
Effects of heat treatment on morphological, optical and
electrical properties of ITO films by sol-gel technique
LI Zhi-hua(李芝华), KE Yu-peng(柯于鹏), REN Dong-yan(任冬燕)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 27 April2007; accepted 5 September 2007
Abstract:
Indium-tin-oxide(ITO) films were prepared on the quarts glass by sol-gel technique. Effects of different heat treatment temperatures and cooling methods on the morphological, optical and electrical properties of ITO films were measured by TG/DTA, IR, XRD, SEM, UV-VIS spectrometer and four-probe apparatus. It is found that the crystallized ITO films exhibit a polycrystalline cubic bixbyite structure. The heat treatment process has significant effects on the morphological, optical and electrical properties of ITO films. Elevating the heat treatment temperature can perfect the crystallization process of ITO films, therefore the optical and electrical properties of ITO films are improved. But the further increasing of heat treatment temperature results in the increment of ITO films’ resistivity. Compared with ITO films elaborated by furnace cooling, those prepared through air cooling have following characteristics as obviously decreased crystalline size, deeply declined porosity, more compact micro-morphology, improved electrical property and slightly decreased optical transmission.
Key words:
ITO film; sol-gel technique; heat treatment; electrical property; optical property;
1 Introduction
Indium-tin-oxide(ITO) films have been widely used for transparent conducting layers in various photoelectric components and devices such as liquid crystal displays (LCD)[1-2], solar cells, sensors and organic light emitting diodes(OLED)[3-4]. Many preparation methods of ITO films, such as chemical vapour deposition[5-6], activated reactive evaporation[7-8], magnetron sputtering[9-11] and recently developed sol-gel(sol-gel) technique[12-14], have been reported. Among the different techniques available, the sol-gel technique seems to be the most attractive for its advantages as feasible coating over large area, easy control of doping concentration and structural homogeneity, and without using expensive and complex equipments[15].
ITO films prepared by sol-gel technique present a relatively high resistance. The reasons for the low conductivity of sol-gel processed ITO films may result from their considerable porosity even after sintering at a high temperature. Another problem, which suppresses wide utilization of ITO films by sol-gel process, is the difficulty to obtain metal alkoxides as raw materials for their unavailability and high price.
In this work, the deposition of ITO films was described by sol-gel technique using low-cost metal salts and organic solvents, and the aim was to investigate heat treatment effects on morphology, structure, and optical and electrical properties of the ITO films directly deposited on quartz substrates.
2 Experimental
Anhydrous indium nitrate (In2(NO3)3) was dissolved in acetylacetone and the solution was maintained in reflux at 80 ℃ for 3 h. Anhydrous tin chloride (SnCl4) was dissolved in ethanol. Then the two solutions were mixed and stirred at room temperature. Finally a stable sol with 10%Sn (mole fraction) was obtained. Quartz substrates were dipped into the stirring sol and withdrawn at the rate of 4 cm/min. Coated substrates were heated at different temperatures for 20 min after each dipping. Thickness of the film was approximately 25 nm for each dipping. By repeating the procedures above, ITO films with different thicknesses were prepared. During heat treatment process, two cooling methods for densification and crystallization were used. One was furnace cooling denoted as Route 1, namely taking both heat treatment and cooling in the furnace, the other was air cooling denoted as Route 2, namely getting heat treatment in the furnace but cooling in air.
The infrared spectra of the ITO sol at the range of 4 000 cm-1 to 400 cm-1 were recorded by a 60XB type FTIR spectrometer. Thermogravimetric analysis(TGA) and differential thermal analysis(DTA) were performed on a PTC-1 type thermal analysis system under the following conditions: in atmospheric air and heating rate of 5 ℃/min. Phase composition of ITO films was determined by a X-ray diffraction instrument (SIMENS D500X) and the average crystal grain size was deduced from the peak width in X-ray diffraction patterns using Scherrer equation. Surface morphology as well as the crystal grain size was directly observed by a KYKY-Ammray2800 type scanning electronic microscopy(SEM). The transmittance of the films was measured by the UV-VIS spectrometer (TU-1800SPC). Electrical property was surveyed with a WS-1 type four-probe apparatus.
3 Results and discussion
3.1 Effect of heat treatment temperature on crystalli- zation
IR spectra of ITO xerogel powder treated at 100, 200 and 500 ℃ are shown in Fig.1, which characterize the transformation of ITO films in composition and structure under different heat treatment temperatures. As we can see in this figure, for ITO xerogel powder treated at 100 ℃ there are a flexural vibration peak of C—H bonds at 1 388.03 cm-1, a stretching vibration peak of C=O bonds at 2 975.18 cm-1, stretching vibration peaks of C—H bonds at 2 922.63 cm-1 and 2 975.18 cm-1 and a stretching vibration peak of O—H bonds at 3 474.45 cm-1. With the elevation of temperature the vibration bands of organic groups gradually decrease. When the temperature reaches 200 ℃, stretching vibration peak of saturated C—H bonds disappears due to the volatilization and decomposition of the organic materials. There is significant attenuation with stretching vibration peak of C=O bonds. After the samples are heat-treated at 500 ℃, crystal water and organic groups in the ITO xerogel powder are eliminated only with faint absorption peak of In—O bonds at 499.85 cm-1.
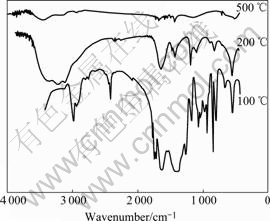
Fig.1 IR spectra of ITO xerogel heat-treated at different temperatures
Under heat treatment at a certain temperature, gel films that form after withdrawing and drying lose their organic components and intermolecular water gradually, and translate into crystalline state. Describing the transformation process of ITO films from gel to crystalline state, TG/DTA curves of ITO xerogel powder are presented in Fig.2. As we can see in TG curve, the mass of ITO xerogel powder exhibits continuous and significant decrease with the rising of heat treatment temperature before 530 ℃. Coupled with DTA curve, there is a distinct exothermic peak at 335.37 ℃, which is determined attributing to the heat release from charring and decomposition of organic groups and components. Another conspicuous exothermic peak also exists at 483.05 ℃ and corresponds to the oxidation of element carbon. An exothermic peak at 578.21 ℃ can also be found, but the mass of ITO xerogel powder does not change at this temperature, which is proposed resulting from the transformation of In2O3 to polycrystalline cubic bixbyite structure. The mass of the ITO xerogel almost keeps constant at the temperature higher than 530 ℃.
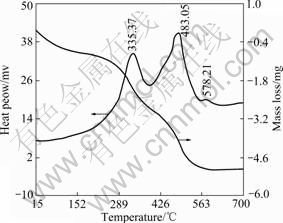
Fig.2 TG/DTA curves of ITO organic gel dried at 100 ℃
3.2 Effect of heat treatment temperature on phase composition
Fig.3 shows the XRD patterns of ITO films heat-treated at 200, 300, 400 and 550 ℃, respectively. As shown in this figure, when the heat treatment temperature is above 200 ℃, gel on the substrate begins to lose the intermolecular water and exhibit characteristic peaks of In2O3. With elevating of heat treatment temperature the characteristic peaks of In2O3 turn to be much sharper, which suggests obvious growing up of the crystalline grain. For the pattern corresponding to ITO gel heat-treated at 300 ℃, typical cubic bixbyite structure of ITO films appears. The crystal size of ITO films heat-treated at 550 ℃ is calculated to be about 20 nm according to Scherrer equation.
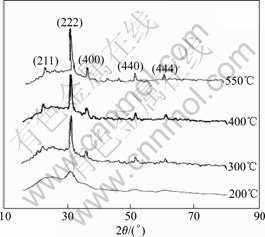
Fig.3 XRD patterns of ITO films heat-treated at different temperatures
Fig.4 presents the XRD pattern of ITO xerogel powder heat-treated at 600 ℃ for 1 h. After the samples are heat-treated at 600 ℃, the XRD pattern embodies sharp and characteristic peaks, indicating that the crystal grains of ITO xerogel become considerably larger and its crystallization process almost finishes. When comparing the XRD data with the JDPDs database, it is found that xerogel heat-treated at 600 ℃ has polycrystalline cubic bixbyite structure and the (222) plane shows the strongest characteristic peak. There are no characteristic peaks of other phase such as SnO2 or SnO in the pattern, which can be inferred that the dopant SnO2 has dissolved into the lattice of In2O3 and solid solution with uniform structure is formed.
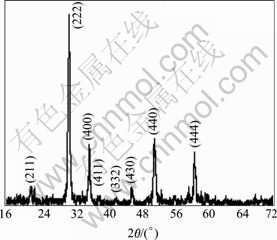
Fig.4 XRD pattern of ITO xerogel heat-treated at 600 ℃
3.3 Effect of cooling method on micro-morphology
Fig.5 shows the SEM surface micrographs of ITO films that were maintained at 600 ℃ for 1 h and cooled through different cooling methods. As shown in Fig.5, ITO films by sol-gel technique have porous structure accumulated by spherical grains and pores uniformly distribute among crystal grains. The grain size is about 20 nm and in well consistence with that calculated from the XRD peak width. When the ITO films are prepared through air cooling, decrease in both the grain size and the porosity can be easily seen, which leads to the densification of microstructure, hence decrease in the resistivity of ITO films.
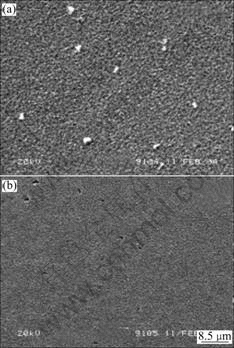
Fig.5 SEM surface photographs of ITO films prepared at 600 ℃ by different cooling methods: (a) Furnace cooling; (b) Air cooling
As also shown in Fig.5, when the furnace cooling is applied to heat-treated ITO films, the main defects are the large In2O3 particles separating out besides the porous structure. It is deemed as the growth up of small amount of In2O3 particles during the long temperature holding process. When air-cooling is adopted to heat-treated ITO films, the main defects are the pores much lager than the average size. These pores are engendered for the aggregation of vacancies at the time of rapid increase of pore concentration during air-cooling.
3.4 Optical properties
3.4.1 Effect of different heat treatment temperatures on optical properties
Optical properties of the ITO films after heat treatment at different temperatures were investigated by UV-VIS spectrometer. High transparency for the ITO films in the visible light range is required for transparent electrode. Fig.6 shows the optical transmission for 10% (mole fraction) Sn-doped ITO films with a thickness of 100 nm. According to Fig.6, the average optical transmission in the visible light region improves with increasing heat treatment temperature, exhibiting 80% for films heat-treated at 200 ℃, and 85%, 90% for 400 ℃ and 600 ℃ ones, respectively.
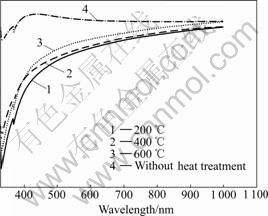
Fig.6 UV-VIS optical transmission of ITO films at different heat treatment temperatures
The improvement of optical properties caused by increasing heat treatment temperature is due to the enhanced formation and crystallization of the ITO films. Higher temperature heat treatment leads to a better crystallization and lower level of defects near grain boundary, thus resulting in the improvement of structural homogeneity and the decrease of light reflection.
3.4.2 Effect of different heat treatment routes on optical properties
Variance of optical transmission, brought from different cooling methods at a certain heat treatment temperature, is shown in Figs.7-9. Under the heat treatment at 200 ℃ (see Fig.7), the two transmission curves are almost the same, indicating that effect of different cooling methods on optical transmission is rather small at this temperature. When ITO films are heat-treated at 400 ℃, as shown in Fig.8, variance caused by cooling method is the most conspicuous. These ITO films by air-cooling have a 80% transparency at the wavelength range from 500 to 1000 nm, but the transparency of furnace cooled ITO films is higher than 90%. When being heat-treated at 600℃, ITO films by air- cooling have a little lower optical transmission than those by furnace cooling.
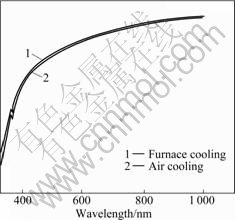
Fig.7 UV-VIS optical transmission of ITO films heat-treated at 200 ℃ with different cooling methods
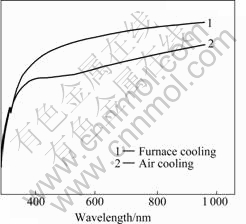
Fig.8 UV-VIS optical transmission of ITO films heat-treated at 400 ℃ with different cooling methods
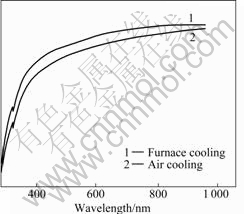
Fig.9 UV-VIS optical transmission of ITO films heat-treated at 600 ℃ with different cooling methods
Cooling rates influence the optical transmission of the films mainly through crystal size and surface rough- ness. When the heat treatment temperature is low, e.g. 200 ℃, the films’ transformation from gel to crystalline barely commences, therefore cooling methods have rather little effect on the crystal size and shape. But it is another case for heat treatment temperature at 400 ℃, the phase transformation has completed and the crystal size and shape are determined by the heat treatment temperature and the time maintained. Compared with air cooling, the temperature holding time of furnace cooling is longer, and lager crystal grain size and homogeneous grain shape can be obtained, so the ITO films exhibit better optical properties. When the heat treatment temperature is 600 ℃, the phase transformation has also completed and so has the growth up of the crystal. Although furnace cooling can provide longer temperature holding time, its effect on the crystal size and shape is not as obvious as that in Fig.8.
Coupled with the SEM surface photograph in Fig.5, air-cooling leads to the surface densification and inhomogeneity of the crystallites, which is the main reason for the decrease in optical transmission of ITO films through air-cooling. In addition, there are causes for the weakened optical properties such as microcracks on the film surface and defects in the films and the interface between the film and the substrate. The microcracks are due to thermal stress that is resulted from the rapid cooling rate during the air-cooling, and the defects are attributed to the different coefficients of thermal expansion between the substrate and the film. Both of them can add up to the dispersion factor, so the transmission of the ITO films decreases.
3.5 Electrical properties
3.5.1 Effects of heat treatment temperature on electrical properties
ITO films for electrical property testing all took the same heat treatment process as follows. The temperature was gradually elevated at first, then ITO films were maintained at their corresponding temperature for 1 h, and at last were cooled in atmospheric air. Fig.10 presents the influence of different heat treatment temperatures on the sheet resistance of ITO films with 10%(mole fraction) Sn doping and one time coating. As shown in this figure, the sheet resistance previously decreases then grows with a minimum at 600 ℃. After heat treatment at 600 ℃, the sheet resistance of 1.7 kΩ is obtained for 10%(mole fraction) Sn-doped ITO films with one time coating. Especially with 5 times coating, the sheet resistance of 110 Ω is obtained for 10%(mole fraction) Sn-doped ITO films with a thickness of 100 nm.
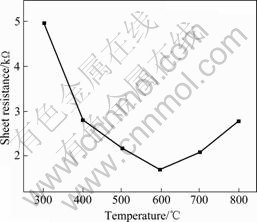
Fig.10 Sheet resistance of ITO films heat-treated at different temperatures
Since the ITO films are composed of small crystal grains, there are inevitably many grain boundaries that may behave as barriers against the electron moving and result in the high resistance of the films. With the rising of heat treatment temperature, the grains grow up. This contributes to the improvement of crystallization as well as the films’ compactification and the conductivity. But when the temperature is higher than 600 ℃, the crystallites of the films become stable and the hole mobility may decrease, which leads to the increase of film resistance. So the minimum of film sheet resistance can be obtained at 600 ℃. Further elevation of heat treatment temperature induces the increase of the sheet resistance for the reasons of both decrease of vacancy mobility and decomposition of In2O3 at higher temperature.
3.5.2 Effect of cooling method on electrical properties
The effect of cooling rate on electrical properties of ITO films is shown in Fig.11. ITO films by air-cooling present systematically higher conductivity than those obtained by furnace cooling. This improvement in conductivity for air-cooled ITO films is due to a better percolation between grains and the surface densification, which reduces the porosity of the films.
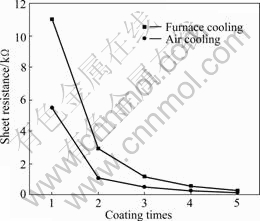
Fig.11 Sheet resistance of ITO films with different cooling methods
The main reason for this is the effect of different cooling rates on the distribution of oxygen vacancy in the films. Under higher heat treatment temperature the oxygen vacancies are formed due to the escape of oxygen atoms from their locations in the lattice. When air-cooling is used, the oxygen vacancy, which keeps its balance as the function depending on the temperature and the partial pressure of oxygen, has been frozen and holds the concentration as that at the state of higher temperature. So the concentration of oxygen vacancy in the films increases. Oxygen vacancy can trap two electrons that are readily to receive energy and be excited up to conduction band, therefore the charge carrier concentration increases and the electrical properties of the ITO films are improved.
4 Conclusions
1) ITO films have been prepared by sol-gel process using low-cost indium nitrate and tin chloride compound. During the heat treatment process, there are conspicuous transformation happened in the composition of the ITO films, namely transformation from amorphous gel to polycrystalline films with cubic bixbyite structure. The dopant SnO2 is dissolved into the lattice of In2O3 and solid solution with uniform structure is formed. ITO films by sol-gel technique have porous structure accumulated by spherical grains and pores evenly distribute among grains.
2) Heat treatment process has significant influence on the morphological, optical and electrical properties of ITO films. Elevating the heat treatment temperature can perfect the crystallization process of ITO films, therefore the optical and electrical properties of ITO films are improved. But further increase of the heat treatment temperature results in the increment of ITO films’ resistivity. Compared with ITO films elaborated by furnace cooling, those prepared through air cooling have following characteristics as obviously decreased crystalline size, deeply declined porosity, more compact micro-morphology, improved electrical property and decreased optical transmission.
References
[1] SOLIEMAN A, AEGERTER M A. Modeling of optical and electrical properties of In2O3:Sn coatings made by various techniques[J]. Thin Solid Films, 2006, 502(1/2): 205-211.
[2] SON K S, CHOI D L, LEE H N, LEE W G. The interfacial reaction between ITO and silicon nitride deposited by PECVD in fringe field switching device [J]. Current Applied Physics, 2002, 2(3): 229-232.
[3] ISHIBASHI K, WATABE K, SAKURAI T, OKADA O, HOSOKAWA N. Large area deposition of ITO films by cluster type sputtering system [J]. Journal of Non-Crystalline Solids, 1997, 218: 354-359.
[4] SU C, SHEU T K, CHANG Y T, WAN M A, FENG M C, HUNG W C. Preparation of ITO thin films by sol-gel process and their characterizations[J]. Synthetic Metals, 2005, 153(1/3): 9-12.
[5] BISWAS P K, DE A, PRAMANIK N C, CHAKRABORTY P K, ORTNER K, HOCK V, KORDER S. Effects of tin on IR reflectivity, thermal emissivity, Hall mobility and plasma wavelength of sol-gel indium tin oxide films on glass [J]. Materials Letters, 2003, 57(15): 2326-2332.
[6] STOICA T F, TEODORESCU V S, BLANCHIN M G, STOICA T A, GARTNER M, LOSURDO M, ZAHARESCU M. Morphology, structure and optical properties of sol-gel ITO thin films[J]. Materials Science and Engineering B, 2003, 101(1/3): 222-226.
[7] OTA R, SEKI S, SAWADA Y, OGAWA M, NISHIDE T, SHIDA A, IDE M. Indium-tin-oxide films prepared by dip coating using an ethanol solution of indium chloride and tin chloride [J]. Surface and Coatings Technology, 2003, 169/170: 521-524.
[8] SEKI S, SAWADA Y, OGAWA M, YAMAMOTO M, KAGOTA Y, SHIDA A, IDE M. Highly conducting indium-tin-oxide transparent films prepared by dip-coating with an indium carboxylate salt[J]. Surface and Coatings Technology, 2003, 169/170: 525-527.
[9] ZHANG J, AU K H, ZHU Z Q, O’SHEA S. Sol-gel preparation of poly (ethylene glycol) doped indium tin oxide thin films for sensing applications[J]. Optical Materials, 2004, 26(1): 47-55.
[10] BISWAS P K, DE A, ORTNER K, KORDER S. Study of sol-gel-derived high tin content indium tin oxide (ITO) films on silica-coated soda lime silica glass[J]. Materials Letters, 2004, 58(10): 1540-1545.
[11] DAOUDI K, SANDU C S, MOADHEN A, GHICA C, CANUT B, TEODORESCU V S, BLANCHIN M G, ROGER J A, M. OUESLATI, B. BESSA?S. ITO spin-coated porous silicon structures [J]. Materials Science and Engineering B, 2003, 101(1/3): 262-265.
[12] AL-DAHOUDI N, AEGERTER M A. Comparative study of transparent conductive In2O3:Sn (ITO) coatings made using a sol and a nanoparticle suspension[J]. Thin Solid Films, 2006, 502(1/2): 193-197.
[13] ALAM M J, CAMERON D C. Characterization of transparent conductive ITO thin films deposited on titanium dioxide film by a sol-gel process[J]. Surface and Coatings Technology, 2001, 142/144: 776-780.
[14] DAOUDI K, CANUT B, BLANCHIN M G, SANDU C S, TEODORESCU V S, ROGER J A. Tin-doped indium oxide thin films deposited by sol-gel dip-coating technique [J]. Materials Science and Engineering, 2002, 21: 313-317.
[15] LI Zhi-hua, REN Dong-yan. Preparation of ITO transparent conductive film by sol-gel method [J]. Trans Nonferrous Met Soc China, 2006, 16(6): 1358-1361.
Foundation item: Project(50271084) supported by the National Natural Science Foundation of China
Corresponding author: LI Zhi-hua; Tel: +86-731-8830838; E-mail: ligfz@mail.csu.edu.cn


