
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1217-1223
Synergistic extraction of zinc from ammoniacal solutions using β-diketone mixed with CYANEX923 or LIX84I
HU Jiu-gang, CHEN Qi-yuan, HU Hui-ping, YIN Zhou-lan
Key Laboratory of Resources Chemistry of Nonferrous Metals (Ministry of Education),
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 19 April 2011; accepted 20 February 2012
Abstracts: The extraction behaviors of zinc from ammoniacal solutions were investigated using β-diketone (HA) and their mixtures with CYANEX923 or LIX84I. The effects of pH, total ammonia concentration, extractant concentration, anion species and temperature on zinc extraction were examined. The synergistic mechanism was discussed with regard to the structure of extractant and the extracted zinc complexes. It is found that the increase of total ammonia concentration and pH inhibits zinc extraction for all extraction systems due to the formation of zinc ammine complexes in aqueous phase. This effect of HA with CYANEX923 is evidently smaller than that of HA with LIX84I or HA alone system. Effect of anion species on the zinc extraction by HA with CYANEX923 can be neglected, but this effect of HA alone and the mixture of HA with LIX84I decreases in the order of (NH4)2SO4 > NH4NO3 > NH4Cl.
Key words:
synergistic extraction; zinc; ammoniacal solution; β-diketone; CYANEX923; LIX84I;
1 Introduction
Due to the increasing demands of zinc in industry and the urgent supply of zinc concentrate, exploiting and utilization of oxidized zinc ores, such as zinc-bearing low grade feeds [1], tailings [2] and wastes [3], have greatly attracted the interest of metallurgists. Among various hydrometallurgical processes, the “ammonia leaching-solvent extraction-electrowinning” process [4] is one of the most promising technologies because zinc can be dissolved easily through the formation of zinc ammine complexes. Furthermore, the major wasteful components in ores, such as Fe2O3, SiO2, CaO and MgO, are insoluble in ammoniacal solutions. As a result, selective recovery of zinc is possible with leaving the undesirable components in the residues [5].
Among the various separation technologies, solvent extraction is rapidly reaching extensive commercial acceptance for the separation and recovery of non-ferrous metals from leaching liquors, due to the attractive characteristics including simple operation and easy large-scale industrial application [6,7]. In the case of the extraction of zinc, various extractants, such as carboxylic acid [8], organophosphorus acid [9,10] and hydroxyoxime [11], have been extensively examined in acidic solutions. But these extractant cannot be used to extract zinc from ammoniacal solutions because of severe emulsification or low extraction efficiency. β-diketone is the most alternative for recovering zinc from ammoniacal media [12]. Acetylacetone (Hacac) and thenoyltrifluoroacetone (Htta) were widely investigated for preconcentration of transition metal ions in analytical and hydrometallurgical applications [13]. Several commercial β-diketone extractants including Hostarex?DK-16 [14], Henkel?LIX51 [15] and LIX54 [12,16] were developed for technical applications, but none of them is commercially available. The extraction behaviors of LIX 54 had systematically studied for copper [17], nickel [18] and zinc [12,19] from ammoniacal solutions. Although exhibiting good performance in some cases, LIX54 is prone to chemical deterioration, forming the ketimine at the octanoyl carbon atom in the presence of high levels of NH3 [20]. Reagents with sterically hindered acyl groups show much greater resistance to chemical deterioration and better strip kinetics than LIX 54. In our previous work [4], a sterically hindered β-diketone, 1-(4’-dodecyl)- phenyl-3-tertiarybutyl-1,3-octadione, was successfully developed to overcome the problem of extractant deterioration in ammoniacal solutions.
However, it should be paid attention that the extraction efficiency of various β-diketones mentioned above is still too low for industrial application and decreases dramatically with the increase of pH or ammonia concentration [14,18,19]. In order to improve the extraction and separation ability, several studies show that co-use of one extractant with another (e.g., solvating extractant and chelating extractants) can effectively enhance both the extractability and extraction selectivity [21]. For instance, evident synergistic effect was found when the Zn(II) was extracted from chloride medium with mixtures of sec-nonylphenoxy acetic acid (CA-100) and bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272) [22]. Completed separation of cadmium over zinc was achieved with a synergistic mixture of nonylsalicylic acid and triisobutylphosphine sulphide [23]. And a method for the separation of copper and zinc during their transfer from hydrochloric acid to sulphuric acid medium had been developed using the mixed extractant of Alamine 336 and LIX 54 [24]. CYANEX923 and LIX84I are commercially available extractants and widely used in solvent extraction for the separation of various metal ions, such as nickel [25], copper [26,27] and zinc [28] in ammoniacal solutions. In the present work, the extraction behaviors of zinc from ammoniacal solutions with mixtures of sterically hindered β-diketone with CYANEX923 or LIX84I were investigated. The effects of the concentrations of total ammonia and the extractant were examined. The dependence of extraction efficiency on aqueous phase pH value, ammonium salts with different anions and temperature was also studied. The results could provide a fundamental understanding to design the “ammonia leaching-solvent extraction-electrowinning” process for dealing with the low grade oxidized zinc ores.
2 Experimental
2.1 Reagents and feed solutions
Sterically hindered β-diketone, 1-(4’-dodecyl)- phenyl-3-tertiarybutyl-1,3-octadione (donated as HA) was made by Claisen condensation as Ref. [29]. Sulphonated kerosene was used as diluent which was purchased from Shanghai Rare Earth Chemical Co., Ltd. CYANEX923 (a mixture of four trialkylphosphine oxides) and LIX84I (2-hydroxy-5-nonylacetophenone oxime) were supplied by Cytec Corporation and Cognis Corporation, respectively. And they were used as-received. All other chemicals were of analytical grade and were used without further purification.
Organic solutions including HA with CYANEX923 or LIX84I in sulfonated kerosene were prepared for the extraction trials. The total concentration of mixed extractant was kept at 45% (volume fraction), unless otherwise stated. Synthetic leaching solutions containing 0.2 mol/L Zn(II) were prepared with the corresponding zinc salt, ammonium salt and aqueous ammonia. The aqueous pH was adjusted by the addition of acid or sodium hydroxide.
2.2 General extraction procedure
Extraction experiments were carried out in a thermostated vessel using a batch technique. The temperature was maintained at (25±0.5) °C, unless otherwise stated. Equal volumes (each of 10 mL) of aqueous solution and organic solution were magnetically stirred for at least 30 min in order to obtain the extraction equilibrium. When the emulsification phenomenon occurred, the phase separation was handled by centrifugation. After phase separation, the metal concentration in aqueous phase was determined by EDTA standard titration and the balance of the mass was calculated in order to estimate the metal concentration in organic phase. The pH values of aqueous phase were measured on a calibrated Shanghai Rex-3C digital pH meter before and after extraction. The distribution coefficient (D) was taken as the ratio of the metal concentration in the organic phase to that in the aqueous phase.
3 Results and discussion
The extraction of zinc by chelating extractant can be represented by the following equilibrium [19]:
![]() (1)
(1)
where HA represents the chelating extractant molecules, i.e. β-diketone or LIX84I; and the subscript aq and org denote the aqueous phase and organic phase, respectively. When the neutral extractant is added, the following equilibrium can be obtained:
![]() (2)
(2)
where B represents the neutral extractant, CYANEX923.
In ammoniacal solutions, complexation equilibrium of Zn (II) with ammonia proceeds as the concentration of free ammonia increases:
![]() , i=1-4 (3)
, i=1-4 (3)
The solvent extraction of zinc is obviously dependent on the concentration of total ammonia, aqueous pH and the concentration of extractant, etc.
3.1 Synergistic effect of extractant mixtures
The extraction behaviors of two extractant mixtures, HA with CYANEX923 and HA with LIX84I, were examined to elucidate their synergistic effects on the extraction of Zn(II) from ammoniacal sulfate solutions (cT(NH3) = 3 mol/L and pH=8.25). Figure 1 presents the relationship between the distribution coefficient of zinc and the molar fraction of HA in the extractant mixtures. It can be found that the synergistic extraction behavior is different for two extraction systems. The evident synergistic effect on zinc extraction was found for the HA with CYANEX923, but this effect is slight for HA with LIX84I. The maximum distribution coefficient of HA with CYANEX923 is obtained when the molar ratio of β-diketone to CYANEX923 is about 2. It means that the Zn(II) could react with HA and CYANEX923 as a stoichiometric ratio of 1:2:1 to form the ZnA2B synergistic adduct. The maximum distribution coefficient is obtained when the molar ratio of β-diketone to LIX84I is about 1.
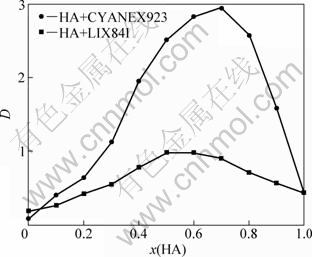
Fig. 1 Relationship between distribution coefficient (D) of Zn(II) and molar fraction of HA in extractant mixture
The discrepancy of extraction behavior could be explained by the different structures of extractants and the different extraction mechanisms. The evident synergistic effect by HA with CYANEX923 can be attributed to the formation of ZnA2B complex (Eq. 2). Because CYANEX923 is a neutral extractant, the formation of synergistic adduct can result in the increase of the stability and the solubility of zinc complexes in organic solution [30]. But the LIX84I is a chelating extractant, its extraction behavior is similar to β-diketone. The synergistic extraction of HA with LIX84I could be explained by the structure change of HA induced by the addition of LIX84I. Due to the keto-enol tautomerism of β-diketone, the enol configuration is found to play a crucial role in metal extraction [31]. Because LIX84I contains hydroxyl group, the content of enol of β-diketone can be increased by intramolecular hydrogen bond and intermolecular hydrogen bond when LIX84I is added. This synergistic role could be similar to that of alcohols and phenols as modifiers [31-33].
3.2 Effect of extractant concentration
The concentration of mixed extractant was investigated when the extraction of zinc from ammoniacal sulfate solutions was carried out (cT(NH3)= 3 mol/L and pH=8.25). The molar ratios of β-diketone to CYANEX923 and LIX84I in extractant mixtures were kept at 2 and 1, respectively. For comparison, the dependency of HA concentration on extraction efficiency was also studied. As shown in Fig. 2, the extraction efficiency of three extraction systems increases with the increase of extractant concentration. When the extractant concentration increases to above 40%, the increment of extraction efficiency is obviously reduced. The extraction performance of HA with CYANEX923 is better than that of the mixture of HA with LIX84I or HA alone. When the concentrations of HA, HA with LIX84I and HA with CYANEX923 are kept at 45%, the extraction efficiencies are 75.2%, 48.8% and 30.6%, respectively. It is necessary to note that emulsification phenomenon occurs when the concentration of HA with CYANEX923 increases to beyond 50%.
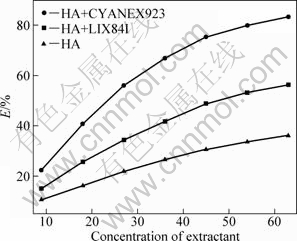
Fig. 2 Relationship between extraction efficiency (E) and concentration of extractant (volume ratio)
3.3 Effect of aqueous pH
The extraction behaviors using HA in the absence and presence of CYANEX923 or LIX84I were examined on the extraction of zinc from ammoniacal sulfate solutions (cT(NH3)= 3 mol/L and aqueous initial pH= 7.6-8.8). The total extractant concentrations were kept at 45%. The molar ratio of β-diketone to CYANEX923 or LIX84I in mixed extractant is kept at 2 or 1. The relationship between the extraction efficiency and aqueous initial pH is illustrated in Fig. 3. The extraction efficiencies of three extraction systems decrease as initial aqueous pH increases. The extraction efficiency of HA with CYANEX923 is evidently greater than that of HA or HA with LIX84I, especially at higher pH. It still reaches about 68% at pH 8.8. The similar phenomenon was also observed in other works [14,19], which suggests that the extraction efficiency of zinc with β-diketone from ammoniacal solutions increases with increasing the initial aqueous pH and reaches a maximum at pH= 7.8-8.1 and then declines. The maximum extraction efficiency is not observed in the present work, due to the different structures of β-diketone and the different conditions of aqueous solutions. Compared with the extraction of zinc in acidic solutions using the similar extractants, this phenomenon is mainly attributed to the complicated zinc ammine complexation reaction (Eq. (3)). Ammonia in the aqueous solution as a competitive ligand coordinates with Zn(II) to form the zinc ammine complexes, which could not be extracted, resulting in a decrease of the extraction of zinc.

Fig. 3 Relationship between extraction efficiency and initial pH of aqueous solution
3.4 Effect of total ammonia concentration
The concentration of total ammonia is a key factor that affects the recovery of zinc from oxidized ores. So, the extraction behaviors of zinc from ammoniacal sulfate solutions with different total ammonia concentrations (0.2 mol/L Zn(II), cT(NH3)= 2-6 mol/L) at fixed pH 8.25 were examined. In Fig. 4, the relationship between the extraction efficiency and total ammonia concentration shows that the extraction performance of three extractant systems decreases as the total ammonia concentration increases. It is worth noting that zinc is hardly extracted by HA alone when total ammonia concentration increases to 6 mol/L, but the extraction percentage of Zn (II) by HA with CYANEX923 still reaches 42%. Similar to the effect of aqueous solution pH, the increase of total ammonia concentration at fixed pH can promote the zinc ammine complexation reaction and reduce accordingly the concentration of free zinc ion. Although high ammonia concentration and pH are generally beneficial to increasing the leaching ratio of oxidized zinc ores [4], the extraction performance of the present extractant systems are obviously restricted under these conditions.
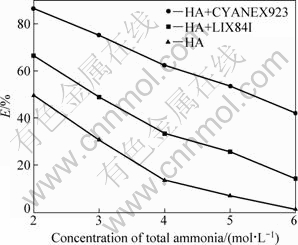
Fig. 4 Relationship between extraction efficiency and total ammonia concentration
3.5 Effect of temperature
The extraction behavior of zinc (II) with three extractant systems from ammoniacal sulfate solution has been studied in the temperature range from 298 K to 323 K. 10 mL of aqueous phase (0.2 mol/L Zn(II), 3 mol/L total ammonia and pH 8.25) was balanced for 1 h with an equal volume of organic phase (45% extractant concentration). The actual temperature of reaction system was measured after extraction equilibrium was reached. As shown in Table 1, the extraction performance of three extractant systems decreases when the temperature increases. The effect of temperature on zinc extraction for HA system and HA with LIX84I system is more evident than that for HA with CYANEX923.
Table 1 Effect of temperature on extraction of zinc

The data were subjected to least square analysis and the fitting straight lines were obtained. The enthalpy change of extraction reaction in each case was evaluated using Van’t Hoff’s equation given below:
![]() (4)
(4)
As shown in Fig. 5, an increase of temperature decreases the zinc extraction from ammoniacal sulfate solutions. The calculated ΔH values for the extraction reaction of HA, HA with LIX84I and HA with CYANEX923 are -16.98, -11.37, -6.06 kJ/mol, respectively. This suggests that these extraction reactions proceed exothermically. The enthalpy change values of HA with CYANEX923 are 10.92 kJ/mol and 5.31 kJ/mol which are greater than those of HA and HA with LIX84I, indicating that the extracted complex, ZnA2, is prone to reaction with CYANEX923 to form the synergistic product, ZnA2B.

Fig. 5 Arrhenius plots for Zn(II) extraction
3.6 Effect of anion species
As ammoniacal solutions with various anions are usually used for leaching of oxidized zinc ores in hydrometallurgical industry, zinc extraction was carried out from different ammoniacal solutions to determine the extraction dependence on the nature of the counter anions of ammonium salts. Ammonium sulfate, ammonium chloride and ammonium nitrate were used to prepare the feed solutions at pH=8.25, which contain 0.2 mol/L Zn (II) and 3 mol/L total ammonia. Extractant concentrations in organic phase were kept at 45%. As shown in Fig. 6, the extraction of Zn(II) by HA with CYANEX923 is slight dependence on the anions in different ammonium salt solutions. However, the discrepancy was observed for HA alone or HA with LIX84I in the order of (NH4)2SO4 > NH4NO3 > NH4Cl. This may be attributed to the different complexation abilities of anions with zinc in aqueous solutions. As chloride ion can coordinate with zinc to form zinc chloride complexes [5], the effect of chloride ion is more evident than that of sulfate or nitrate ion. The results further confirm that HA with CYANEX923 is more efficient than HA with LIX84I or HA alone for Zn(II) extraction from ammoniacal solutions.

Fig. 6 Effect of anion species on extraction efficiency of Zn(II)
4 Conclusions
1) Synergistic effect on the zinc extraction from ammoniacal solutions was found by co-use of β-diketone with other commercial extractants, i.e., CYANEX923 and LIX84I. HA with CYANEX923 is more effective for the zinc extraction than HA with LIX84I, especially at a higher total ammonia concentration or aqueous solution pH. The synergistic effect for HA with CYANEX923 can be attributed to the formation of more stable synergistic adduct.
2) The extraction efficiencies of HA, HA with CYANEX923 and HA with LIX84I decrease with the increase of total ammonia concentration and pH in the aqueous phase. This can be attributed to the formation of nonextractable zinc ammine complexes.
3) The extraction reactions proceed exothermically for three extractant systems. The more positive enthalpy change of HA with CYANEX923 indicates that the extracted complex, ZnA2, is prone to reaction with CYANEX923 to form the synergistic adduct, ZnA2B, which increases the stability and the solubility of zinc complex in organic solution.
4) Effect of ammonium salts with different anion species on the extraction of HA with CYANEX923 can be neglected but this effect for HA alone or the mixture of HA with LIX84I systems decreases in the order of (NH4)2SO4 > NH4NO3 > NH4Cl.
References
[1] ZHANG Yu-mei, LI Jie, CHEN Qi-yuan, DING Hong-qing. Influence of ultrasonic irradiation on ammonia leaching of zinc from low-grade oxide zinc ore [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(5): 960-966. (in Chinese)
[2] ZHANG Jiang-hui, LU Zhong-wu. Status and approach of recycle of zinc resources in China [J]. Resources Science, 2007, 29(3): 86-93. (in Chinese)
[3] JHA M. K, KUMAR V, SINGH R J. Review of hydrometallurgical recovery of zinc from industrial wastes [J]. Resources, Conservation and Recycling, 2001, 33(1): 1-22.
[4] CHEN Qi-yuan, LI Liang, BAI Lan, HU Hui-ping, LI Jian, LIANG Qi-wen, LING Jiang-hua. Synergistic extraction of zinc from ammoniacal ammonia sulfate solution by a mixture of a sterically hindered beta-diketone and tri-n-octylphosphine oxide (TOPO) [J]. Hydrometallurgy, 2010, 105(3-4): 201-206.
[5] MENG X, HAN K N. The principles and applications of ammonia leaching of metals—A review [J]. Mineral Processing and Extractive Metallurgy Review, 1996, 16(1): 23-61.
[6] KUMAR V, SAHU S K, PANDEY B D. Prospects for solvent extraction processes in the Indian context for the recovery of base metals. A review [J]. Hydrometallurgy, 2010, 103(1-4): 45-53.
[7] HE Jing, HUANG Ling, CHEN Yong-ming, TANG Mo-tang, JIN Sheng-ming, FENG Rui-shu, WU Sheng-nan. Solvent extraction of zinc from Zn(II)-NH3 complex system by new extractant YORS [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(3): 687-693. (in Chinese)
[8] DEEP A, de CARVALHO J M R. Review on the recent developments in the solvent extraction of zinc [J]. Solvent Extraction and Ion Exchange, 2008, 26(4): 375-404.
[9] INOUE K, GOYA M, TANIGUCHI M. Extraction equilibrium and extraction kinetics of nickel from aqueous ammonium nitrate solution with versatic 10 in n-hexane [J]. Hydrometallurgy, 1984, 13(2): 155-167.
[10] LONG Huai-zhong, CHAI Li-yuan, QIN Wen-qing,TANG Shuang-hua. Solvent extraction of zinc from zinc sulfate solution [J]. Journal of Central South University of Technology, 2010, 17(4): 760-764.
[11] QIN Wen-qing, LAN Zhuo-yue, LI Wei-zhong, QIU Guan-zhou. Selective extraction of zinc from sulfate leach solution of zinc ore [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(6): 1435-1439.
[12] PARIJA C, BHASKARA S P V R. Separation of nickel and copper from ammoniacal solutions through co-extraction and selective stripping using LIX84 as the extractant [J]. Hydrometallurgy, 2000, 54(2-3): 195-204.
[13] ALGUACIL F J, ALONSO M. The effect of ammonium sulphate and ammonia on the liquid-liquid extraction of zinc using LIX 54 [J]. Hydrometallurgy, 1999, 53(2): 203-209.
[14] NASH K L, CHOPPIN G R. The thermodynamics of synergistic solvent extraction of zinc(II) [J]. Journal of Inorganic and Nuclear Chemistry, 1977, 39(1): 131-135.
[15] RAO K S, SAHOO P K, JENA P K. Extractions of zinc from ammoniacal solutions by Hostarex DK-16 [J]. Hydrometallurgy, 1992, 31(1-2): 91-100.
[16] JYOTHI A, RAO G. Solvent extraction of metals with a commercial fluorinated β-diketone (LIX51) extractant [J]. Journal of Chemical Sciences, 1988, 100(6): 455-457.
[17] MICKLER W, UHLEMANN E. Liquid-liquid extraction of copper from ammoniacal solution with β-diketones [J]. Separation Science and Technology, 1992, 27(12): 1669-1674.
[18] ALGUACIL F J, ALONSO M. Recovery of copper from ammoniacal/ammonium sulfate medium by LIX 54 [J]. Journal of Chemical Technology & Biotechnology, 1999, 74(12): 1171-1175.
[19] ALGUACIL F J, COBO A. Solvent extraction equilibrium of nickel with LIX54 [J]. Hydrometallurgy, 1998, 48(3): 291-299.
[20] ALGUACIL F J, COBO A. Extraction of zinc from ammoniacal/ammonium sulphate solutions by LIX 54 [J]. Journal of Chemical Technology & Biotechnology, 1998, 71(2): 162-166.
[21] TASKER P A, PLIEGER P G, WEST L C. Metal complexes for hydrometallurgy and extraction [C]//MCCLEVERTY J A, MEYER T J. Comprehensive Coordination Chemistry II. Oxford: Pergamon, 2003: 759-808.
[22] FLETT D S. New reagents or new ways to use old reagents [J]. Journal of Chemical Technology and Biotechnology, 1999, 74(2): 99-105.
[23] WANG Y G, WANG L G, LI D Q. Synergistic extraction of zinc(II) with mixtures of CA-100 and cyanex 272 [J]. Separation Science and Technology, 2003, 38(10): 2291-2306.
[24] MORADKHANI D, URBANI M, CHENG C Y O, ASKARI M, BASTANI D. The separation of cadmium from zinc with a synergistic mixture of nonylsalicylic acid and triisobutylphosphine sulphide [J]. Hydrometallurgy, 2005, 78(1-2): 129-136.
[25] MISHONOV I, KYUCHOUKOV G. Separation of copper and zinc during their transfer from hydrochloric acid to sulphuric acid medium using a mixed extractant [J]. Hydrometallurgy, 1996, 41(1): 89-98.
[26] PARIJA C, REDDY B R, SARMA P V R. Recovery of nickel from solutions containing ammonium sulphate using LIX 84-I [J]. Hydrometallurgy, 1998, 49(3): 255-261.
[27] GAMEIRO M L, MACHADO R M, ISMAEL M R, REIS M T, CARVALHO J M. Copper extraction from ammoniacal medium in a pulsed sieve-plate column with LIX 84-I [J]. Journal of Hazardous Materials, 2010, 183(1-3): 165-175.
[28] SENGUPTA B, BHAKHAR M, SENGUPTA R. Extraction of zinc and copper-zinc mixtures from ammoniacal solutions into emulsion liquid membranes using LIX 84I [J]. Hydrometallurgy, 2009, 99(1-2): 25-32.
[29] SARANGI K, PARHI P K, PADHAN E, PALAI A K, NATHSARMA K C, PARK K H. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX 84I and Cyanex 923 [J]. Separation and Purification Technology, 2007, 55(1): 44-49.
[30] BARKLEY L, LEVINE R. The synthesis of certain ketones and α-substituted β-diketones containing perfluoroalkyl groups[J]. Journal of the American Chemical Society, 1953, 75(9): 2059-2063.
[31] SHIGEMATSU T, HONJYO T, TABUSHI M, MATSUI M. Synergistic effect in solvent extraction-effect of oxygen and nitrogen containing organic bases on the stability of zinc (II) and cobalt (II) beta-diketonate adducts [J]. Bulletin of the Chemical Society of Japan, 1970, 43(3): 793-796.
[32] SUZUKI N, AKIBA K. Roles of the oxygen-containing solvents in the distribution of zinc thenoyltrifluoroacetonate [J]. Journal of Inorganic and Nuclear Chemistry, 1971, 33(6): 1897-1907.
[33] KATSUTA S, SUZUKI N. Solvent extraction of 8-quinolinolato-iron (III) with the aid of various phenols as hydrogen-bonding donors [J]. Talanta, 1993, 40(2): 231-235.
β-二酮与CYANEX923或LIX84I混合萃取剂从氨性体系中协同萃锌
胡久刚,陈启元,胡慧萍,尹周澜
中南大学 化学化工学院,有色金属资源化学教育部重点实验室,长沙 410083
摘 要:研究β-二酮(HA)与CYANEX923或LIX84I的混合萃取剂在氨性溶液中的萃锌行为;系统讨论有机相萃取剂浓度、水相pH、总氨浓度、温度和阴离子等对萃取性能的影响,从锌萃合物及β-二酮萃取剂结构方面讨论2个混合萃取体系的协同萃取机理。结果表明,HA与CYANEX923混合比与LIX84I混合具有更加明显的协同效应;水相pH和总氨浓度的升高均使萃取性能降低,因为升高pH或氨浓度会促使锌氨配合物的生成,降低水相自由锌离子的浓度,但HA与CYANEX923混合体系在较高pH或总氨浓度下仍具有明显的萃取性能;不同阴离子对HA与CYANEX923混合萃取体系的性能没有明显影响,但在HA与LIX84I混合萃取体系和单独使用HA萃取过程中以(NH4)2SO4>NH4NO3>NH4Cl顺序降低。
关键词:协同萃取;锌;氨性溶液;β-二酮;CYANEX923;LIX84I
(Edited by YANG Hua)
Foundation item: Project (2007CB613601) supported by the National Basic Research Program of China; Project (CX2010B112) supported by Hunan Provincial Innovation Foundation for Postgraduate, China
Corresponding author: CHEN Qi-yuan; Tel/Fax: +86-731-88879616; E-mail: cqy@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61308-3


