:2:1 were dispersed in N-methyl-2- pyrrolidone (NMP) solution to prepare the slurry, which was coated on an aluminum foil and dried in a vacuum oven at 90 °C for 20 h to make the cathode. The half-cells were assembled in a glove box (Mbraun, Germany) filled with ultrahigh purity argon, using polypropylene membrane as the separator and 1 mol/L LiPF6 dissolved in ethylene carbonate/dimethyl carbonate (V(EC)/V(DMC)=1:1) as the electrolyte. Cyclic voltammetry measurement was performed on a CHI660C electrochemical workstation (CHI660C, Shanghai, China) at a scan rate of 0.1 mV/s in the voltage range of 3.0-4.9 V (vs Li/Li+). The galvanostatic charge-discharge characteristics of the cells were recorded with a Land battery tester (Land CT 2001A, Wuhan, China) at room temperature, and the specific capacity is based on the mass of active material only.
3 Results and discussion
3.1 Thermal analysis
The TG and DSC measurements were carried out for the precursor obtained by solution combustion synthesis method to find out the exact complete crystallization temperature of the LiNiVO4 powder. The TG and DSC curves of the precursor are shown in Fig. 1. In the TG curve, the mass loss before 250 °C is due to the removal of water molecule present in the precursor, and the DSC curve shows one distinguishable endothermic peak at about 100 °C. The decomposition process mainly occurs in the temperature range of 250-500 °C. The mass loss after 250 °C is attributed to the complex decomposition of lithium, vanadium and nickel citrates. The DSC curve shows three exothermic peaks with a peak maximum at 478 °C. The combustion nature of citric acid together with the presence of nitrate in the precursor gives rise to enormous heat energy for initiating the crystallization of LiNiVO4. At temperature higher than 500 °C, there is no mass loss. This indicates that the decomposition of precursor is completed at 500 °C.
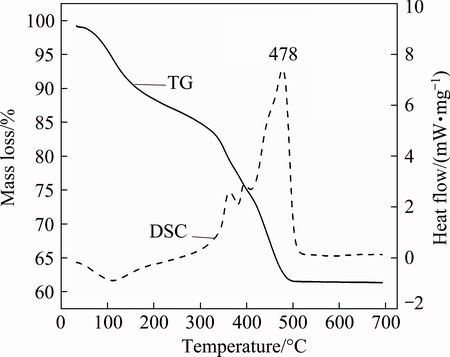
Fig. 1 TG and DSC curves of precursor
3.2 Structure and morphology
Figure 2 shows the XRD patterns of LiNiVO4 obtained by calcining the precursor at different temperatures. From Fig. 2, it can be seen that some visible diffraction peaks of LiNiVO4 compound appear in the pattern recorded at 400 °C. By increasing the heating temperature, the crystallinity of the samples increases and the main diffraction peaks become sharper.
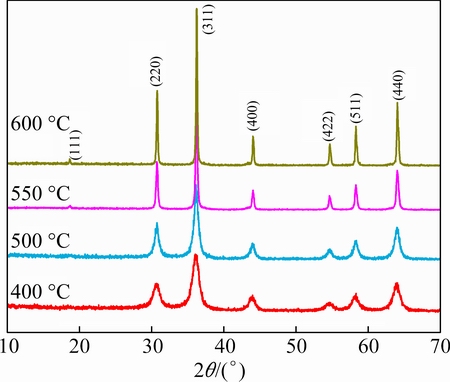
Fig. 2 XRD patterns of LiNiVO4 powders obtained at various temperatures
From 500 °C, all the peaks of samples exhibit the characteristic diffraction lines of LiNiVO4 without any miscellaneous phase, which is completely consistent with the pattern of JCPDS card No. 38-1395 [2] and indexed to the cubic system with space group Fd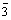 m. The main peaks for the material are labeled with (hkl) indexes. The XRD patterns also display a strong (220) line but (111) line is so weak that it can be hardly distinguished, which is ascribed to the inverse spinel structure of Vtetra(LiNi)octaO4 [21,22]. The XRD results show that the LiNiVO4 is synthesized successfully by calcining the precursor at 500 °C, and the temperature required for the formation of LiNiVO4 is much lower compared with that required for the conventional solid state product [6].
m. The main peaks for the material are labeled with (hkl) indexes. The XRD patterns also display a strong (220) line but (111) line is so weak that it can be hardly distinguished, which is ascribed to the inverse spinel structure of Vtetra(LiNi)octaO4 [21,22]. The XRD results show that the LiNiVO4 is synthesized successfully by calcining the precursor at 500 °C, and the temperature required for the formation of LiNiVO4 is much lower compared with that required for the conventional solid state product [6].
The FT-IR spectra of the synthesized powders are shown in Fig. 3. In the sample obtained at 400 °C, the band at 3397 cm-1 is characteristic of the O—H group, and those at 1500 and 1434 cm-1 result from the absorption of C=O group [23]. The band in 900-600 cm-1 region is associated with the stretching vibrations of V—O bonds of VO4 tetrahedron [9] in LiNiVO4. The presence of the organic remnants is probably originated from citric acid or citrate. With increasing the sintering temperature, the absorption intensities of C=O and O—H groups significantly decrease. The products obtained at 500, 550 and 600 °C show well-defined V—O bands. The results of FT-IR spectra also show that LiNiVO4 can be well formed at 500 °C.
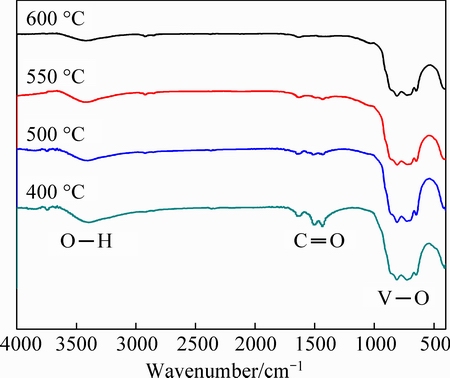
Fig. 3 FT-IR spectra of LiNiVO4 powders obtained at various temperatures
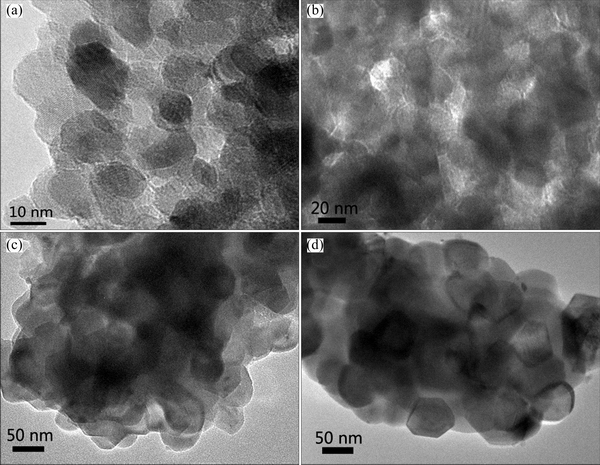
Fig. 4 TEM images of LiNiVO4 powders obtained at 400 °C (a), 500 °C (b), 550 °C (c) and 600 °C (d)
The influence of temperature on the morphology of LiNiVO4 was studied by TEM. As shown in Fig. 4, the synthesized LiNiVO4 is composed of flaky nano-sized particles. The particle size becomes larger as the calcination temperature increases, which agrees with the results of XRD. The particles of product calcined at 400 °C are coated by a layer of organic remnants with the particle size about 10 nm. The product obtained at 500 °C has a large amount of porous structures between the particles with the particle size about 20 nm. The porosity between particles probably results from the decomposition of the residual organics. The particle size increases to around 40 nm as the calcination temperature rises to 550 °C and there is an aggregation of small flaky particles. When the temperature further increases to 600 °C, the morphology is more regular and the particle size is about 60 nm.
3.3 Electrochemical performance
The electrochemical properties of LiNiVO4 powders were evaluated by cyclic voltammetry (CV). Figure 5 shows the first cyclic voltammogram of the electrode prepared from the porous LiNiVO4 powders obtained at 500 °C in the voltage range of 3-4.9 V (vs Li/Li+) at a scan rate of 0.1 mV/s. An anodic peak at 4.81 V and a cathodic peak at 4.72 V can be observed, which correspond to the electrochemical lithium deinsertion and insertion processes, respectively. The appearance of redox peaks illustrates the considerable structure reversibility of the sample.
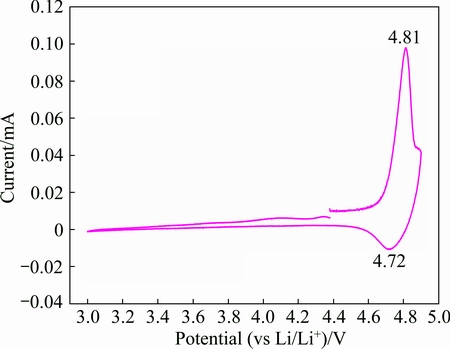
Fig. 5 Cyclic voltammogram of LiNiVO4 powder electrode at scan rate of 0.1 mV/s
The charge-discharge technique was used to study the electrochemical performance of LiNiVO4 as a cathode material for lithium-ion batteries. Figure 6 shows the charge-discharge curves of the 1st, 2nd and 5th cycles of the LiNiVO4 nanoparticle obtained at 500, 550 °C and a current density of 15 mA/g in the potential window of 3-4.9 V (vs Li/Li+). The products display a voltage plateau at 4.6-4.9 V corresponding to Li deinsertion/insertion process [24,25], which is consistent with the CV results. As shown in Fig. 6(a), the specific discharge capacities of 28, 21 and 14 mA·h/g for the 1st, 2nd and 5th cycles can be obtained for the product obtained at 500 °C, respectively. While for the product obtained at 550 °C, the specific discharge capacities are 30, 21 and 8 mA·h/g, respectively (Fig. 6(b)). The product obtained at 500°C exhibits better electro- chemical performance, which should be attributed to the fact that the product obtained at 500 °C has smaller particle sizes with more porous structure between the particles (Fig. 4(b)). The small particle size results in a short diffusion path for lithium ions and restrains the concentration polarization effectively, which should benefit the battery with high discharge capacity. Moreover, the porous structure would facilitate the electrolyte penetration and increase the contact area between the active materials and the electrolyte. Furthermore, the porous structure may be also of advantage to accommodate the volume variations during the lithium ion intercalation and deintercalation. The products synthesized by solution combustion synthesis method display much higher discharge capacity compared with the conventional solid state product [5]. However, compared with the theoretical capacity of LiNiVO4, the discharge capacity of the products is still low. PRAKASH et al [7] proposed that the decomposition of electrolyte at high voltage is the main reason for low capacity of LiNiVO4. There is scope for further investigations to understand the mechanism of Li extraction/insertion and improve the electrochemical properties of LiNiVO4.
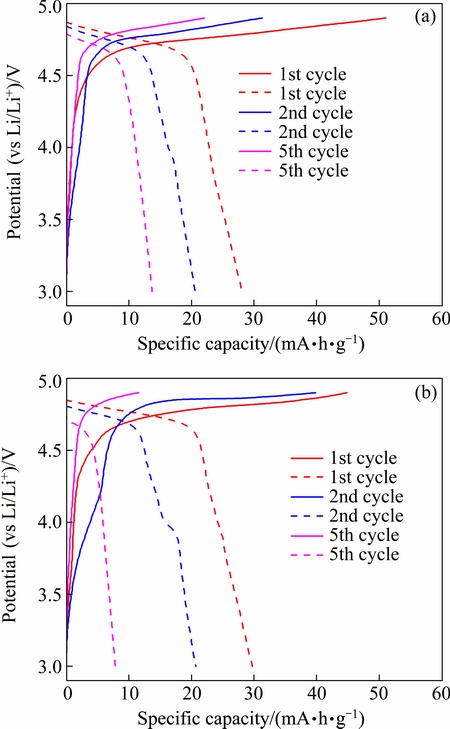
Fig. 6 Galvanostatic charge-discharge curves of samples obtained at 500 °C(a) and 550 °C(b)
4 Conclusions
1) LiNiVO4 nanoparticles with the particle size around 20 nm were synthesized using solution combustion synthesis method by calcining the precursor at 500 °C for 2 h.
2) As a cathode material for lithium-ion batteries, the porous LiNiVO4 powder exhibits good structural reversibility.
3) The solution combustion synthesis not only endows this work simplicity and feasibility but also provides a superiority of processing condition over the conventional solid state method.
References
[1] FEY G T K, LI W, DAHN J R. LiNiVO4: A 4.8 volt electrode material for lithium cells [J]. Journal of the Electrochemical Society, 1994, 141(9): 2279-2282.
[2] LIU J R, WANG M, LIN X, YIN D C, HUANG W D. Citric acid complex method of preparing inverse spinel LiNiVO4 cathode material for lithium batteries [J]. Journal of Power Sources, 2002, 108(1-2): 113-116.
[3] REDDY W V, WANNEK C, PECQUENARD B, VINATIER P, LEVASSEUR A. LiNiVO4–Promising thin films for use as anode material in microbatteries [J]. Journal of Power Sources, 2003, 119-121: 101-105.
[4] REDDY M V, WANNEK C, PECQUENARD B, VINATIER P, LEVASSEUR A. Preparation and characterization of LiNiVO4powder and non-stoichiometric LiNixVyOz films [J]. Materials Research Bulletin, 2008, 43(6): 1519-1527.
[5] FEY G T K, DAHN J R, ZHANG M J, LI W. The effects of the stoichiometry and synthesis temperature on the preparation of the inverse spinel LiNiVO4and its performance as a new high voltage cathode material [J]. Journal of Power Sources, 1997, 68(2): 549-552.
[6] FEY G T K, PENG W B. A new preparation method for a novel high voltage cathode material: LiNiVO4[J]. Materials Chemistry and Physics, 1997, 47(2-3): 279-282.
[7] PRAKASH D, MASUDA Y, SANJEEVIRAJA C. Synthesis and structure refinement studies of LiNiVO4electrode material for lithium rechargeable batteries [J]. Ionics, 2013, 19(1): 17-23.
[8] LIU R S, CHENG Y C, GUNDAKARAM R, JANG L Y. Crystal and electronic structures of inverse spinel-type LiNiVO4 [J]. Materials Research Bulletin, 2001, 36(7-8): 1479-1486.
[9] LU C H, LEE W C, LIOU S J, FEY G T K. Hydrothermal synthesis of LiNiVO4cathode material for lithium ion batteries [J]. Journal of Power Sources, 1999, 81-82: 696-699.
[10] LU C H, LIOU S J. Hydrothermal preparation of nanometer lithium nickel vanadium oxide powder at low temperature [J]. Materials Science and Engineering B, 2000, 75: 38-42.
[11] FEY G T K, CHEN K S. Synthesis, characterization, and cell performance of LiNiVO4 cathode materials prepared by a new solution precipitation method [J]. Journal of Power Sources, 1999, 81-82: 467-471.
[12] CAO Xiao-yu, XIE Ling-ling, ZHAN Hui, ZHOU Yun-zhou. Rheological phase synthesis and characterization of LiNiVO4 as a high voltage cathode material for lithium ion batteries [J]. Journal of New Materials for Electrochemical Systems, 2008, 11(3): 193-198.
[13] HAN Xiao-yan, TANG Wen-chao, YI Zong-hui, SUN Ju-tang. Synthesis, characterization and electrochemical performance of LiNiVO4anode material for lithium-ion batteries [J]. Journal of Applied Electrochemistry, 2008, 38: 1671-1676.
[14] THONGTEM T, KAOWPHONG S, THONGTEM S. Malic acid complex method for preparation of LiNiVO4nano-crystallites [J]. Journal of Materials Science, 2007, 42(11): 3923-3927.
[15] THONGTEM T, KAOWPHONG S, THONGTEM S. Preparation of LiNiVO4nano-powder using tartaric acid as a complexing agent [J]. Ceramics International, 2007, 33(8): 1449-1453.
[16] QIAO X B, HUANG Y L, SEO H J. Optical property and visible-light-driven photocatalytic activity of inverse spinel LiNiVO4 nanoparticles prepared by Pechini method [J]. Applied Surface Science, 2014, 321: 488-494.
[17] SUBRAMANIA A, ANGAYARKANNI N, KARTHICK SN, VASUDEVAN T. Combustion synthesis of inverse spinel LiNiVO4nano-particles using gelatine as the new fuel [J]. Materials Letters, 2006, 60(25-26): 3023-3026.
[18] KALYANI P, KALAISELVI N, MUNIYANDI N. An innovative soft-chemistry approach to synthesize LiNiVO4 [J]. Materials Chemistry and Physics, 2003, 77(3): 662-668.
[19] PRABAHARAN S R S, MICHAEL MS, RADHAKRISHNA S, JULIEN C. Novel low-temperature synthesis and characterization of LiNiVO4forhigh-voltage Li ion batteries [J]. Journal of Materials Chemistry, 1997, 7(9): 1791-1796.
[20] VIVEKANANDHAN S, VENKATESWARLU M, SATYANARAYANA N. Glycerol-assisted gel combustion synthesis of nano-crystalline LiNiVO4powders for secondary lithium batteries [J]. Materials Letters, 2004, 58(7-8): 1218-1222.
[21] CHITRA S, KALYANI P, YEBKA B, MOHAN T, HARO- PONIATOWSKI E, GANGADHARAN R, JULIEN C. Synthesis, characterization and electrochemical studies of LiNiVO4cathode material in rechargeable lithium batteries [J]. Materials Chemistry Physics, 2000, 65(1): 32-37.
[22] REDDY M V, PECQUENARD B, VINATIER P, LEVASSEUR A. Effect of substrate temperature on morphology and electrochemical performance of radio frequency magnetron sputtered lithium nickel vanadate films used as negative electrodes for lithium microbatteries [J]. Journal of Physical Chemistry B, 2006, 110(9): 4301-4306.
[23] BARBOUX P, TARASCON J M, SHOKOOHI F K. The use of acetates as precursors for the low-temperature synthesis of LiMn2O4 and LiCoO2intercalation compounds [J]. Journal of Solid State Chemistry, 1991, 94(1): 185-196.
[24] LI X, WEI Y J, EHRENBERG H, LIU D L, ZHAN S Y, WANG C Z, CHEN G. X-ray diffraction and Raman scattering studies of Li+/e--extracted inverse spinel LiNiVO4 [J]. Journal of Alloys and Compounds, 2009, 471: L26-L28.
[25] REDDY M V, LEVASSEUR A. Sputtered lithium nickel vanadium oxide (LiNiVO4) films: Chemical compositions, structural variations, target history, and anodic/cathodic electrochemical properties [J]. Journal of Electroanalytical Chemistry, 2010, 639: 27-35.
锂离子电池用多孔高电压正极材料LiNiVO4粉末的简便合成
秦牡兰1,刘万民1,梁叔全2,3,潘安强2,3
1. 湖南工程学院 化学化工学院,湘潭 411104;
2. 中南大学 材料科学与工程学院,长沙 410083;
3. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083
摘 要:以硝酸锂、硝酸镍、偏钒酸铵和柠檬酸为原料,采用溶液燃烧合成方法制备多孔LiNiVO4粉末。采用热重-差示扫描量热法(TG-DSC)、X射线衍射技术(XRD)、傅里叶变换红外光谱(FT-IR)和透射电镜(TEM)对样品的结构和形貌进行表征。结果表明,煅烧温度对样品的结晶度和形貌有显著影响。将前驱体在空气中于500 °C煅烧2 h可制备薄片型LiNiVO4纳米颗粒,其颗粒尺寸约为20 nm。作为锂离子电池正极材料,多孔LiNiVO4粉末具有较好的结构可逆性。
关键词:锂离子电池;LiNiVO4;正极材料;溶液燃烧合成;纳米颗粒
(Edited by Wei-ping CHEN)
Corresponding author: Mu-lan QIN; Tel: +86-15274946795; E-mail: qinmulan@126.com
DOI: 10.1016/S1003-6326(16)64455-2
Abstract: Porous LiNiVO4 powder was synthesized via solution combustion synthesis method using lithium nitrate, nickel nitrate, ammonium metavanadate and citric acid as raw materials. Thermogravimetry (TG) and differential scanning calorimetry (DSC), X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectroscopy and transmission electron microscopy (TEM) were used to evaluate the structures and morphologies of samples. The results show that the calcination temperature has significant effect on the crystallinity and morphologies. Pure LiNiVO4 flaky nanoparticles with a mean particle size around 20 nm can be readily prepared by calcining the precursor in air at 500 °C for 2 h. As a cathode material for lithium-ion batteries, the porous LiNiVO4 powder exhibits a good structural reversibility.


