J. Cent. South Univ. (2012) 19: 1685-1692
DOI: 10.1007/s11771-012-1194-z![]()
Dyestuff wastewater treatment by combined SDS-CuO/TiO2 hotocatalysis and sequencing batch reactor
XU Xuan(徐璇)1, JI Fang-ying(吉芳英)1, FAN Zi-hong(范子红)2,
HE Li(何莉)1, HU Xue-bin(胡学斌)1, ZHANG Kun(张琨)1
1. Key Laboratory of The Three Gorges Reservoir Region’s Eco-Environment (Chongqing University),
Ministry of Education, Chongqing 400045, China;
2. Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
Combined technology of SDS-CuO/TiO2 photocatalysis and sequencing batch reactor (SBR) were applied to treating dyestuff wastewater. Photocatalysis was carried out in a spiral up-flow type reactor as pre-treatment. SDS-CuO/TiO2 photocatalyst was prepared by modification of nano-TiO2 using CuO and sodium dodecyl sulfate (SDS). Results show that the SDS-CuO/TiO2 photocatalyst contains two kinds of crystals, including TiO2 and CuO. The band gap of this photocatalyst is 1.56 eV, indicating that it can be excited by visible light (λ<794.87 nm). And characterization also shows that there are alkyl groups on its surface. It takes 40 min to improve the biodegradability of dyestuff wastewater. Five-day biochemical oxygen demand (BOD5) and dehydrogenase activity (DHA) of wastewater reach the maximum value when dissolved oxygen is higher than 2.97 mg/L. SBR reactor was used to treat this biodegradability improved wastewater. Chemical oxygen demand (COD) and colority decline to 72 mg/L and 20 times, respectively, when the sludge loading is 0.179 kg(COD)/[kg(MLSS)·d], dissolved oxygen is 4.09 mg/L and aeration time is 10 h.
Key words:
biodegradability; photocatalysis; sequencing batch reactor; dyestuff wastewater;
1 Introduction
Dyestuff wastewater has many refractory organic pollutants, including kinds of synthetic dyes. It is very difficult to remove these pollutants by traditional biochemical methods [1-2]. In recent years, photocatalysis is widely researched in the field of pollutant removal. LIU et al [3] reported that methamidophos can be degraded by TiO2 effectively, and the amount of the photocatalyst, illumination time, pH of the system, reaction temperature, initial concentration, electron acceptors, metal ions and presence of anions strongly influence the degradation. CRISTINA et al [4] and KOHTANI et al [5] reported that phenol and benzo[a]pyrene can be degraded by photocatalyst. Studies show that almost all organic pollutants can be degraded by certain photocatalysts.
However, it is hard to purify high concentration wastewater by photocatalysis, because photocatalyst does not have enough catalytic ability to degrade all pollutants simultaneously within a short irradiation time [6]. And the operational costs of photocatalysis are relatively high compared to the biochemical methods. Advantages of photocatalysis focus on the degradation of refractory organic pollutants. Therefore, photocatalysis is considered to be an effective pre- or post-treatment for biochemical technology [7]. When it is used as pre-treatment, usually, it is proposed to improve the biodegradability of wastewater [8]. BOLDUC and ANDERSON [9] reported that TiO2 can be used to degrade m-dinitrobenzene, diphenylamine and resorcinol to improve the biodegradability of model wastewater.
However, photocatalyst does not have the ability to select refractory organic pollutants from wastewater, and to degrade it preferentially [10]. We insist that photocatalyst has to be modified to have selective surface for refractory organic pollutants. Thus, these refractory pollutants will be preferentially removed, but biodegradable contaminants can be left. This means that the catalytic ability of photocatalyst is improved efficiently, and the biodegradability of wastewater will be improved faster than using common photocatalyst.
According to these analyses, we develop a photocatalytic-biochemical combined system to treat high concentration dyestuff wastewater. In this system, photocatalysis is still used as pre-treatment unit. But the photocatalyst is modified to have hydrophobic surface. The primarily purpose is to degrade refractory organic pollutants selectively, and to improve the biodegradability of wastewater. And this process takes place in a spiral up-flow type reactor [11]. The following biochemical unit is sequencing batch reactor (SBR), which is designed to remove common pollutants. Because of the pre-treatment of photocatalysis, it becomes much easier for biochemical unit to achieve the effluent standard. The main aim of this work is to develop a general method for dyestuff wastewater treatment that can combine the advantages of photocatalysis and biochemical methods.
2 Materials and methods
2.1 Chemicals
Tetra-n-butyl titanate was purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Sodium dodecyl sulfate (SDS), copper nitrate, anhydrous ethanol and acetic acid were from Tianjin Tiantai Fine Chemicals Co., Ltd. (China). Tetra-n-butyl titanate was of chemical grade. The other reagents were all of analytical grade.
2.2 Preparation of photocatalyst
SDS-CuO/TiO2 was prepared as the following method. 10 mL tetra-n-butyl titanate was dissolved in 40 mL anhydrous ethanol. This solution was signed as A. 5 mL acetic acid, 23.5 g copper nitrate and 0.1 g SDS were dissolved in 40 mL distilled water. This mixture was signed as B. B was added in A drop by drop. These suspension systems were stirred at room temperature for 2 h. It became blue gelatin after 48 h aging. The gelatin was dried at 110 °C. The solid particles were ground to pass 75 μm sieve, and calcined at 450°C (heating rate is 2 °C/min) for 5 h. The resultant particles were SDS- CuO/TiO2. CuO/TiO2 and TiO2 were prepared by the same method but without sodium dodecyl sulfate or without copper nitrate and sodium dodecyl sulfate, respectively.
2.3 Photocatalysts characterization
X-ray diffraction (XRD) patterns of photocatalysts were conducted in a XD-2 instrument using Cu Kα radiation (Persee, China). Scanning electron microscopy (SEM) images were collected on an S-4800 field emission scanning electron microscope (Hitachi, Japan). Brunauer-Emmett-Teller (BET) surface areas were measured by nitrogen adsorption at 77.35 K on an ASAP-2010 adsorption apparatus (Micromeritics, USA). Fourier-transform infrared spectroscopy (FTIR) was performed on a IRPrestige-21 type instrument (Shimadzu, Japan) running at 2 cm-1 resolutions. The UV-Vis spectra were collected on a UV-3010 UV-visible spectrometer (Hitachi, Japan) using BaSO4 as reference.
2.4 Degradation experiment
Figure 1 shows the schematic diagram of photocatalytic-biochemical treatment system. Photocatalysis was carried out in a homemade spiral up- flow reactor with a 500 W Xe-arc lamp (λmax≥420 nm). Irradiation intensity on the surface of the reactor was about 5 000 lx. The volume of this photocatalytic reactor is 20 L. Wastewater and air were pumped in it from the bottom. This meant that the photocatalyst concentration was 2 g/L in the reactor. The hydraulic remaining time (HRT) of wastewater in the reactor was dependent on the flow and volume. In order to find a better HRT, several flows were experimented and the relationship between effluent quality and HRT was researched. The effect of dissolved oxygen (DO) was also studied by changing the air flow.
Effluent of photocatalysis was treated in a SBR unit. The volume of SBR is 100 L. Mixed liquor suspended solid (MLSS) in SBR was designed as 4 000 mg/L. Every cycle took 12 h, including 0.5 h infall, 10 h aeration, 1 h sediment and 0.5 h draining. Effects of sludge loading and DO were researched by changing the filling ratio and air flow, respectively. Concentrations of chemical oxygen demand (COD), 5 d biochemical oxygen demand (BOD5) and total organic carbon (TOC) were respectively determined by HACH-COD/DR2010 (HACH, America) and Oxitop (WTW, America) LiquiTOC (Elementar, Germany). Dehydrogenase activity (DHA) was measured as reported in Ref. [12]. Colority was determined by visual colorimetry.
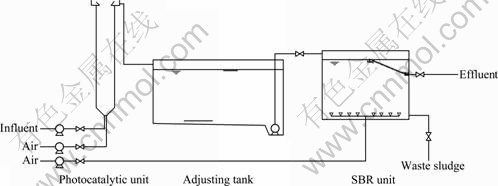
Fig. 1 Photocatalytic-biochemical treatment system
3 Results and discussion
3.1 Photocatalysts characterization
3.1.1 XRD characterization
The XRD patterns of photocatalysts are compared in Fig. 2. Diffraction peaks of anatase TiO2 are observed with 2θ at 25.2°, 37.7° and 48.1° in all three photocatalysts. Diffraction peaks of CuO appear at 35.5°, 38.7° and 48.7° in CuO/TiO2 and SDS-CuO/TiO2. This means that anatase TiO2 crystal always geminates no mater copper nitrate or/and sodium dodecyl sulfate is/are added or not. And copper nitrate and sodium dodecyl sulfate do not change the crystal of TiO2. When copper nitrate is added, CuO diffraction peaks are observed, and they are not affected by sodium dodecyl sulfate. XRD characterization indicates that there are two crystals in SDS-CuO/TiO2 photocatalyst. It is reported that photocatalyst with combined crystals may have excellent catalytic activity because of its isolating function on hole (h+)-electron (e-) pair [13-14]. It is known that the band gap of CuO crystal is about 1.4-1.7 eV [15-16], which is much smaller than TiO2 (about 3.2 eV) [17-18]. CuO can be excited by visible light, and then transports h+ to the valence band (VB) of TiO2, but remains e- in its own conduction band (CB). Thus, hole-electron pair is separated in CuO/TiO2 and SDS-CuO/TiO2 photo- catalysts. This phenomenon can promote the using efficiency of h+ and e-, and enhance the catalytic ability of photocatalyst.
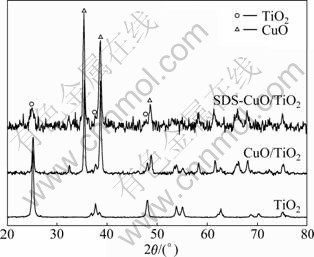
Fig. 2 XRD patterns of TiO2, CuO/TiO2 and SDS-CuO/TiO2
3.1.2 BET characterization
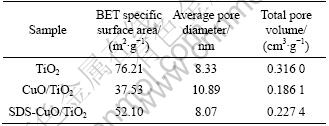
Table 1 gives the N2 adsorption-desorption characterization results. BET specific surface area decreases from 76.21 m2/g to 37.53 m2/g and the total pore volume declines to 0.1861 m3/g when CuO forms in photocatalyst. These variations are caused by the increase of average pore diameter. This diameter is decreased to 8.07 nm when SDS is added, so BET area improves to be 52.1 m2/g. BET area is an important factor of photocatalytic reaction. Larger BET area leads to better adsorption ability and more activity position. This means that the photocatalytic efficiency of TiO2 and SDS-CuO/TiO2 may be higher than that of CuO/TiO2.
Table 1 BET surface area, average pore diameter and maximum pore volume of TiO2, CuO/TiO2 and SDS-CuO/TiO2
3.1.3 SEM characterization
SEM images of TiO2, CuO/TiO2 and SDS- CuO/TiO2 are presented in Fig. 3. As shown in Fig. 3, particle sizes of these three photocatalysts are 0.3, 1.0 and 0.5 μm, respectively. Particle size of CuO/TiO2 and SDS-CuO/TiO2 is bigger than that of TiO2, indicting that copper nitrate affects the forming of photocatalyst crystal. It can also be seen that agglomeration occurs when SDS is added. We speculate that SDS plays the role of bridging adsorption in solution and gelatin during the preparation of photocatalyst. Ti(OH)4 gelatin and SDS are adsorbed by each other. Thus, dispersion property of SDS-CuO/TiO2 is worse than that of TiO2 and CuO/TiO2.
3.1.4 UV-Vis characterization
The UV-Vis absorption properties of TiO2, CuO/TiO2 and SDS-CuO/TiO2 are shown in Fig. 4. There is a clear redshift of absorption peak of CuO/TiO2 and SDS-CuO/TiO2. UV-Vis absorption intensity of CuO/TiO2 is much lower than that of SDS-CuO/TiO2. SEM characterization (Fig. 3) shows that particle size of CuO/TiO2 is bigger than that of SDS-CuO/TiO2. We consider that the bigger particle size leads to the lower absorption. It was reported by Al-ANI and HIGAZY that the UV-Vis absorption angle and band gap of semiconductor followed the law [19]:
![]() (1)
(1)
where a and B are constants; hv is the energy of photon, Eg is the band gap; r is a constant related to the electron transition in semiconductor material band. WANG et al proved that r value of TiO2 is 2 [20]. We plot (ahν)2 versus hν, and then evaluate the band gap Eg by extrapolating the straightest line to the hν axis. As shown in Fig. 5, Eg evaluation of TiO2 is 3.22 eV, and that of CuO/TiO2 and SDS-CuO/TiO2 is 1.56 eV. So the maximum excited wavelength of TiO2 is 385.06 nm, and it is 794.87 nm for CuO/TiO2 and SDS-CuO/TiO2. UV-Vis characterization indicates that photocatalysts modified by CuO can be excited by visible light, and the addition of SDS does not affect the band gap and the maximum excited wavelength of photacatalyst.
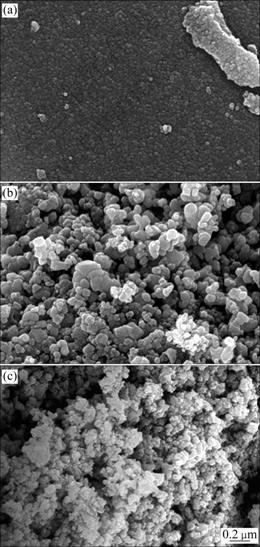
Fig. 3 SEM images of different catalysts: (a) TiO2; (b) CuO/TiO2; (c) SDS-CuO/TiO2
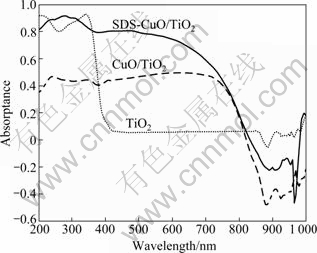
Fig. 4 UV-Vis absorption spectra of TiO2, CuO/TiO2 and SDS-CuO/TiO2

Fig. 5 Band gap of TiO2, CuO/TiO2 and SDS-CuO/TiO2
3.1.5 FTIR characterization
Functional groups on photocatalysts surface were confirmed by Fourier-transform infrared spectroscopy. Figure 6 shows the FTIR characterization results. It is shown from the spectra that the peaks of C—C stretching vibration around 1 089.78 cm-1 and 1 116.78 cm-1 are enhanced when the SDS is added. This phenomenon demonstrates that C—C groups on photocatalysts surface become apparent. Deformation vibration of —CH— around 827.46 cm-1 also increases obviously when using SDS as modification reagent. We speculate that there are alkyl groups on SDS-CuO/TiO2 surface. Peaks at 534.28 cm-1 and 3 337.36 cm-1 are assigned to Ti—O and —OH stretching vibrations, respectively. Photo- catalyst with alkyl groups on its surface may exhibit better adsorption of hydrophobic organic compounds. Refractory pollutants in dyestuff wastewater mostly have hydrophobic organic groups. Thus, refractory organic pollutants will be preferentially adsorbed and degraded by SDS-CuO/TiO2, and biodegradability of wastewater will be improved.
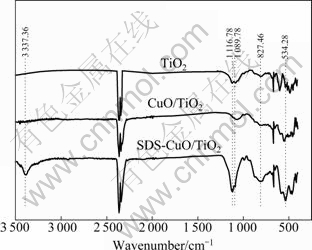
Fig. 6 FTIR spectra of TiO2, CuO/TiO2 and SDS-CuO/TiO2
3.2 Treatment efficiency of photocatalysis
3.2.1 Influence of HRT
Static experiments were carried out to analyze the influence of hydraulic remaining time. After a certain irradiation time, COD, BOD5, TOC and DHA concentrations were determined. Results are shown in Fig. 7. Unusually, COD concentration increases at the first 30 min. It goes to the maximum value of 1 303 mg/L, and then declines. BOD5 varies with COD. It stays below 100 mg/L at the first 30 min, and then reaches its maximum concentration after 40 min irradiation. TOC always decreases during the reaction. DHA value almost does not change at the first 30 min. Then, it reaches about 28 μg(TF)/(mL·h) quickly and maintains at this level.
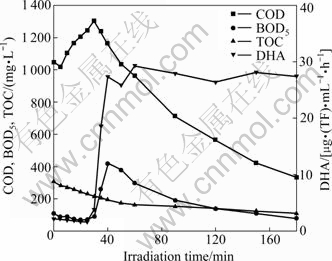
Fig. 7 COD, BOD5, TOC and DHA concentration with different irradiation time
COD variation indicates that refractory organic pollutants convert to biodegradable organics when being catalyzed by photocatalyst. Dyestuff wastewater in this work contains many kinds of refractory organic pollutants, such as disperse blue BGL. Disperse blue BGL is a kind of anthraquinone dyes, and it can not be oxidized by potassium dichromate when determining COD. In fact, it can be obtained by oxidation of anthracene using dichromate [21]. So it does not contribute to the COD of wastewater. In the first 30 min, refractory organic pollutants as disperse blue BGL are initially degraded by photocatalysts. The products are not structure-stable as raw materials. They can be oxidized by potassium dichromate and reflected in COD. Thus, COD concentration increases at the beginning of photocatalysis. This phenomenon does not show the increase of pollutants. On the contrary, it exhibits the advantages of photocatalysis in degradation of refractory organic pollutants. TOC curve proves this explanation. TOC concentration decreases all the reaction time, indicating that the organic pollutants are removed continually. This capability of SDS-CuO/TiO2 photocatalyst is based on its surface property with alkyl groups (Fig. 6).
Although refractory organic pollutants begin to degrade in beginning of photocatalysis, biodegradability of wastewater is not improved until 30 min later. BOD5 and DHA values are still at a low level, and even decline. This illustrates that intermediates of these pollutants still can not be used by microbes, or toxic to microbes. With further reaction, these toxic intermediates are effectively degraded, so BOD5 and DHA increase significantly. After 40 min reaction, almost all refractory organic pollutants convert to biodegradable organics. Photocatalysts begin to degrade these simple pollutants, so BOD5 declines with irradiation time and DHA remains at its high level. We speculate that biodegradability of wastewater reaches the highest value after 40 min irradiation.
In the photocatalytic-biochemical treatment system, SDS-CuO/TiO2 photocatalytic unit is carried out to degrade the refractory organic pollutants selectively and preferentially. Its purpose is to improve the biodegradability of wastewater. Thus, the best HRT of photocatalytic unit is 40 min.
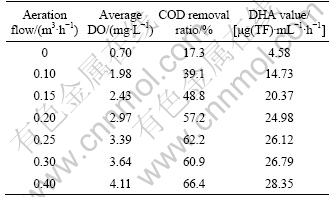
3.2.2 Influence of DO
Influence of dissolved oxygen on COD removal ratio and DHA value were examined in spiral up-flow type reactor. The HRT of wastewater was 40 min. Results under different DO conditions are presented in Table 2. COD removal ratio increases from 17.3% to 66.4% when average DO concentration is from 0.72 mg/L to 4.11 mg/L. This shows that high DO concentration is favorable to COD removal. DHA value has the same variation as COD removal ratio. ZHANG et al [22] reported that additional oxidant will promote the catalytic activity of photocatalyst, because it can capture electron on conduction band of photocatalyst, and then produce hydroxyl radical as the following laws:
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
![]() (5)
(5)
![]() (6)
(6)
Influence of DO in this work is consistent with the report. It can also be seen from Table 2 that COD removal ratio and DHA value increase slowly when DO concentration is more than 3 mg/L. Much more aeration and DO cannot improve effluent quality significantly, but waste power. So we identify that the best aeration flow of the spiral up-flow type reactor is 0.2 m3/h.
Table 2 COD removal ratio and DHA value in photocatalytic unit with different DO
3.3 Treatment efficiency of SBR
3.3.1 Influence of sludge loading
We adjusted the sludge loading of SBR reactor by changing the filling ratio. We employed four filling ratios, which were 0.08, 0.23, 0.38 and 0.54. The average sludge loadings obtained were 0.037, 0.110, 0.179 and 0.245 kg(COD)/[kg(MLSS)·d], respectively. Experiment results are shown in Fig. 8. From Fig. 8 we find that COD concentration and colority of effluent increase obviously, when sludge loading is changed from 0.037 kg(COD)/[kg(MLSS)·d] to 0.245 kg(COD)/ [kg(MLSS)·d]. The COD concentration of effluent is about 72 mg/L, and colority is about 20 times, when sludge loading is 0.179 kg(COD)/[kg(MLSS)·d]. These indicators achieve the I-level standard of “Discharge Standard of Water Pollutants for Dyeing and Finishing of Textile Industry” (GB 4287—92). If sludge loading increases to 0.245 kg(COD)/[kg(MLSS)·d], COD and colority reach 92 mg/L and 45 times, respectively. The colority does not achieve the standard.
Because of the low biodegradability of dyestuff wastewater, traditional biochemical treatment takes a very low loading [23]. In this photocatalytic-biochemical system, biodegradability of wastewater is improved by photocatalytic pre-treatment. So the SBR unit can operate in a higher sludge loading condition. Even the loading increases to 0.245 kg(COD)/[kg(MLSS)·d], and COD of effluent can also fit for the I-level standard.
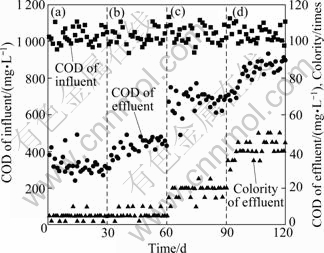
Fig. 8 COD and colority removal efficiency in SBR unit under different sludge loadings: (a) 0.037 kg(COD)/[kg(MLSS)·d]; (b) 0.110 kg(COD)/[kg(MLSS)·d]; (c) 0.179 kg(COD)/ [kg(MLSS)·d]; (d) 0.245 kg(COD)/[kg(MLSS)·d]
3.3.2 Influence of DO
COD of effluent treated under different DO conditions are shown in Fig. 9. It is shown that COD concentration of effluent declines when DO increases. When average DO increases to 2.74 mg/L or above, average concentration of COD is below 100 mg/L, meeting the quest of I-level standard of “Discharge Standard of Water Pollutants for Dyeing and Finishing of Textile Industry”. Besides, Fig. 10 shows that the colority of effluent has the same variation trends of COD efficiency removal (Colority of influent maintains at about 300 times during these experiments). However, it does not fit for the I-level standard until DO in SBR reactor reaches 4.09 mg/L. This indicates that it needs higher DO for colority removal than COD.
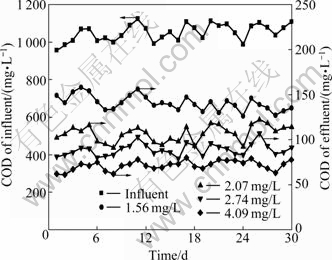
Fig. 9 COD removal efficiency in SBR unit under different DO conditions
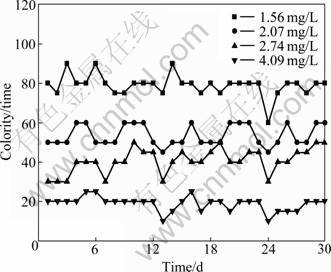
Fig. 10 Colority removal efficiency in SBR unit under different DO conditions
3.3.3 Influence of aeration time
Figure 11 shows the COD removal efficiency in SBR reactor. It can be found from Fig. 11 that COD continues to be degraded in 10 h of aeration time. However, COD degradation rate gradually slows down with the reaction time. COD degradation ratio of 8 h is below 100 mg/L, fitting for the quest of the I-level standard. But colority at this point is higher than 20 times. When aeration time lasts for 10 h, it can meet the standard.
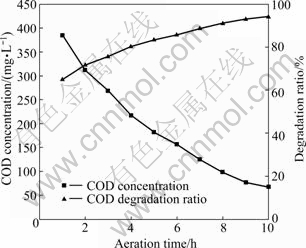
Fig. 11 Influence of aeration time on COD degradation in SBR unit
It needs a long HRT for treatment of dyestuff wastewater when biochemical technology is used alone. JIN et al [24] reported that the effluent concentration of COD cannot reach the I-level standard when influent concentration is higher than 1 000 mg/L, despite the HRT of “Hydrolytic acidification-SBR” system is 16 h. HRT of our photocatalytic-biochemical system is less than 12.5 h. A shorter HRT and better effluent quality are obtained.
3.4 Treatment efficiency of comparative systems
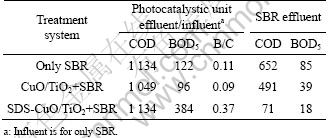
Single SBR reactor and CuO/TiO2+SBR were used to treat dyestuff wastewater as comparative systems of SDS-CuO/TiO2+SBR. These parallel systems were operated at the same time, and took 30 d. The parameters follow the results of Sections 3.2 and 3.3. Table 3 gives the average COD and BOD5 concentration of each unit. According to the BOD5/COD (B/C) value, it is known that the biodegradability of influent is poor. If SBR is used to treat this wastewater alone, average COD concentration of its effluent is 652 mg/L. CuO/TiO2 photocatalysis can degrade COD and BOD5 to some extent. But biodegradability of wastewater is not improved. Effluent quality of this system also does not reach the standard. After being treated by SDS-CuO/TiO2 photocatalysis, biodegradability is improved greatly, because of the obvious increase of BOD5. Although COD is as high as that in CuO/TiO2+SBR system, it provides a good degradation condition for the following biochemical treatment unit. Thus, SBR unit in SDS-CuO/TiO2+SBR system can degrade the wastewater to the I-level standard. The difference between CuO/TiO2+SBR and SDS-CuO/TiO2+SBR is the photocatalyst. As shown in Section 3.1, SDS-CuO/TiO2 has organic groups on its surface. It can adsorb and degrade refractory organic pollutants preferentially. Via this process, biodegradability of wastewater is improved.
Table 3 COD and BOD5 of effluent in comparative treatment systems
4 Conclusions
1) After being modified by sodium dodecyl sulfate, the SDS-CuO/TiO2 photocatalyst contains TiO2 crystals and CuO crystals. And there are alkyl groups on its surface. The band gap of SDS-CuO/TiO2 is 1.56 eV.
2) After being treated by SDS-CuO/TiO2 for 40 min at DO of 2.97 mg/L, BOD5 concentration of dyestuff wastewater increases obviously. B/C value improves from 0.11 to 0.37, indicating that the biodegradability of wastewater becomes much better.
3) Effluent of photocatalytic unit is treated by SBR reactor. The best degradation conditions are: sludge loading of 0.179 kg(COD)/[kg(MLSS)·d]; average DO of 4.09 mg/L; aeration time of 10 h.
References
[1] ROBINSON T, CHANDRAN B, NIGAM P. Removal of dyes from a synthetic textile dye effluent by biosorption oon apple pomace and wheat straw [J]. Water Research, 2002, 36: 1743-1748.
[2] AHN D H, CHANG W S, YOON T I. Dyestuff wastewater treatment using chemical oxidation, physical adsorption and fixed bed biofilm process [J]. Process Biochemistry, 1999, 34: 429-439.
[3] LIU Wei, CHEN Shi-fu, ZHAO Wei, ZHANG Su-juan. Titanium dioxide mediated photocatalytic degradation of methamidophos in aqueous phase [J]. Journal of Hazardous Materials, 2009, 164: 154- 160.
[4] CRISITINA A, ARTURO M A, SIXTO M, ANA B. New insights on solar photocatalytic degradation of phenol over Fe-TiO2 catalysts: Photo-complex mechanism of iron lixiviates [J]. Applied Catalysis B: Environmental, 2009, 93(1/2): 96-105.
[5] KOHTANI S, INAOKA Y, HAYAKAWA K, NAKAGAKI R. Degradation of benzo[a]pyrene using TiO2 and Ag-loaded BiVO4 photocatalysts: Evaluation by the Ames mutagenicity assay [J]. Journal of Advanced Oxidation Technologies, 2007, 10(2): 381-386.
[6] YAMASHITA H, HARADA M, MISAKA J, TAKEUCHI M, NEPPOLIAN B, ANPO M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2 [J]. Catalysis Today, 2003, 84: 191-196.
[7] HU Chun, WANG Yi-zhang. Decolorization and biodegradability of photocatalytic treated azo dyes and wool textile wastewater [J]. Chemosphere, 1999, 39(12): 2107-2215.
[8] JONSTRUP M, WAERJERSTAM M, MURTO M, MATTIASSON B. Immobilisation of TiO2 for combined photocatalytic-biological azo dye degradation [J]. Water Science & Technology, 2010, 62(3): 525- 531.
[9] BOLDUC L, ANDERSON W A. Enhancement of the biodegradability of model wastewater containing recalcitrant or inhibitory chemical compounds by photocatalytic pre-oxidation [J]. Biodegradation, 1997, 8(4): 237-249.
[10] TURCHI C S, OLLIS D F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack [J]. Journal of Catalysis, 1990, 122(1): 178-192.
[11] XU Xuan, JI Fang-ying, FAN Zi-hong. Design of spiral up-flow tower-type photocatalysis reactor [J]. China Water & Wastewater, 2009, 25(23): 79-81. (in Chinese)
[12] WANG Jian-fang, JIN Wen-biao, ZHAO Qing-liang, LIU Zhi-gang, LIN Ji-kan. Peformance of treating wastewater and anti-shockloading in oxic-settling-anaerobic (OSA) process for minimization of excess sludge [J]. Environmental Science, 2007, 28(11): 2488-2493. (in Chinese)
[13] WU Ling, YU J C, FU Xian-zhi. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation [J]. Journal of Molecular Catalysis A: Chemical 2006, 244(1/2): 25-32.
[14] GOPIDAS K R, BOHORQUEZ M, KAMAT P V. Photophysical and photochemical aspects of coupled semiconductors: Charge-transfer processes in colloidal CdS-TiO2 and CdS-AgI systems [J]. J Phys Chem, 1990, 94(16): 6435-6440.
[15] GHIJSEN J, TJENG L H, VANELP J, ESKES H, WESTERINK J, SAWATZKY G A, CZYZYK M T. Electronic structure of Cu2O and CuO [J]. Physical review B: Condensed Matter and Materials Physics, 1988, 38(16): 11322-11330.
[16] KOFFYBERG F P, BENKO F A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO [J]. Journal of Applied Physics, 1982, 53(2): 1173-1177.
[17] ROBERT D. Photosensitization of TiO2 by MxPy and MxSy nanoparticles for heterogeneous photocatalysis applications [J]. Catalysis Today, 2007, 122(1/2): 20-26.
[18] TIAN Guang-lei, HE Hong-bo, SHAO Jian-da. Effect of Microstructure of TiO2 thin films on optical band gap energy [J]. Chinese Physics Letters, 2005, 22(7): 1787-1789.
[19] AL-ANI S K J, HIGAZY A A. Study of optical absorption edges in MgO-P2O5 glasses [J]. Journal of Materials Science, 1991, 26: 3670-3674.
[20] WANG Zhi-yu, TANG Pei-song, JIANG Yu-long, FAN Xian-ping, QIAN Guo-dong, HONG Zhang-lian. Study on fluorescence and diffuse reflection spectrum of nanosized TiO2 [J]. Rare Metal Materials and Engineering, 2004, 33(Suppl.3): 162-168. (in Chinese)
[21] FRIEDMAN L, FISHEL DL, SHECHTER H. Oxidation of alkylarenes with aqueous sodium dichromate: A useful method for preparing mono-and polyaromatic carboxylic acids [J]. The Journal of Organic Chemistry, 1965, 30(5): 1453-1457.
[22] ZHANG Wen, WANG Xu-xu, LIN Hua-xiang, FU Xian-zhi. Influence of magnetic field on the formation rate of hydroxyl radical in photocatalysis [J]. Acta Chimica Sinica, 2005, 63(18): 1765-1768.
[23] OH Y K, KIM Y J, AHN Y, SONG S K, PARK S. Color removal of real textile wastewater by sequential anaerobic and aerobic reactors [J]. Biotechnology and Bioprocess Engineering, 2004, 9(5): 419- 422.
[24] JIN Yi-zhong, WEI Yan-yan, CHEN Xiao-ping. Studies on treatment of printing and dying wastewater by hydrolysis and acidification- SBR technology [J]. China Environmental Science, 2004, 24(4): 489-491. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Project(CDJZR11210009) supported by the Fundamental Research Funds for the Central Universities of China
Received date: 2011-07-26; Accepted date: 2011-11-14
Corresponding author: XU Xuan, Associate Professor, PhD; Tel: +86-18701488468; E-mail: xuxuan@cqu.edu.cn
Abstract: Combined technology of SDS-CuO/TiO2 photocatalysis and sequencing batch reactor (SBR) were applied to treating dyestuff wastewater. Photocatalysis was carried out in a spiral up-flow type reactor as pre-treatment. SDS-CuO/TiO2 photocatalyst was prepared by modification of nano-TiO2 using CuO and sodium dodecyl sulfate (SDS). Results show that the SDS-CuO/TiO2 photocatalyst contains two kinds of crystals, including TiO2 and CuO. The band gap of this photocatalyst is 1.56 eV, indicating that it can be excited by visible light (λ<794.87 nm). And characterization also shows that there are alkyl groups on its surface. It takes 40 min to improve the biodegradability of dyestuff wastewater. Five-day biochemical oxygen demand (BOD5) and dehydrogenase activity (DHA) of wastewater reach the maximum value when dissolved oxygen is higher than 2.97 mg/L. SBR reactor was used to treat this biodegradability improved wastewater. Chemical oxygen demand (COD) and colority decline to 72 mg/L and 20 times, respectively, when the sludge loading is 0.179 kg(COD)/[kg(MLSS)·d], dissolved oxygen is 4.09 mg/L and aeration time is 10 h.


