网络首发时间: 2015-05-04 11:06
稀有金属2016年第5期
湿法分离锆铪的研究进展
李攀红 徐志高 池汝安 赵骏 王力军 徐源来
摘 要:
锆铪分离是制备核级锆和铪的技术关键。简单综述了工业上生产核级锆铪的主要溶剂萃取分离工艺,即甲基异丁基酮(MIBK)法、磷酸三丁酯(TBP)法、三辛胺或三烷基叔胺(TOA或N235)法,并对各种工艺流程的优劣进行了技术分析,重点介绍了中性萃取体系、酸性萃取体系、螯合萃取体系、碱性萃取体系和协同萃取体系等溶剂萃取分离锆铪技术的新进展。另外,还阐述了其他锆铪湿法分离的方法如分步结晶和沉淀法、吸附分离法、膜分离法、微溶剂萃取法、双水相萃取法和液膜萃取法对锆铪进行富集和分离的现状。可以发现,新开发的锆铪湿法分离技术大都是优先分离锆铪原料中含量较多的锆,且大多仅进行了分离工艺的研究,而对锆铪分离的机制、热力学和动力学的相关研究较少。为了提高分离效率和保护环境,开发优先萃取铪且环境友好型的绿色分离体系显得尤为必要。
关键词:
锆;铪;湿法分离;
中图分类号: O652.6
作者简介:李攀红(1987-),男,湖北汉川人,硕士研究生;;研究方向:有色湿法冶金;E-mail:lph412841@sina.com;;徐志高,副教授;电话:027-87194980;E-mail:xuzhigaotc@126.com;
收稿日期:2014-12-09
基金:国家自然科学基金项目(51174146,51374158);“十二五”国家科技部科技支撑计划项目(2012BAB10B10);教育部新世纪优秀人才支持计划项目(NCET-13-0941);湖北省教育厅优秀中青年科技创新团队项目(T201506);武汉市青年科技晨光计划项目(2014070404010217)资助;
Research Progress in Hydrometallurgical Separation of Zirconium and Hafnium
Li Panhong Xu Zhigao Chi Ruan Zhao Jun Wang Lijun Xu Yuanlai
Key Laboratory for Green Chemical Process of Ministry of Education,Wuhan Institute of Technology
Division of Mineral Resources,Metallurgy and Materials,General Research Institute for Nonferrous Metals
Abstract:
The separation between zirconium and hafnium is the key technology to prepare nuclear-grade zirconium and hafnium. Several solvent extraction separation processes to produce nuclear-grade zirconium and hafnium in industry,such as methyl isobutyl ketone( MIBK),tributyl phosphate( TBP) and trio ctylamine or trialkyl tertiary amine( TOA or N235) methods,were briefly reviewed,and their advantages and disadvantages were discussed in the view of technology. Besides,several latest research progress in zirconium and hafnium separation by solvent extraction methods,such as neutral extraction system,acidic extraction system,chelating extraction system,alkaline extraction system,and synergistic extraction system,were emphatically introduced,respectively. In addition,some of other hydrometallurgical separation techniques,including fractional crystallization and precipitation method,adsorption separation method,membrane separation method,micro solvent extraction method,aqueous biphasic system method and liquid membrane method were also presented. It could be found that newly developed hydrometallurgical separation methods were mainly focused on the separation of zirconium prior to hafnium. Moreover,most of works were mainly concerned with separation process rather than mechanism,thermodynamic and kinetic. In order to improve the separation efficiency and protect environment as well,the development of a new extraction system separating hafnium as well as friendly to environment was particularly on the trend.
Keyword:
zirconium; hafnium; hydrometallurgical separation;
Received: 2014-12-09
随着能源危机的不断加剧和节能减排的巨大需求,人们对清洁能源的需求不断增加。核电作为一种技术成熟的清洁能源,受到了各国研究者的广泛关注,而锆和铪是构建核反应堆的关键材料,已被列入国家战略发展的重点研究对象[1]。锆和铪因具有截然不同的核性能,而被用作核反应堆的不同方面。然而,在自然界中锆和铪总是伴生的,没有单独存在的锆或铪,且锆中铪含量一般约为1%~3%。由于镧系收缩,锆和铪的原子半径和离子半径相近,且理化性质也比较相似,使其分离比较困难。因此,锆铪分离是制备核级锆铪的技术关键。为了有效分离锆和铪,国内外科技工作者先后提出了不同的方法,以湿法分离技术为主,包括溶剂萃取法、分步结晶法和吸附分离法等[2],其中,溶剂萃取法因具有生产量大,设备简单,便于自动化,成本低等优势而被广泛的应用于核级锆铪的生产之中[3]。自1947年Fisher首次利用二乙醚在硫氰酸介质中萃取分离锆和铪以来,溶剂萃取法得到了长远的发展和进步。目前,已相继开发出了几种比较成熟核级锆铪生产的溶剂萃取分离体系:MIBK-HSCN体系、TBP-HCl-HNO3体系和TOA/N235-H2SO4体系[4,5,6,7]。
1 主要的工业湿法分离工艺
经过多年的不断工艺优化和改进,目前,已形成了以上3种稳定的溶剂萃取分离工艺,其各自所采用工艺参数如表1所示。
1.1 MIBK-HSCN法
MIBK-HSCN法是20世纪70年代工业上应用最为广泛的锆铪分离生产工艺,全球近2/3的核级锆和铪均由此法生产。MIBK法主要利用Zr4+和Hf4+与SCN-离子络合能力的不同而优先萃取铪,而锆则留在水相,从而实现锆和铪的分离。但MI-BK法存在一些缺点:(1)MIBK在水中溶解度高(1.7%(质量分数)),溶剂损失大;(2)工业排放的废水中含有硫化物和氰化物等,对环境具有一定的危害;(3)MIBK具有一定的气味,使得操作环境较差。针对MIBK工艺存在的弊端,先后有不少的单位对其工艺进行了改进。江西晶安高科公司设计了MIBK+萃取剂L双溶剂萃取法制备原子级氧化锆和氧化铪的工艺,该工艺可适用于高浓度、低酸度的锆料液的萃取,降低萃取剂和硫氰酸铵的浓度,以及减缓HSCN的分解[4]。
1.2 TBP-HCl-HNO3法
TBP法是有别于MIBK法的另一种高效萃取体系,该法利用Zr4+和Hf4+与NO3-和Cl-络合物稳定性的差异而优先萃取锆。目前主要采用的是改进的TBP-HNO3-HCl体系,该法以四氯化锆为原料,加硝酸直接配置成锆(铪)的硝酸-盐酸溶液。经过改进后,锆对铪的分离系数可高达30~40,一次萃取后可以同时获得原子级的二氧化锆和二氧化铪,但该体系酸度较大,设备腐蚀强,且TBP存在严重的乳化等问题,直接影响了萃取操作的正常运行。林振汉[5]发现原料中硅含量对TBP的乳化有很大的影响,是造成TBP乳化的主要因素,在硅含量较低情况下不会发生乳化现象,而当硅含量大于0.2 g·L-1时,TBP容易乳化。中国核工业集团二七二铀业有限公司[6]根据多年湿法冶金的经验,开发出了TBP+HCl+X分离锆铪新工艺,锆的回收率可达99%以上。该工艺保留了混酸萃取体系的优点,同时克服了TBP-HNO3-HCl混酸体系易腐蚀设备和易乳化等缺点。
表1 几种传统工业化锆和铪溶剂萃取分离工艺的比较Table 1 Comparison of several traditional solvent extraction systems for Zr and Hf separation 下载原图
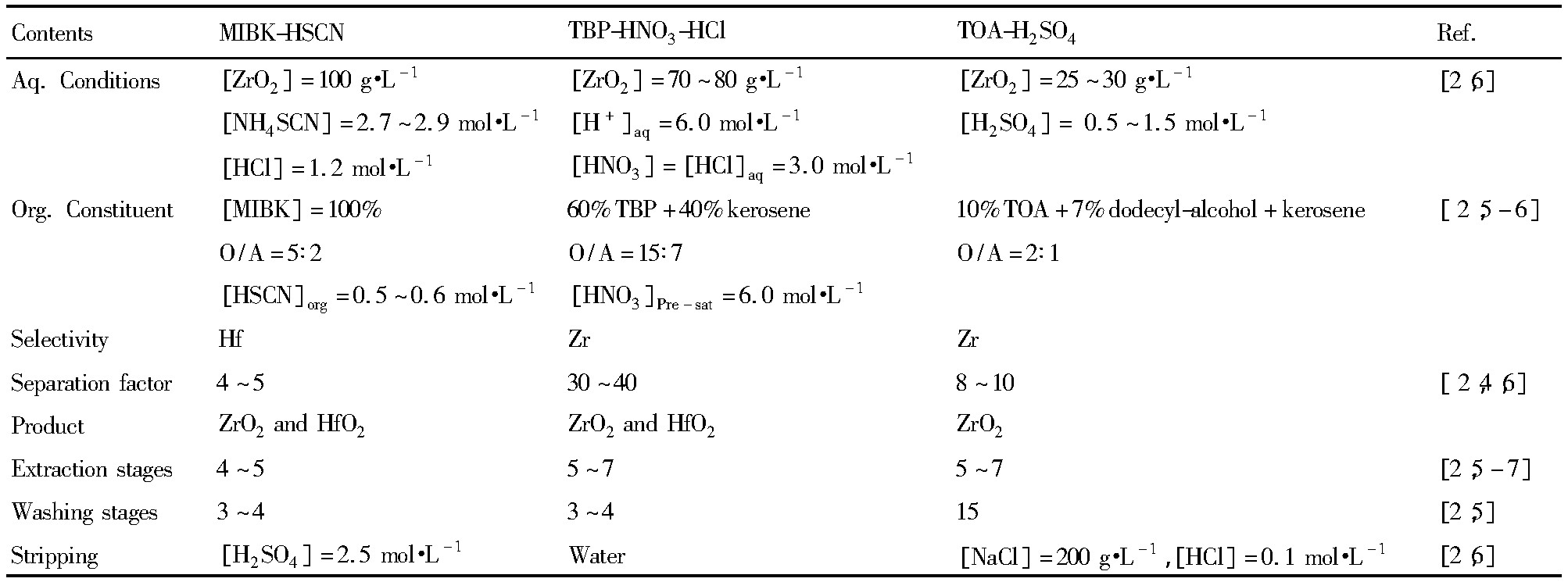
表1 几种传统工业化锆和铪溶剂萃取分离工艺的比较Table 1 Comparison of several traditional solvent extraction systems for Zr and Hf separation
1.3 TOA/N235-H2SO4法
TOA法是日本矿业公司于1971年提出的继MIBK法和TBP法之后的又一种锆铪分离生产工艺。该法以硫酸为介质,优先萃取锆,且锆铪的分离系数达8~10。TOA法虽然具有污染小、放射性物质集中易于处理、设备简单和投资费用低等优点,但该法也存在锆铪萃取饱和容量小,分离系数不高等缺点。鉴于TOA法的局限性,北京有色金属研究院以添加剂伯醇β-A1416代替仲辛醇及用低酸料液代替高酸料液对N235工艺进行了改进,小型连续型工业试验结果表明,改进的N235法具有产品纯度高、锆萃取率高、萃取分相好、设备产能大、操作简单、污染小等特点[7]。
尽管以上几种工艺能够实现锆和铪分离的要求,但因其自身存在一些弊端,这极大地限制其在工业上的应用。开发分离能力高的锆铪分离工艺是当前的主要研究目标和发展方向。
2 溶剂萃取体系研究进展
根据徐光宪理论,萃取体系可主要分为中性萃取体系,螯合和酸性萃取体系,胺类萃取体系和协同萃取体系等[8,9]。本文将从中性萃取体系、酸性萃取体系、螯合萃取体系、碱性萃取体系和协同萃取体系5个方面,分别阐述溶剂萃取法在锆铪分离的研究进展。
2.1 中性萃取体系
中性萃取剂按照其络合原子的不同可分为中性含磷萃取剂,如TOPO,TBP,Cyanex923,Cyanex925和三苯基氧化磷(TPPO)等;中性含氧萃取剂,如MIBK、环己酮、二异丁基酮、4-甲基-3-戊烯-2-酮、3,3-己烷基撑-双-(1-苯基-4-乙酰基-5-酮)、3-苯基-4-酰基-5-恶唑酮类、冠醚和夹醚等;中性含氮萃取剂,包括吡啶类和取代酰胺类等,如N,N,N'N'-tetraoctyldiglycolamide(TODGA)等;中性含硫萃取剂,如石油亚砜和二正辛基亚砜等。
徐志高等[10]以氧氯化锆为原料,研究了在不同酸度条件下MIBK萃取分离锆和铪的机制,并发现硫酸铵能够显著提高锆和铪的分离系数,在其最佳加入量为0.8 mol·L-1时,分离系数达到14。Nayl等[11]发现经Cyanex925萃取锆铪的饱和有机相,以0.5 mol·L-1硝酸为反萃剂,经两级反萃,锆和铪的反萃率分别达到97.5%和10.2%,分离系数为40.7。Saleh[12]研究了萃取剂TODGA从硝酸介质中萃取和分离锆铪的性能,结果表明,在硝酸浓度大于3.0 mol·L-1时,TODGA对锆和铪具有较好的萃取能力,但锆对铪的最大分离系数仅为2.8。
2.2 酸性萃取体系
酸性萃取剂是萃取剂中重点的研究对象,常见的酸性含膦萃取剂如P204、P507(PC-88A)、丁基磷酸二丁酯、Cyanex301、Cyanex302和Cyanex272等及羧酸类萃取剂,如Verstic10、环烷酸和Li X63等。
Saberyan等[13]研究了Cyanex301萃取分离锆铪的行为,发现锆和铪的分离系数随着初始p H的增加而增加,而随着温度的升高而降低。以氯化钠为盐析剂,p H为4.0时,锆和铪分离系数达到最大值7.0。Cyanex272在盐酸和硝酸介质中萃取锆的机制有所不同,在硝酸介质中,为溶剂化机制[14],而在盐酸中为阳离子交换萃取机制[15]。此外,还有关于Cyanex272反萃相关研究[16]。Lee等[17]研究了Versatic Acid 10在盐酸介质中萃取锆和铪的机制,得Versatic Acid 10与锆和铪的萃合物组成分别为Zr OR2·2HR和HfR4。Reddy等[18]研究了PC-88A在盐酸介质中萃取铪的行为,发现铪的分配系数随着酸度的增加而减小,各种钠盐对铪萃取的盐析能力按Na SCN<Na Cl<Na NO3依次减小。
2.3 螯合萃取体系
根据络合的原子不同,螯合萃取剂主要可分为含氧类和含氮类螯合萃取剂。过去主要关注含氧类螯合剂,如8-羟基喹啉、β-双酮,包括2-噻吩甲酰三氟丙酮(HTTA)和双安替比林基甲烷及其衍生物。近年来,研究重点转为含氮类螯合萃取剂,如2-羟基-5-壬基乙酰苯羟胺(LIX84-IC)和2-羟基-5-壬基苯甲醛肟(LIX 860N-IC)等。
Reddy等[19,20]发现LIX 84-IC在低酸度(<1.0 mol·L-1)时萃取锆和铪为阳离子反应机制,并发现各种钠盐对锆和铪盐析能力也有所差异。对于锆,有Na2SO4>Na SCN>Na NO3>Na Cl;而对于铪,则有Na SCN>Na NO3>Na Cl>Na2SO4。此外,还研究了LIX 860N-IC萃取锆和铪机制,结果表明为阳离子交换机制[21]。
2.4 碱性萃取体系
常见的碱性萃取剂主要为胺类萃取剂和季铵盐类萃取剂。胺类萃取剂如三辛胺TOA(N235)、三苯胺、TEHA、Alamine336、Primene-JMT、Tetradentate Naphthol-derivative Schiff Base[22]和N-正辛基苯胺等;季铵盐类萃取剂,如氯化甲基三烷基铵(N236)、三葵基甲基氯化铵(Aliquat336)和三辛基甲基氯化铵等。
Poriel等[23]发现Aliquat336在较低盐酸浓度(<5.0 mol·L-1)下几乎不萃取锆和铪,而在盐酸浓度高于5.0 mol·L-1时,对锆和铪的萃取能力逐步增加。在盐酸浓度为7.0 mol·L-1,锆的萃取达到50%,而铪的萃取率小于5%,分离系数达到最大值。Banda等[24]研究发现TEHA在高浓度的盐酸介质中优先萃取锆,且在盐酸浓度为8.0 mol·L-1时,锆对铪的分离系数达到最大值。此外,Banda等[25]还比较了不同的胺类萃取剂Aliquat336,Alamine300,Alamine308和Alamine336对锆和铪的分离性能,发现Alamine336对锆和铪具有最佳的分离性能。
2.5 协同萃取体系
协同萃取不仅可以提高萃取金属离子的分配系数和分离系数,而且可以改善一些分相的问题,成为近年来人们研究的热点,而其中以二元协萃体系是研究最多,也最为常见。
Wang等[26]研究了D2EHPA,PC88A和Cyanex272及其两者的组合在盐酸介质中萃取和分离锆和铪的行为,发现TOPO的加入,对锆和铪萃取具有协同作用,且其分配系数随着盐酸浓度的增加而增加,但分离系数却呈现相反的趋势,且相对于其他协萃体系而言,TOPO-Cyanex272对锆和铪具有最大的协萃能力。Wang和Lee[27]还研究了Lix63和PC-88A单独及其混合物在硝酸介质中萃取和分离锆和铪的性能,发现在单一或混合体系中,均优先萃取锆;PC-88A相对于Lix63对锆和铪具有较大的萃取能力,但后者对锆和铪具有较高的分离能力,在其最佳条件,分离系数达到186;二元体系对锆和铪萃取具有协同效应,但其分离能力不如Lix63。Banda等[28]研究了单一的Cyanex272和P204及其与LIX63的混合物,在硫酸介质中分离锆和铪的性能,发现P204和Cyanex272均优先萃取铪,且前者对铪具有较强的萃取能力;相对于单一萃取剂而言,Lix63与上述两种萃取剂的混合物对铪和锆的萃取选择性无协同作用;以Cyanex272为萃取剂,在硫酸浓度为4.0 mol·L-1时,锆和铪的分离系数达到30。此外,Banda等[29]还研究了TOPO在盐酸介质中单独萃取锆和铪的性能及与DOS,D2EHPA,Aliquat 336,Alamine 336和Alamine 308组成二元体系萃取锆和铪的行为,发现TOPO与D2EHPA具有协同效应,而与胺类萃取剂具有反协同效应。Reddy等[30]研究了不同的冠醚CEs(18C6,DC18C6和B15C5)与HPBI组成的二元体系在盐酸介质中对锆和铪萃取行为,发现CEs与HPBI的协萃物为Zr(Hf)O(PBI)2·CE,且CEs对HPBI萃取锆和铪具有协同效应,能增加锆和铪分离系数,尤其是B15C5,使分离系数由2.09提高到5.28。此外,Reddy等[31]还研究了4-酰基双-(1-苯基-3-甲基-5-吡唑啉酮(HPBI,HFBPI和HTPI)在盐酸介质中萃取锆和铪的行为,发现锆(铪)的萃合物组成为Zr OA2和Hf OA2,且其萃合物的稳定常数按HFBPI>HPBI>HTPI次序递减。此外,还对HPBI和中性磷类萃取剂(TOPO,TBPO和TBP)协同萃取锆(铪)的行为进行了研究,发现中性含磷萃取剂的加入能够提高锆和铪的分离系数,且其协萃物的稳定常数按TOPO>TRPO>TBP[32]顺序递减。近几年还开发现新的协萃体系,如DIBK+P204[33],TBP+P204[34,35],TOPO+TOA[36],Cyanex923+TOA[37],DIBK+TBP[38,39],TBP+Cyanex923[40]。
经过几十年不断的研究和改进,溶剂萃取分离工艺无论是在工艺改进还是新型溶剂萃取体系的研发上都取得了一定的发展和进步,但关于溶剂萃取体系中“三废”处理的相关文献报道相对较少。随着工业化进程不断向前推进,环境问题的矛盾也将日益突出,愈来愈成为人们关注的焦点,国家和行业对化工生产提出了更多新的要求,以实现化工生产过程的绿色化,因而,减小和控制溶剂萃取分离工艺中的环境污染将是大势所趋。
3 其他湿法分离锆铪方法研究进展
溶剂萃取分离锆和铪技术经过几十年的发展,在核级锆铪生产上占据了主导地位,但溶剂萃取法本身也存在一定的问题,如有机溶剂易污染环境、易乳化和设备腐蚀严重等。近年来,其他的湿法分离方法,如分步结晶和沉淀法、吸附分离法、膜分离法、微溶剂萃取法、双水相萃取法和液膜萃取法等也取得了一定的研究进展,为开发新的锆铪分离技术提供了新的思路和方向。
3.1 分步结晶和沉淀法
分步结晶/沉淀法均是基于锆和铪化合物所形成的晶体或沉淀的溶解度的微小差异来实现两者的分离方法。Branken等[41]考察了水溶液的组成,包括KF和HF对锆/铪的氟锆(铪)酸钾盐结晶和分离的影响,发现KF的加入使得氟锆/铪酸钾盐的晶体粒径减小,且容易生成K3Zr(Hf)F7共聚物,降低了锆铪分离系数,但加入2.5 mol·L-1HF后,K3Zr(Hf)F7的共聚物将受到抑制,其分离锆和铪的HF和KF最佳加入量分别为2.5和0.45mol·L-1。解西京[42]控制水相酸度p H=1.3,以柠檬酸为沉淀剂,考察了柠檬酸与锆(铪)的摩尔比(M4+/H3Cit)在0.8~1.8范围内对锆和铪分离的影响,发现锆和铪的沉淀率均随着摩尔比的增加先增加后减小,当摩尔比为1.75时,锆和铪的沉淀率分别达到最大值70.07%和12.97%,而在摩尔比为1.7时,锆和铪分离系数达到最大值9.3。
3.2 吸附分离法
3.2.1 离子交换法
离子交换法是基于锆和铪水解所形成的阴离子或阳离子与带相反电荷的交换树脂的吸附能力的差异而实现锆与铪分离的方法。Smolik等[43]研究了螯合阳离子交换树脂Diphonix从酸性介质如次磷酸、磺酸和羧酸中吸附和分离锆和铪的行为,发现降低温度不利于锆和铪的分离,而降低流速有利于锆和铪分离。在最佳分离条件,铪中锆的含量将减少10倍,锆萃取率达45%。Favre-Reguillon等[44]研究了大孔阴离子交换树脂Amberjet 4200 Cl在0.1~12.0 mol·L-1盐酸介质中吸附和分离锆和铪的性能,发现在盐酸浓度为9.5 mol·L-1时,锆铪分离系数达到最大值9.5,并采用不同的等温吸附模型对Amberjet4200 Cl吸附锆和铪的结果进行了拟合。以Langmuir方程式计算得锆的最大饱和吸附量为0.94mmol·g-1。
3.2.2 萃淋树脂法
萃淋树脂法将溶剂萃取和离子交换结合起来的一种分离方法,它是分离科学中的一个重要研究领域,受到人们的广泛关注。张力等[45]以自制的TBP萃淋树脂填充色层柱分离锆和铪得到了较纯铪和锆。周新木等[46]利用改性的P507萃淋树脂在硫酸介质中进行了锆、铪分离性能的研究,发现最佳反应时间为30 min,且锆分配系数随着硫酸浓度的增加而减小,而铪则有所不同,随着硫酸浓度增加,先增加后减小;锆和铪分离系数随着温度的增加而减小,在较低温度(10℃)下,分离系数达到最大值6.81;铪的浓度越低越有利于锆和铪的分离。此外,Marek等[47]开发了Purolite S-957树脂等。
3.2.3 硅胶吸附法
硅胶吸附法是基于硅胶与锆铪之间产生的物理和化学作用不同而实现两者分离的方法。Qin等[48]通过硅烷偶联剂将二-苯并-18-冠醚-6键合在硅胶上,制得固相萃取剂SGN18,并以此固相萃取剂进行锆铪饱和吸附容量实验,发现SGN18对锆和铪的饱和吸附量分别为420.88和18.71μmol·g-1。Donia等[49]以膦酸或磺酸对硅胶进行处理,制备了改性硅胶,依次考察了p H、时间、温度等条件对该改性的硅胶吸附锆和铪性能的影响,结果表明磷酸和磺酸改性硅胶对锆和铪最大吸附量分别为72.2和45 mg·g-1,其吸附行为符合Langmuir等温吸附模型,吸附动力学为准二级反应;负载锆和铪的磷酸和磺酸改性硅胶,可分别用2.5和1.6 mol·L-1HNO3实现其再生。Yin等[50]合成了几种不同类型的TODGA改性的大孔硅树脂,并研究了该改性硅树脂在盐酸介质中对锆和铪吸附的选择性,依次考察了盐种类和浓度及温度对TODGA改性的大孔硅树脂吸附锆和铪的影响,发现该改性树脂优先吸附锆,且H+浓度对改性树脂吸附锆和铪有很大的影响,其吸附动力学满足Langmuir等温吸附模型,吸附过程为吸热反应。
3.2.4 磁性分离法
磁性分离法以纳米或微米级的磁性微粒为载体,利用结合于微粒表面的有机物或高分子所提供的特异的亲和特性,在外加磁场的控制下,通过亲和吸附、洗涤、解吸从复杂的体系中提取和分离某种目标物。磁性分离技术具有简单、方便,快速的特点。
Aliakbari等[51]利用粒径为10 nm的TBP改性的磁性纳米粒子分离锆和铪,考察了酸浓度和种类(硝酸和盐酸)和金属离子浓度对锆和铪萃取和分离的影响,发现MACS(磁性辅助分离)技术能够有效的分离锆和铪,当锆和铪浓度分别为10.0和7.5 mg·g-1,盐酸浓度为4.0 mol·L-1下,锆和铪萃取和反萃的分离系数分别为1.4和1.5。Donia等[52]合成了胺/硫醇磁性树脂,并以该磁性树脂考察了对锆和铪的吸附性能,发现该磁性树脂对锆和铪饱和吸附容量分别为1.96和1.35 mmol·g-1,其吸附机制可用树脂和金属离子的相互作用来解释,吸附动力学为准二级反应。此外,反萃的实验结果表明,用0.5 mol·L-1HNO3洗涤树脂可实现树脂的再生,且再生的树脂具有较好的耐受力,经过八次循环,树脂性能变化不大。
3.3 膜分离法
Poriel等[53]采用纳滤技术,在配体辅助条件下分离锆和铪,发现超滤和纳滤对锆和铪具有较高的截留率,且当氨基配体乙二胺四乙酸二钠(ED-TA)的加入时,锆和铪的传质速率显著提高,截留率随乙二胺四乙酸二钠与Zr4+(Hf4+)的物质的量比(EDTA/M)比例增加而减小,当EDTA/M为0.2时,铪对锆的分离系数高于2.5。
3.4 微溶剂萃取法
Rezaee等[54]提出了一种新型反液-液微萃取分离锆和铪的新技术。将反相溶剂(丙酮)和萃取剂(乙腈)快速加入到含有2.0 mol·L-1硝酸和Cyanex272负载锆和铪有机相的固相悬浊液中,以形成浑浊的溶液,考察了萃取剂、反相溶剂、配体和酸的种类和浓度对锆和铪萃取和分离的影响,发现Cyanex272相对P204和Cyanex301具有较好的分离能力,在最佳条件下,锆铪的分离系数最大达13。该方法具有萃取时间短、费用低、有机溶剂消耗小等优点。
Hosseini和Alizadeh[55]提出了一种以1,3-二氰丙烷作为萃取剂分离锆和铪的方法。该方法先将温度升高至萃取剂与锆和铪水溶液混溶的温度,使其混溶,然后降温冷却,根据锆和铪离子的水解和聚合随温度变化的差异而实现两者的分离。
浊点萃取法是基于带有憎水和疏水基团的表面活性剂,在临界胶束浓度下,通过改变温度和p H等条件,形成密度不同的富胶束和贫胶束而实现溶质彼此分离的方法。Shariati和Yamini[56]提出了一种在螯合剂醌茜素存在下,以Triton X-114为非离子表面活性剂,浊点萃取、测定锆和铪的方法。在最佳条件下,醌茜素,3.4×10-3mol·L-1,Triton X-114,0.1%(W/V),55℃下,标准曲线呈较好的线性关系,且锆和铪最低检测浓度分别为0.26和0.31μg·L-1。该方法与传统的液液萃取相比具有有机溶剂用量少,易于操作,对环境影响小等优势,是一种新型的环境友好型分离技术。
3.5 双水相萃取法
高聚物/无机盐双水相体系是双水相体系中研究较多的一种,它是基于溶质在互不相溶的两相(富含高聚物水相和富含盐水相)中的溶解度差异而实现彼此的分离。Smolik和Jakobik-Kolon[57]研究了聚乙二醇(PEG)的无机盐双水相体系萃取分离锆铪的行为,依次考察了温度、盐酸浓度、反应时间等对锆和铪分配的影响,发现硫酸和柠檬酸根与锆(铪)的络合物选择性富集于含盐较高的水相,而氯离子与锆(铪)的络合物则倾向富集于含高聚物的有机相,当加入水溶性有机配体钛试剂后,锆和铪的分配系数分别增加到3.75和4.31,而锆对铪的分离系数则由1.03降低到0.85。该方法具有分相时间短、条件温和、对环境危害小、投资成本低等优点,但对其机制的研究不多,尚不是很明确。
3.6 液膜萃取法
Yang等[58]以中空纤维膜为支撑体,三正辛基铝(TNOA)或Aliquat336为载体分离锆和铪,发现TNOA和Aliquat336负载的中空纤维(HFSLM)分离锆和铪能力与液-液单级萃取相当。Karve和Gholave[59]以Amberlite XAD-2000树脂和Cyanex272为填充物和载体,在硝酸或盐酸介质中富集锆,发现在0.01 mol·L-1硝酸或盐酸介质中,锆能被定量吸附,且Cyanex272最佳加入量为100 mg,较佳的淋洗液为1.5 mol·L-1H2SO4。此外,树脂的耐受性实验结果表明,经过25次重复性吸附和脱附实验,该树脂对锆的吸附性能变化不大。
4 结语
1.锆和铪相似的理化性质以及其在原子能领域上的显著差异使得锆铪分离成为制备核级锆铪的技术关键。传统的锆铪溶剂萃取分离工艺,虽经过一定的改进,但仍存在一定的缺点,这在一定程度上限制了溶剂萃取法在工业的应用。
2.大部分的文献报道集中于锆铪湿法分离工艺的研究,而对锆和铪的萃取机制、热力学和动力学的研究相关较少。已开发的锆铪溶剂萃取分离体系,大多优先萃取含量较多的锆,溶剂消耗较大,且溶剂大都存在一定的水溶性和挥发性,对大气和水存在一定的污染,从环保角度考虑,要求分离工艺在技术经济指标之外,还要达到处理过程接近绿色化。因此,开发优先萃取锆化合物中含量较少的铪(2%左右)和环境友好型的绿色萃取剂及萃取体系是大势所趋。
3.溶剂萃取法本身存在的缺点促进了其他的湿法分离方法的发展,已在分步结晶法、离子交换树脂法、膜分离法、微溶剂萃取法、双水相萃取法、液膜萃取法和磁分离法等技术上取得了一定的研究成果,为开发新的锆铪分离技术提供了供新的途径。
参考文献
[1] China Nonferrous Metals Industry.Association,titanium,zirconium and hafnium branch.Studies on the“12th Five”development plan for zirconium industry of China[J].Titanium Industry Progress,2011,28(5):9.(中国有色金属工业协会钛锆铪分会.中国锆产业“十二五”发展规划研究[J].钛工业进展,2011,28(5):9.)
[2] Xiong B K,Wen W G,Yang X M,Li H Y,Luo F C,Zhang W,Guo J M.Metallurgy of Zirconium and Hafnium[M].Beijing:Metallurgical Industry Press,2006.146.(熊炳昆,温旺光,杨新民,李蕙媛,罗方承,张伟,郭靖茂.锆铪冶金[M].北京:冶金工业出版社,2006.146.)
[3] Xu Z G,Wu Y K,Zhang J D,Wang X,Zhang L,Wang L J.Research progress in separation technique of zirconium and hafnium[J].Chinese Journal of Rare Metals,2010,34(3):444.(徐志高,吴延科,张建东,王鑫,张力,王力军.锆铪分离技术的研究进展[J].稀有金属,2010,34(3):444.)
[4] Luo F C,Chen Z X,Sun X L,Chen M L.The craft of the MIBK solvent pairs separation system produces the atomic energy level zirconium oxide and the oxidized hafnium[J].Rare Letters,2007,26(1):89.(罗方承,陈忠锡,孙小龙,谌美玲.MIBK双溶剂萃取法制备原子能级氧化锆和氧化铪的工艺设计[J].稀有金属快报,2007,26(1):89.)
[5] Lin Z H.Process research for the extraction separation of zirconium and hafnium with TBP[J].Rare Letters,2004,23(11):21.(林振汉.用TBP萃取分离锆和铪的工艺研究[J].稀有金属快报,2004,23(11):21.)
[6] Huang D F,Zhou M,Li C X,Kong D C,Yang L F,Xiao S H.Current technological states in the preparation of atomic-grade Zr O2and Hf O2and its research for new process[A].Progress Report on China Nuclear Science&Technology[C].Beijing:Chinese Nuclear Society,2009.188.(黄代富,周密,李春湘,孔冬成,杨立峰,肖少华.制备原子能级二氧化锆(铪)工艺技术现状与新工艺的研究[A].中国核科学技术进展报告[C].北京:中国核学会,2009.188.)
[7] Ma R J.Extraction Metallurgy[M].Beijing:Metallurgical Industry Press,2009.654.(马荣骏.萃取冶金[M].北京:冶金工业出版社,2009.654.)
[8] Che D H,Xu G,Xu S M.Research progress in solvent extraction and separation of zirconium and hafnium[J].Nonferrous Metals Engineering,2014,4(1):75.(车德华,徐刚,徐盛明.溶剂萃取法分离锆铪技术研究进展[J].有色金属工程,2014,4(1):75.)
[9] Banda R,Lee M S.Solvent extraction for the separation of Zr and Hf from aqueous solutions[J].Separation&Purification Reviews,2015,44(3):199.
[10] Xu Z G,LüB Q,Zhang L,Xiong B K,Wang L J.Process research of the extraction and separation of zirconium and hafnium by MIBK method and its treatments to the“three waste”[A].Proceedings of the Conference of the Zirconium Industry-2008[C].Beijing:China Nonferrous Metals Industry Association,2008.33.(徐志高,吕标起,张力,熊炳昆,王力军.甲基异丁基酮(MIBK)萃取分离锆铪的工艺研究及三废处理[A].2008-锆行业大会论文集[C].北京:中国有色金属工业协会,2008.33.)
[11] Nayl A A,El-Nadi Y A,Daoud J A.Extraction and separation of Zr(IV)and Hf(IV)from nitrate medium by some cyanex extractants[J].Separation Science and Technology,2009,44(12):2956.
[12] Saleh A S.Solvent extraction of Zr(IV)and Hf(IV)with N,N,N',N'-tetraoctyldiglycolamide[J].Journal of Radioanalytical and Nuclear Chemistry,2012,292(3):1109.
[13] Saberyan K,Meysami A H,Rashchi F,Zolfonoun E.Proposal of a new Hf(IV)/Zr(IV)separation system by the solvent extraction method[J].Chinese Journal of Chemistry,2008,26(11):2067.
[14] Taghizadeh M,Ghasemzadeh R,Ashrafizadeh S N.Stoichiometric relation for extraction of zirconium and hafnium from acidic nitrate solutions with Cyanex272[J].Hydrometallurgy,2009,96(1-2):77.
[15] Reddy B R,Kumar J R,Reddy A V.Liquid liquid extraction of tetravelent zirconium from acidic chloride solutions using Cyanex272[J].Analytical Sciences March,2004,20(3):501.
[16] Taghizadeh M,Ghasemzadeh R,Ashrafizadeh S N,Saberyan K,Ghannadi Maragheh M.Selective zirconium stripping of a loaded Cyanex272 using Taguchi orthogonal array design[J].Minerals Engineering,2007,20(15):1401.
[17] Lee H Y,Kim S G,Oh J K.Stoichiometric relation for extraction of zirconium and hafnium from acidic chloride solutions with versatic acid 10[J].Hydrometallurgy,2004,73(1):91.
[18] Reddy B R,Kumar J R,Reddy A V.Solvent extraction of tetravalent hafnium from acidic chloride solutions using 2-ethyl hexyl phosphonic acid mono-2-ethyl hexyl ester(PC-88A)[J].Minerals Engineering,2004,17(4):553.
[19] Reddy B R,Kumar J R.Studies on liquid-liquid extraction of tetravalent hafnium from weakly hydrochloric acid solutions by LIX84-IC[J].Separation and Purification Technology,2005,42(2):169.
[20] Reddy B R,Kumar J R,Reddy A V.Solvent extraction of zirconium(IV)from acid chloride solutions using LIX84-IC[J].Hydrometallurgy,2004,74(1-2):173.
[21] Reddy B R,Jyothi R K.Liquid liquid extraction of tetravalent zirconium and hafnium from acidic chloride solutions using 2-hydroxy-5-nonylsalysildehydeoxime(LIX860N-IC)[J].Solvent Extraction Research and Development Japan,2006,13:37.
[22] Saberyan K,Shamsipur M,Zolfonoun E,Salavati-Niasari M.Liquid-liquid distribution of the tetravalent zirconium,hafnium and thorium with a new tetradentate naphthol-derivative Schiff base[J].Bull.Korean Chem.Soc,2008,29(1):94.
[23] Poriel L,Favre-Rguillon A,Pellet.-Rosting S,Lemaire M.Zirconium and hafnium separation,part 1.liquidliquid extraction in hydrochloric acid aqueous solution with Aliquat336[J].Separation Science and Technology,2006,41(9):19.
[24] Banda R,Lee H Y,Lee M S.Separation of Zr and Hf from strong hydrochloric acid solution by solvent extraction with TEHA[J].Journal of Radioanal of Nuclear Chemistry,2013,295(2):1537.
[25] Banda R,Lee H Y,Lee M S.Separation of Zr from Hf in hydrochloric acid solution using amine-based extractants[J].Industrial&Engineering Chemistry Research,2012,51(28):9652.
[26] Wang L Y,Lee H Y,Lee M S.Solvent extraction of zirconium and hafnium from hydrochloric acid solutions using acidic organophosphorus extractants and their mixtures with TOPO[J].Materials Transactions,2013,54(8):1460.
[27] Wang L Y,Lee M S.Separation of zirconium and hafnium from nitric acid solutions with LIX63,PC 88A and their mixture by solvent extraction[J].Hydrometallurgy,2014,150:153.
[28] Banda R,Min S H,Lee M S.Selective extraction of Hf(IV)over Zr(IV)from aqueous H2SO4solutions by solvent extraction with acidic organophosphorous based extractants[J].Journal of Chemical Technology and Biotechnology,2014,89(11):1712.
[29] Banda R,Lee H Y,Lee M S.Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants[J].Journal of Radioanal ofNuclear Chemistry,2013,298(1):259.
[30] Reddy K J,Kumar J R,Reddy M L P,Reddy A V,Park H S,Choo K H.Synergistic enhancement and separation of zirconium(IV)and hafnium(IV)with 3-phenyl-4-benzoyl-5-isoxazolone in the presence of crown ethers[J].Separation Science and Technology,2009,44(9):2022.
[31] Reddy K J,Kumar J R,Reddy A V,Reddy M L P.Synergistic extraction of zirconium(IV)and hafnium(IV)with 4-acyl bis-(1-phenyl-3-methyl-5-pyrazolones)in the presence of neutral organophosphorus extractants[J].Solvent Extraction and Ion Exchange,2006,24(3):419.
[32] Reddy K J,Reddy A V,Shaibu B S,Reddy M L P.Enhanced extraction and separation of zirconium(IV)and hafnium(IV)with 3-phenyl-4-benzoyl-5-isoxazolone in presence of various neutral organophosphorus extractants[J].Radiochimica Acta,2007,95(5):289.
[33] Jirandehi V R,Fatmehsari D H,Firoozi S,Taghizadeh M,Alamdari E K.Statistical modeling of Zr/Hf extraction using TBP-D2EHPA mixtures[J].The Minerals,Metals&Materials Society and ASM International,2012,43B:1262.
[34] Xu Z G,Wang L J,Wu Y K,Chi R A,Li P H,Yang H F.Mechanisms of extraction and separation of zirconium and hafnium by DIBK-P204 system[J].Transactions of Nonferrous Metals Society of China,2013,23(7):2061.(徐志高,王力军,吴延科,池汝安,李攀红,阳慧芳.DIBK-P204萃取分离锆和铪机理的研究[J].中国有色金属学报,2013,23(7):2061.)
[35] Xu Z G,Wang L J,Chi R A,Zhang L,Wu M.Extraction kinetics of zirconium and hafnium in DIBK-P204system[J].Nonferrous Metals(Extractive Metallurgy),2013,(2):28.(徐志高,王力军,池汝安,张力,吴明.DIBK-P204体系萃取锆和铪的动力学[J].有色金属(冶炼部分),2013,(3):28.)
[36] Bhatta B C,Panda N,Mishra S.Extraction of Zr(IV)from hydrochloric acid with trioctyl amine and Cyanex921 in kerosene[J].International Journal of Minerals,Metallurgy and Materials,2013,20(9):823.
[37] Bhatta B C,Mishra S.Binary mixture of trioctyl amine and Cyanex923 as extractant for the extraction of Zr(IV)from acidic chloride medium[J].International Journal of Nonferrous Metallurgy,2014,3(2):9.
[38] Xu Z G,Wang L J,Wu Y K,Chi R A,Zhang L,Wu M.Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system[J].Transactions of Nonferrous Metals Society of China,2012,22(7):1760.
[39] Xu Z G,Wu M,Chi R A,Li P H,Wang L J,Yang H F.Extraction kinetics of hafnium and zirconium in DIBK-TBP system[J].Nonferrous Metals(Extractive Metallurgy),2014,(2):35.(徐志高,吴明,池汝安,李攀红,王力军,阳慧芳.DIBK-TBP体系萃取铪和锆的动力学研究[J].有色金属(冶炼部分),2014,(2):35.)
[40] Taghizadeh M,Ghanadi M,Zolfonoun E.Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923[J].Journal of Nuclear Materials 2011,412(3):334.
[41] Branken D J,Lachmann G.,Krieg H M,Bruinsma D S.Influence of KF and HF on the selectivity of Zr and Hf separation by fractional crystallization of K2Zr(Hf)F6[J].Ind.Eng.Chem.Res.,2010,49(2):797.
[42] Jie X J.Separation of Zirconium and Hafnium[D].Guangzhou:South China University of Technology,2010.47.(解西京.锆和铪的分离[D].广州:华南理工大学,2010.47.)
[43] Smolik M,Jakóbik-Kolon A,Porański M.Separation of zirconium and hafnium using Diphonixchelating ionexchange resin[J].Hydrometallurgy,2009,95(3):350.
[44] Favre-Reguillon A,Fiaty K,Laurent P,Pellet-Rostaing S,Lemaire M.Solid/liquid extraction of zirconium and hafnium in hydrochloric acid aqueous solution with anion exchange resin kinetic study and equilibrium analyses[J].Industrial&Engineering Chemistry Research,2007,46(4):1286.
[45] Zhang L,Wang L J,Lang S L,Chen S,Luo Y H,Huang Y Z.A method for the separation zirconium and hafnium by extraction chromatograph with tributyl phosphate[P].Chinese Patent:CN101209858,2008.(张力,王力军,郎书玲,陈松,罗远辉,黄永章.磷酸三丁酯萃取色层法分离锆铪的方法[P].中国专利,CN101209858,2008.)
[46] Zhou X M,Dong X P,Chen H Q,Guo S J.Experiments of static adsorption of zirconium and hafnium fromH2SO4medium with improved P507[J].Nonferrous Metals(Extractive Metallurgy),2009,(4):26.(周新木,董雪平,陈慧勤,郭树军.改进的P507在H2SO4体系中对锆铪的静态吸附实验[J].有色金属(冶炼部分),2009,(4):26.)
[47] Marek S,Siepietowski,Jakóbik-Kolon A.The effects of concentrations of zirconium(IV)sulphate and sulphuric acid on sorption of zirconium(IV)and hafnium(IV)on purolite S-957 resin:adsorption isotherms of zirconium(IV)and hafnium(IV)ions[J].Solvent Extraction and Ion Exchange 2014,32(4):437.
[48] Qin W,Xu S,Xu G,Xie Q,Wang C,Xu Z.Preparation of silica gel bound crown ether and its extraction performance towards zirconium and hafnium[J].Chemical Engineering Journal,2013,225:528.
[49] Donia A M,Atia A A,Daher A M,Desouky O A,Elshehy E A.Extraction and separation of Zr(IV)from hydrochloric acid solution using modified silica gel produced from waste solution of sodium silicate[J].Separation Science and Technology,2011,46(8):1329.
[50] Yin X B,Wei Y Z,Zu J H.Adsorption behavior of Zr(IV)and Hf(IV)on a silica-based macroporous TODGA adsorbent[J].Nuclear Science and Techniques,2013,24(040203):1.
[51] Aliakbaria M,Saberyan K,Noaparast M.Separation of hafnium and zirconium using TBP modified ferromagnetic nanoparticles:effects of acid and metals concentrations[J].Hydrometallurgy,2014,146:72.
[52] Donia A M,Atia.A A,Daher A M,Desouky O A,Elshehy E A.Synthesis of amine/thiol magnetic resin and study of its interaction with Zr(IV)and Hf(IV)ions in their aqueous solutions[J].Journal of Dispersion Science and Technology,2011,32(5):634.
[53] Poriel L,Chitry F,Pellet-Rostaing S,Lemaire M,Favre-Réguillon A.Zirconium and hafnium separation,part 3:ligand-enhanced separation of zirconium and hafnium from aqueous solution using nanofiltration[J].Separation Science and Technology,2006,41:2883.
[54] Rezaee M,Yamini Y,Khanchi A.Extraction and separation of zirconium from hafnium using a new solvent micro extraction technique[J].Journal of the Iranian Chemical Society,2012,9(1):67.
[55] Hosseini M H,Alizadeh N.Coalescence extraction system for rapid efficient and selective separation of zirconium and hafnium[J].Industrial&Engineering Chemistry Research,2010,49:7068.
[56] Shariati S,Yamini Y.Cloud point extraction and simultaneous determination of zirconium and hafnium using ICP-OES[J].Journal of Colloid and Interface Science,2006,298:419.
[57] Smolik M K,Jakobik-Kolon A.Extraction of zirconium and hafnium in polyethylene glycol-based aqueous biphasic system[J].Separation Science and Technology,2007,42(8):1831.
[58] Yang X J,Fane A G,Pin C.Separation of zirconium and hafnium using hollow fibers,part I.supported liquid membranes[J].Chemical Engineering Journal,2002,88(1):37.
[59] Karve M,Gholave G V.Amberlite XAD-2 impregnated with Cyanex272 for zirconium(IV)enrichment followed by spectrophotometric determination[J].Desalination and Water Treatment,2014,52(1-3):452.



