
First principles calculation of stable structure and
adhesive strength of plated Ni/Fe(100) or Cu/Fe(100) interfaces
Ryota NAKANISHI1, Koji SUEOKA1, Seiji SHIBA1, Makoto HINO2, Koji MURAKAMI2, Ken MURAOKA2
1. Department of System Engineering, Okayama Prefectural University, Soja, 719-1197, Japan;
2. Metallic Materials Group, Department of Mechanical Engineering,
Industrial Technology Center of Okayama Prefecture, Okayama, 701-1296, Japan
Received 18 June 2008; accepted 10 March 2009
Abstract:
A study the with first principles calculation of the interfaces of the Ni layer or Cu layer on the Fe(100) surface formed with metal plating was performed. Ni or Cu atoms were shown to adopt the corresponding position to the bcc structure of the Fe(100) substrate. Other calculations showed that the interfaces of Ni (5 atomic layers)/Fe(100) (5 layers) or Cu (5 atomic layers)/Fe(100) (5 layers) had square lattices. The orientation relationship of Ni/Fe(100) interface corresponds to fcc-Ni(100)//bcc-Fe(100), Ni[011]//Fe[010], and![]() Similar results were obtained for Cu/Fe(100) interfaces. This structure was supported by TEM analysis of plated Ni layer on Fe(100) surfaces. The adhesion strength of the Ni/Fe(100) interface evaluated by first principles calculation was higher than that of the Cu/Fe(100) interface. The experimental results of Hull cell iron plated with Ni or Cu supported the results of the calculation. These results indicate that the first principles calculation, which deals with the ideal interface at the atomic scale, has the potential to evaluate the adhesion strength of metallic material interfaces.
Similar results were obtained for Cu/Fe(100) interfaces. This structure was supported by TEM analysis of plated Ni layer on Fe(100) surfaces. The adhesion strength of the Ni/Fe(100) interface evaluated by first principles calculation was higher than that of the Cu/Fe(100) interface. The experimental results of Hull cell iron plated with Ni or Cu supported the results of the calculation. These results indicate that the first principles calculation, which deals with the ideal interface at the atomic scale, has the potential to evaluate the adhesion strength of metallic material interfaces.
Key words:
first principles calculation; metal interface; adhesion strength; TEM;
1 Introduction
The adhesion strength between the plated film and the substrate is important in many technological fields [1]. However, it is difficult to evaluate the adhesion strength quantitatively in experiments[2]. Although the first principles calculation deals with the ideal interface at the atomic scale, it has the potential to predict the adhesion strength without any fitting parameters. With this background, we studied the adhesion strength of metallic material interfaces, Ni layer or Cu layer with the first principles calculation on Fe(100) surface formed with metal plating.
2 Calculation procedure
The first principles calculation used was based on density functional theory[3]. In this method, the ground state of the system was found by solving the Kohn-Sham equation[4], which is a rule equation of the electronic system for given atom placement. The wave function was expanded as plane-waves, and ultrasoft pseudopotential[5] was used to reduce the plane-wave number. The generalized gradient approximation(GGA) was adopted in the evaluation of the exchange- correlation term, and the function form suggested by PERDEW et al[6] and ZUNGER was used. The density mixing method was used for energy minimization of the electronic system, and the BFGS optimizing structure method[7] was used for optimization of atom placement. The three-dimensionally periodic boundary condition was imposed. CASTEP code was used in the present study. The details of the calculation procedure were described previously[8].
The following procedure was considered in prediction of the adhesion of Ni/Fe(100) and Cu/Fe(100) interfaces: 1) The most stable structures of the interfaces were determined after calculation of the stable position of Ni or Cu atoms on the Fe(100) surface; 2) the total energy curve as a function of the interface distance was obtained; 3) the reduction of total energy to form the stable interface, and the tensile stress to separate the interfaces were calculated using the energy curve; and 4) these two values were used to compare the adhesion strengths of Ni/Fe(100) and Cu/Fe(100) interfaces.
3 Results and discussion
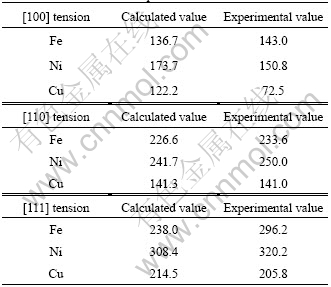
To confirm the accuracy of our calculations, the total energies and the lattice constants of Fe, Ni, or Cu single crystals were first calculated by taking the spin- polarization into consideration[9]. The comparison of crystal structures and lattice parameters between the calculations and the experiments is summarized in Table 1. The calculated crystal structures of these metals agreed with those determined by the experiments[9-10]. The comparison of elastic moduli of Fe, Ni and Cu between the calculations and the experiments is summarized in Table 2. The calculated [100], [110], and [111] elastic moduli of these metals also agreed with experimental values[9, 11-12] with differences of at most 20%, except for the [100] elastic modulus of Cu.
Table 1 Comparison of crystal structures and lattice parameters between calculations and experiments
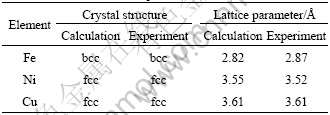
Table 2 Comparison of elastic moduli (GPa) of Fe, Ni and Cu between calculations and experiments
The (100) surface of the Fe substrate was prepared with a vacuum slab of 10 ? in thickness on the (100) plane of the Fe conventional cell. The Fe atoms in the second and third layers from the surface were fixed to model the bulk Fe. This cell included one Fe atom in one atomic layer. The calculated stable position of three Ni atoms on the Fe(100) surface is shown in Fig.1. Ni atoms as well as Cu atoms adopt the corresponding position to the bcc structure of the Fe(100) substrate. That is, the films of Ni and Cu begin to grow heteroepitaxially on the Fe(100) surface[9, 13]. We have reached the same conclusion using a cell with a nine-fold larger surface area[9].

Fig.1 Calculated stable position of three Ni atoms on Fe(100) surface
The stable structure of atomic models of Ni (5 atomic layers)/Fe(100) (5 layers) obtained by the calculation is shown in Fig.2. The interface distance and the interface side length of Ni/Fe(100) or Cu/Fe(100) are summarized in Table 3. The obtained stable structure of the interface had a square lattice[9], in which the orientation relationship corresponded to fcc-Ni(100)// Fe(100), Ni[011]//Fe[010], and ![]() [10]. Similar results were obtained for Cu (5 atomic layers)/ Fe(100) (5 layers). Ni and Cu deformed to fit the lattice constant of Fe.
[10]. Similar results were obtained for Cu (5 atomic layers)/ Fe(100) (5 layers). Ni and Cu deformed to fit the lattice constant of Fe.
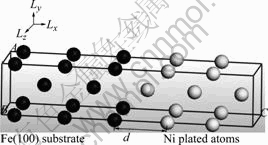
Fig.2 Stable structure of atomic models of Ni (5 atomic layers)/ Fe(100) (5 layers)
Table 3 Calculated interface distance d and interface side length Ly, Lz of Ni/Fe(100) and Cu/Fe(100)

The experimental results of TEM analysis for Ni/ Fe(100) interface formed with metal plating are shown in Fig.3. TEM analysis of the plated Ni layer on the Fe(100) surface[9] supported the calculated results described above.
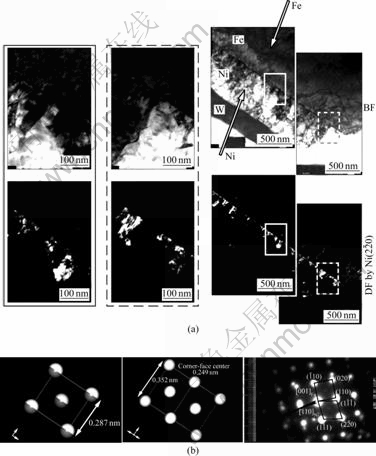
Fig.3 Experimental results of TEM analysis for Ni/Fe(100) interface: (a) TEM image; (b) diffraction pattern of selected area
The relationship between the total energy(E) and the interface distance(d) is shown in Fig.4. The energy curves were obtained by an increase in an interface distance. The energy refers to the sum of the energies of isolated Ni (5 atomic layers) and Fe (5 atomic layers) or isolated Cu (5 atomic layers) and Fe (5 atomic layers). The interaction between each surface disappeared in the case that the interface distance was larger than 5 ?. The positive values of the total energies with large interface distance are due to the lattice strain to fit the lattice constants. Here, we considered that the adhesion strength of Ni/Fe(100) or Cu/Fe(100) can be evaluated with 1) the reduction of the total energy Ed to form the stable interface, and 2) the tensile stress σto separate the interfaces. The value of σ was obtained from the maximum value of positive gradient of the energy curves. Ed and σ obtained from Fig.4 are summarized in Table 4. Both Ed and σ of the Ni/Fe(100) are higher than those of the Cu/Fe(100) interface. These results indicate that the Ni/Fe(100) interface is more stable than the Cu/Fe(100) interface. The photographs of Cu film and Ni film on the Fe substrate after bending test are shown in Fig.5. It was found that only the Cu film was separated from the Fe substrate after bending tests. This result indicates that the adhesion strength of Ni film is higher than that of Cu film on the Fe substrate. Therefore, these experimental results of JIS G 3141 Hull cell iron supported the calculated results described above.
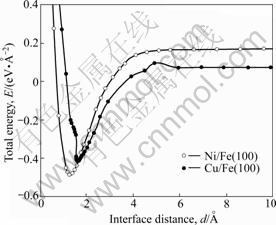
Fig.4 Relationship between total energy and interface distance
Table 4 Reduction of total energy Ed and tensile stress σ to separate interfaces

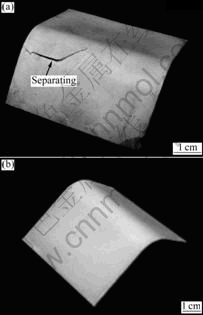
Fig.5 Photographs of Cu film (a) and Ni film (b) on Fe substrate after bending test
4 Conclusions
By the first principles calculation, we evaluated the adhesion strength of interfaces of the Ni layer or Cu layer on the Fe(100) surface formed with metal plating. The results indicated that Ni or Cu atoms adopt the corresponding position to the bcc structure of Fe(100) substrate. The orientation relationship of the interface of Ni/Fe(100) corresponded to the experimental results. The evaluated adhesion strength of the Ni/Fe(100) interface was higher than that of the Cu/Fe(100) interface. The experimental results of Hull cell iron plated with Ni or Cu supported the calculated results. These results indicate that the first principles calculation, which deals with the ideal interface at the atomic scale, has the potential to evaluate the adhesion strength of metallic material interfaces.
References
[1] WATANABE T. Nano plating [M]. Nikkan Kougyou Shinbunsya, 2004. (in Japanese)
[2] IWAMURA E. Improvement of adhesion strength in thin films [J]. Hyomen Gijutsu, 2007, 58: 260-266. (in Japanese)
[3] HOHENBERG P, KOHN W. Inhomogeneous electron gas [J]. Phys Rev, 1964, 136: B864-B871.
[4] KOHN W, SHAM L. Self-consistent equations including exchange and correlation effects [J]. Phys Rev, 1965, 140: A1133-A1138.
[5] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Phys Rev, 1990, B41: 7892-7895.
[6] PERDEW J, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Phys Rev Lett, 1996, 77: 3865-3868.
[7] FISCHER T, ALMLOF J. General methods for geometry and wave function optimization [J]. J Phys Chem, 1992, 96: 9768-9774.
[8] SUEOKA K, SHIBA S, FUKUTANI S. First principles calculation of the mechanism of oxygen precipitation in czochralski silicon crystals [J]. J Solid Mechanics and Materials Engineering, 2007, 1: 1165-1174.
[9] NAKANISHI R, SUEOKA K, SHIBA S, FUKUTANI S, HINO M, MURAKAMI K. First-principles calcultion on the stable structure and adhesive strength of Ni/Fe(100) or Cu/Fe(100) interfaces [J]. J Japan Inst Metals, 2007, 71: 1024-1031.
[10] KITTEL C. Introduction to solid state physics (Charpter I) [M]. Maruzen Kabushiki Gaisya, 1998. (in Japanese)
[11] The Japan Institute of Metals. Kinzoku data book [M]. Sendai, 2004: 31. (in Japanese)
[12] OMI T. Surface science technology series 3 [M]. Riaraizusya, 1996: 989. (in Japanese)
[13] WATANABE T. Structure control theory of plated film—Part of epitaxy [J]. J Japan Inst Metals, 2002, 66: 362-370.
Corresponding author: Ryota NAKANISHI; Tel: +81-866-94-2136; Fax: +81-866-94-2199; E-mail: nakanishi@argon.cse.oka-pu.ac.jp
DOI: 10.1016/S1003-6326(08)60392-1
[1] WATANABE T. Nano plating [M]. Nikkan Kougyou Shinbunsya, 2004. (in Japanese)
[3] HOHENBERG P, KOHN W. Inhomogeneous electron gas [J]. Phys Rev, 1964, 136: B864-B871.
[11] The Japan Institute of Metals. Kinzoku data book [M]. Sendai, 2004: 31. (in Japanese)
[12] OMI T. Surface science technology series 3 [M]. Riaraizusya, 1996: 989. (in Japanese)


