
Synthesis and grain growth kinetics of in-situ FeAl matrix nanocomposites(Ⅰ): Mechanical alloying of Fe-Al-Ti-B composite powder
REN Rong(任 榕), WU Yu-cheng(吴玉程), TANG Wen-ming(汤文明),
WANG Feng-tao(汪峰涛), WANG Tu-gen(王涂根), ZHENG Zhi-xiang(郑治祥)
School of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China
Received 16 April 2007; accepted 16 July 2007
_________________________________________________________________________________
Abstract:
Three nanocrystalline alloys, Fe50Al50, Fe42.5Al42.5Ti5B10 and Fe35Al35Ti10B20 (molar fraction, %), were synthesized from elemental powders by high-energy ball milling. The structural evolutions and morphological changes of the milled powders were characterized by X-ray diffractometry(XRD), transmission electron microscopy(TEM) and scanning electron microscopy(SEM). The effects of different Ti, B additions on the structure and phase transformation in these alloys were also discussed. It is observed that the diffusion of Al, Ti, B atoms into Fe lattice occurs during milling, leading to the formation of a BCC phase identified as Fe(Al) or Fe(Al, Ti, B) supersaturated solid solution. Fe-based solid solution with nanocrystalline structure is observed to be present as the only phase in all the alloy compositions after milling. Furthermore, the contents of Ti, B affect the formation of mechanical alloying products, changes in the lattice parameter as well as the grain size.
Key words:
FeAl; composite; mechanical alloying; structural evolution; nanocrystalline;
_________________________________________________________________________________
1 Introduction
Iron aluminides are of considerable interest due to their excellent oxidation and sulphidation resistance especially at elevated temperatures, in combination with their relatively low density and cost. However, concurrent drawbacks such as low ductility at room temperature and low mechanical strength above 600 ℃ limit their use for structural applications. Since refining of grain size to nanocrystalline state has been suggested as a way of improving the ductility of intermetallic compounds, a considerable amount of work has therefore been concentrated on the synthesis of nanocrystalline intermetallic compounds, and in particular, of iron aluminides[1]. Mechanical alloying (MA) is one of the most promising techniques for the preparation of nanocrystalline alloys and compounds from elemental powders[2-3]. Furthermore, several studies have reported on the successful fabrication of intermetallic matrix composites that are difficult to prepare by conventional processing techniques through MA process followed by heat treatment[4-5]. Obtaining bulk intermetallic matrix composites is of interest not only due to the improved hardness and strength, but also because of expectations of better ductility and toughness[6]. Some investigations have been performed on mechanical alloying of intermetallic matrix composites reinforced with particles such as nitrides, carbides or borides[7-9]. However, investigations of mechanically alloyed Fe-Al-Ti-B have mostly concentrated on the in-situ formation of TiB2 in the Fe3Al matrix[10-11]. Few studies have dealt with the role of a wide range of TiB2 compositions, especially the high Ti and B contents, in the synthesis of in-situ composites based on FeAl and their effect on the structural evolution during fabrication. In the present study, MA technique was employed to prepare several Fe-Al-Ti-B alloys with different Ti, B additions. The structural evolutions of the elemental powders during ball milling were studied in detail. The effects of Ti, B addition on the structure and phase transformation were also discussed.
2 Experimental
Elemental powders of Fe, Al, Ti and B with the purities of 99%, 98%, 99% and 98% respectively were used as starting materials. And the average particle sizes of the powders are 10, 10, 45 and 45 μm, respectively. Three compositions, Fe50Al50, Fe42.5Al42.5Ti5B10 and Fe35Al35Ti10B20 (molar fraction, %) were mixed from the above powders and were designated as Alloy 1, Alloy 2 and Alloy 3, respectively. Mechanical alloying was performed in an argon atmosphere using a GN-2 high-energy ball mill operated at 700 r/min. Hardened steel balls of 12 mm in diameter were employed. The ball-to-powder mass ratio was about 10?1. CH3(CH2)5CH3 (n-heptane) was used as a lubricant agent, in a concentration of 0.015 mL/g to avoid aluminum sticking to the walls of the vial and the milling media during the process. The vial was cooled with forced air during milling to prevent excessive temperature rise. After a certain time, the milling was stopped and a small amount of the powders was removed for structure analysis. All handling of the powders during milling was performed in the glove box in an argon atmosphere.
The morphological changes and the structural evolutions occurred in the powder during milling were characterized using a Sirion200 type scanning electron microscope(SEM) and a D/max-γB type X-ray diffractometer with Cu Kα radiation (λ=0.154 nm). Morphological and structural observations of the milling product were carried out by an H-800 type transmission electron microscope (TEM+SAED) with electronic diffractometer. Average grain sizes of phases were determined by Scherrer formula, using standard method to eliminate the instrumental broadening contribution:
![]() (1)
(1)
where λ is the wavelength of X-ray radiation; B is the angular width at half-maximum intensity and 2θ is the diffraction angle.
3 Results and discussion
3.1 Morphological evolution of particles during milling
Figs.1(a)-(c) show the SEM images of Alloy 2 powders after being milled for 5, 20 and 50 h, respectively. The particle morphology after 5 h of milling is irregular and the size appears to be distributed over a wide range. Some particles are as large as 10 μm, while some others are as small as 0.5 μm. After 20 h, the particles become smaller, appear to approach a spherical shape, and the size range gets narrow evidently, as shown in Fig.1(b). After 50 h of milling, the particles are spherical and uniform in size less than 2 μm mostly. Thus, powder particles become uniform and round, and the average particle size diminishes with increasing milling time. Similar results are also obtained in mechanically alloyed Alloy 1 and Alloy 3.
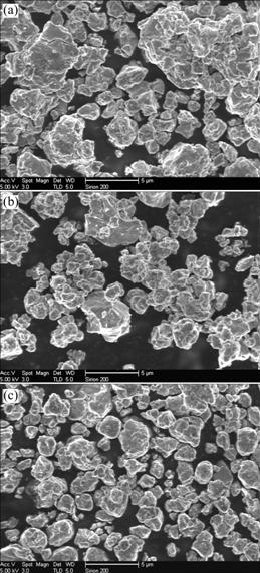
Fig.1 SEM images of particles in Alloy 2 after milling for 5 h (a), 20 h (b) and 50 h (c)
3.2 XRD analysis during milling
The structural evolutions during milling are recorded by XRD for all compositions. Fig.2(a) shows the XRD patterns of Alloy 1 milled for different durations. During mechanical alloying of Fe-Al binary elemental powders, the intensity of Al diffraction peaks decreases, and all Al peaks disappear in the pattern of the alloy milled for 5 h. While a BCC structure Fe(Al) solid solution appears from the BCC characteristic peaks of pure Fe. And this solid solution completes at milling time of 30 h, leaving a single-phase Fe(Al). No significant variation can be observed after further milling for any of the diffraction peaks. XRD patterns of Alloy 2 and Alloy 3 powders milled for different times are shown in Fig.2(b) and (c), respectively. From Fig.2(b), it can be seen that B and Al diffraction peaks disappear at milling of 2 and 5 h, respectively. And no Ti peaks are observed after milling of 10 h. As for Fe, peak broadening as well as peak shifting to low angle increase, which indicates that Al, Ti and B dissolve into Fe. Prolonging the milling time to 50 h, the homogeneous Fe(Al, Ti, B) solid solution phase is formed. There is no obvious change when milling time is further prolonged. As shown in Fig.2(c), it can be seen that the structure evolution of Alloy 3 powders is almost the same as that of Alloy 2, i.e. B, Al and Ti elements gradually dissolve in Fe. The only difference is that the alloying sustains for longer time with higher Ti, B contents. Alloy 3 reaches “milling equilibrium” after 70 h of milling, and the milling product is also Fe(Al, Ti, B) solid solution. From the XRD patterns of Alloy 2 and Alloy 3, TiB2 compound is not formed. And the amorphization reaction has not been observed. It can be concluded that the MA products are metastable BCC Fe-based solid solution. In general, Fe-based solid solution with BCC structure is formed with increasing milling time for all compositions, but the alloying time needed is different. The different complete time of the MA process may be a consequence of the different contents of Ti and B, indicating that the contents of Ti and B affect the evolution of structures during mechanical alloying. They may hinder the diffusion of Fe and Al ions further. Thus, it needs long time for the formation of homogeneous Fe-based solid solution. It is concluded that the alloying rate is lowed and the alloying time is prolonged by the increment of Ti, B contents during mechanical alloying.
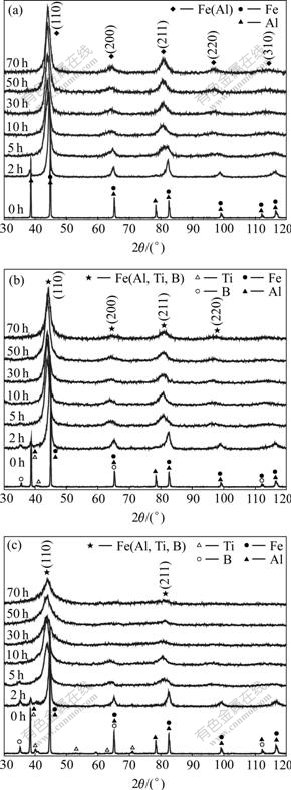
Fig.2 XRD patterns showing structural evolution of powders: (a) Alloy 1; (b) Alloy 2; (c) Alloy 3
For comparison, XRD patterns of different composition powders milled for 70 h are studied in detail, as shown in Fig.3. The peaks of Alloy 2 are broader and less intense than those obtained for Alloy 1. The peaks of Alloy 3 are the broadest with the lowest intensities in the three alloys. An increase in peak broadening as a function of composition is found, i.e. the XRD peaks become much broader as the contents of Ti, B are increased. Furthermore, the diffraction peaks shift to low angle side with the increase in Ti and B contents. According to TANG et al[12], the reaction of Ti33B67 elemental powder during high-energy ball milling can be described as follows: Ti+B→Ti(B) nanocrystalline→Ti(B) amorphous→TiB2 nanocrystalline. That means that the formation of amorphous Ti(B) first takes place followed by transformation from amorphous Ti(B) to TiB2 during mechanical alloying. But in this case, no amorphous phase was observed during mechanical alloying of these powders. Compared with previous studies reported[10], much broader peaks in XRD patterns are assumed to be related to the increased molar fraction of in-situ reinforcement forming elements Ti, B on the same milling condition. The addition of Ti, B enhances amorphization of the MA materials, and the amorphization accelerates as Ti, B contents increase.

Fig.3 XRD patterns of samples milled for 70 h
In Fig.4, the variation in lattice parameters and grain sizes for BCC Fe-based solid solution phase can be observed, as a function of Ti, B content. Fig.4(a) shows the lattice parameter values of Fe-based solid solution phases as a function of milling time. As it can be seen, for all compositions there is an expansion of the parameter. At the early stage of MA process, the value of the lattice parameter increases slightly, then abruptly increases to a steady value and remains nearly constant upon further processing. Furthermore, with increasing Ti and B contents, the value of the lattice parameter increase. The values of lattice parameters are systematically the largest in Alloy 3 of three alloys.

Fig.4 Lattice parameters (a) and grain sizes (b) of Fe-based solid solution of milled powders vs milling time
In all three alloys a systematic variation of the grain sizes of Fe-based solid solution (Fig.4(b)) as a function of milling time is also observed. The grain size for Fe(Al) in Alloy 1 decreases rapidly from the initial size to about 6 nm at first (<10 h), then the grain reduction rate is reduced. Although the change of the grain size of Fe(Al, Ti, B) in Alloy 2 with milling time is similar to that of the size of Fe(Al) in Alloy 1, the decrease of Fe(Al, Ti, B) grain size is more rapid, i.e. the grain size of Fe-based solid solution in Alloy 2 is less than that of Alloy 1. With increasing the content of Ti, B to 30% (molar fraction), the grain size of Fe(Al, Ti, B) reaches the least. After 80 h milling, the grain sizes of Fe-based solid solution are 5.7, 4.5 and 3.6 nm respectively for the Alloy 1, Alloy 2 and Alloy 3. The milled grain size decreases with the increase of the molar fraction of reinforcement forming elements. Note that as reported in earlier studies [13-14], the size decrease can be associated with a segregation of B atoms at grain boundaries. The brittleness of B enables fracture to occur during mechanical alloying. And Ti is also beneficial to the grain refinement of powders. This indicates that the more content of Ti, B results in the more refinement of grains of powders.
3.3 TEM observation
Fig.5 shows the morphologies and SAED patterns of the final stage milling products. Alloy 1 that contains no Ti, B milled for 30 h has a uniform distribution of spherical powders with size of about 3-4 μm (Fig.5(a)). The diffraction pattern shows six strong diffraction rings (Fig.5(b)), which indicates a BCC phase corresponding to Fe(Al). For Alloy 2 milled for 50 h, the average particle size of the alloyed powder is about 2 μm, as shown in Fig.5(c). In Fig.5(d), three diffraction rings refer to (110), (200) and (211) planes of the BCC Fe(Al, Ti, B). For Alloy 3 milled for 70 h, the particles are uniform in size less than 1 μm mostly (Fig.5(e)). A BCC phase corresponding to Fe(Al, Ti, B) appears, suggested by two weak rings belonging to Fe(Al, Ti, B) (110) and (211) respectively (Fig.5(f)).

Fig.5 TEM images of alloyed samples: (a) Alloy 1, milled for 30 h; (b) SAED pattern for Fig.5(a); (c) Alloy 2, milled for 50 h; (d) SAED pattern for Fig.5(c); (e) Alloy 3, milled for 70 h; (f) SAED pattern for Fig.5(e)
By comparing the intensity of diffraction rings of all samples, it can be noted that Alloy 3 is the weakest, and Alloy 2 is much weaker than Alloy 1. Though deformation induced defects introduced by milling seem to contribute significantly to the disorder[15], addition of Ti and B also results in some disordering behavior of mechanically alloyed Fe-Al-Ti-B. This means that the degree of disorder of milling products increases with the increment of Ti, B contents. Then it can be concluded that the amorphization fasters with the increase of Ti and B contents. TEM result agrees with the previous XRD observations.
4 Conclusions
1) Nanocrystalline powder composed of BCC structure Fe(Al) or Fe(Al, Ti, B) supersaturated solid solution is synthesized by ball milling Fe-Al-Ti-B elemental powders, respectively. The diffusion of B, Al, Ti atoms into Fe lattice occurs during milling.
2) The alloying rate is lowered and the alloying time is prolonged with the increment of Ti, B contents during mechanical alloying of the powders. The addition of Ti, B enhances amorphization of the powders, and the amorphization fasters as Ti, B contents increase.
3) In all three alloys, a systematic variation of the parameters of Fe-based solid solutions as a function of milling time is observed. At the early stage of the MA process, the value of the lattice parameter increases slightly, then abruptly increases to a steady value and remains nearly constant upon further processing. With increasing of the molar fraction of Ti and B, the value of the lattice parameter increases.
4) The grain sizes of Fe-based solid solutions as a function of milling time show a systematic variation. The grain size decreases rapidly at first (<10 h), then the grain reduction rate is reduced. The milled grain size decreases with the increment of Ti, B contents. The grain size of the alloyed powders reaches 4-6 nm after 80 h of milling.
References
[1] OEHRING M, BORMANN R. Nanocrystalline alloys prepared by mechanical alloying and ball milling [J]. Mater Sci Eng A, 1991, 134: 1330-1333.
[2] HUANG B, ISHIHARA K N, SHINGU P H. Metastable phases of Al-Fe system by mechanical alloying [J]. Mater Sci Eng A, 1997, 231: 72-79.
[3] HUANG B, PEREZ R J, LAVERNIA E J. Grain growth of nanocrystalline Fe-Al alloys produced by cryomilling in liquid argon and nitrogen [J]. Mater Sci Eng A, 1998, 255: 124-132.
[4] CHARLOT F, GAFFET E, ZEGHMATI B, BERNARD F, NIEPCE J C. Mechanically activated synthesis studied by X-ray diffraction in the Fe-Al system [J]. Mater Sci Eng A, 1999, 262: 279-288.
[5] ZHU S M, IWASAKI K. Microstructure and mechanical properties of mechanically alloyed and HIP-consolidated Fe3Al [J]. Mater Trans, 1999, 40(12): 1461-1466.
[6] KOCH C C. Intermetallic matrix composites prepared by mechanical alloying—A review [J]. Mater Sci Eng A, 1998, 214: 39-48.
[7] KRASNOWSKI M, WITEK A, KULIK T. The FeAl-30%TiC nanocomposite produced by mechanical alloying and hot-pressing consolidation [J]. Intermetallics, 2002, 10: 371-376.
[8] OLESZAK D, MATYJA H. Formation of nanocrystalline FeAl-NbC and FeAl-VC composites by mechanical alloying [J]. Mater Sci Forum, 2000, 343/346: 320-325.
[9] KRASNOWSKI M, KULIK T. FeAl-TiN nanocomposite produced by reactive ball milling and hot-pressing consolidation [J]. Scripta Materialia, 2003, 48: 1489-1494.
[10] PARK B G, KO S H, PARK Y H. In situ formation of TiC and TiB2 in the Fe3Al matrix by MA-PDS process [J]. J Japan Soc Powder Powder Metall, 2000, 47(10): 1080-1084.
[11] PARK B G, KO S H, PARK Y H, LEE J H. Mechanical properties of in situ Fe3Al matrix composites fabricated by MA-PDS process [J]. Intermetallics, 2006, 14, 660-665.
[12] TANG W M, ZHENG Z X, WU Y C, LV J, LIU J W, WANG J M. Synthesis of TiB2 nanocrystalline powder by mechanical alloying [J]. Trans Nonferrous Met Soc China, 2006, 16: 613-617.
[13] RICO M M, GRENECHE J M, PEREZ ALCAZAR G A. Effect of boron on structural and magnetic properties of the Fe60Al40 system prepared by mechanical alloying [J]. J Alloy Compd, 2005, 398: 26-32.
[14] LU L, LAI M O, WANG H Y. Synthesis of titanium diborde TiB2 and Ti-Al-B metal matrix composites [J]. J Mater Sci, 2000, 35: 241-248.
[15] MURTY B S, RANGANATHAN S. Novel materials synthesis by mechanical alloying/milling [J]. Int Mater Rev, 1998, 43: 101-141.
_____________________
Foundation item: Project(050440704) supported by the Natural Science Foundation of Anhui Province, China; Project(103-037016) supported by the Technological Innovation Foundation of Hefei University of Technology, China
Corresponding author: WU Yu-cheng; Tel/Fax: +86-551-2905085; E-mail: ycwu@hfut.edu.cn


