- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 FT-IR spectra of PGF with different treating conditions
- Fig.2 SEM images of different graphite fibers
- Fig.3 Cyclic voltammograms of PGF and TPGF at scan rate of 3 mV/s
- Fig.4 shows a typical series of cyclic voltammo- grams obtained in 1.0 mol/L VOSO4 and 1.6 mol/L H2SO4 solution at different scan rates. As the VO2+/VO2+ reaction is quasi reversible at the TPGF, the peak current Ip is given as follows[19]:
- Fig.5 Relationship between anodic peak current Ip of VO2+/VO2+ and scan rate υ1/2
- Fig.6 Cyclic voltammograms of electrolyte with different H2SO4 concentrations
- Fig.7 EIS of character with different VOSO4 concentrations
- Fig.8 Equivalent circuit of graphite felt electrodes at 100 mV
J. Cent. South Univ. Technol. (2007)01-0051-06
DOI: 10.1007/s11771-007-0011-6
![]()
Electrochemical behavior of diverse vanadium ions at modified graphite felt electrode in sulphuric solution
LI Xiao-gang(李晓刚)1, HUANG Ke-long(黄可龙)1, LIU Su-qin(刘素琴)1, CHEN Li-quan(陈立泉)1, 2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Institute of Physics, Chinese Academy of Sciences, Beijing 100080, China)
Abstract:
PAN-based graphite felt (PGF) treated in 98% sulphuric acid for 5 h and then kept at 450 ℃ for 2 h was evaluated for their electrochemical performance as electrodes of vanadium redox battery (VRB). Structure and characteristic of treated PAN-based graphite felt (TPGF) were determined by means of Fourier Transform Infrared Spectroscopy, Scanning Electron Microscopy, Brunauer-Emmett-Teller surface area analysis and VRB test system. The results show that the acid and heat synergistic effect increase the number of —COOH functional groups on the PGF surface, and the PGF is eroded by sulphuric acid oxidation, resulting in the surface area increases from 0.31 m2/g to 0.45 m2/g. The V(Ⅱ)/V(Ⅲ) redox reaction is electrochemically reversible on the TPGF electrode, while the V(Ⅳ)/V(Ⅴ) couple is a quasi reversible process. The diffusion coefficients of the oxidation for V(Ⅳ)/V(Ⅴ) obtained from the scope of peak current Ip vs scan rate v1/2 is 4.4×10-5 cm2/s. The improvement of electrochemical activity for the electrode is mainly ascribed to the increase of the number of —COOH groups on the TPGF, which behaves as active sites catalyzing the vanadium species reactions and accelerating electron transfer reaction and oxygen transfer.
Key words:
vanadium; redox flow battery; graphite felt; diffusion coefficient.;
1 Introduction
The vanadium redox flow battery proposed by SKYLLAS-KAZACOS et al[1-3], becomes the most practical candidate for energy reserving purposes of solar energy, wind energy and some other renewable power resources[4-5]. In order to further improve the performance of vanadium redox battery (VRB) system, it is necessary to investigate the novel electrode and discover the redox mechanism of diverse vanadium ions in sulphuric solution.
A number of researchers investigated the electrochemical behavior of the V(IV)/V(V) and V(II)/ V(III) couples at noble-metal electrodes[6], glassy-carbon electrodes[7] and conducting plastic composite electrodes[8]. These findings indicated that the redox reactions are irreversible at go1d and glassy-carbon electrodes. At lead and titanium electrodes, however, passivating phenomena were observed over the potential of 1.70 V. Although flat-plate titanium and dimensionally stable anode (DSA) electrodes exhibited better reversibility, the high cost of these materials would not be suitable for large-scale applications of the vanadium battery.
An ideal electrode should possess higher electric conductivity and longer cyclic life in sulfuric acid containing concentrated and oxidizing pentavalent vana-dium ion (VO2+). The PAN-based graphite felt (PGF), due to its greater specific surface area, fine electrochemical activity, wide operating potential range and good stability, is competitive when compared with metal electrode and conductive plastic composite electrode[2, 9-12]. However, the lower electrochemical activity of commercial graphite felt is still one of the major drawbacks limiting power density and voltage efficiency of electrochemical system. But, it was reported that oxygen functional groups on the carbon surface, behave as active sites for many electrochemical reactions[13]. The result inspired researchers to develop other novel modification techniques to improve the electrochemical activity of graphite felt material[14-18].
In this study, the PGF was modified with sulphuric acid and heat treatment, and then was characterized by using scanning electron microscope (SEM) and Fourier transform infrared (FT-IR) spectrum. Its electrochemical activity and the electrochemical behavior of diverse vanadium ions at treated PAN-based graphite felt (TPGF) were evaluated by means of cyclic voltammogram (CV) and electrochemical impedance spectrum (EIS).
2 Experimental
2.1 Treatment of samples
PGF samples were treated in 98% (mass fraction)
sulphuric acid (H2SO4) for 5 h, and washed thoroughly with deionized water. After being dried in a vacuum at 450 ℃ for 1 h, then the PGF samples were treated in muffle furnace at 450 ℃ for 2 h.
2.2 Characterizations
The Fourier transform infrared (FT-IR) spectra were recorded from KBr disks containing the PGF powder on an AVATAR-360 instrument (Licolet Co Ltd, America). Scanning Electron Microscope (SEM) was performed on JSM-5600LV produced by Japan electronic company. Monosorb direct reading specific surface analyzer (Quantachrome Co Ltd, America) was employed for Brunauer-Emmett-Teller(BET) measure- ment in He+30%N2 atmosphere under 0.1 MPa at 150 ℃.
2.3 Electrochemical measurement
The cyclic voltammetry and alternating current (AC) impedance were carried out on CHI660 electrochemical workstation (CH Instruments, Inc. America) with a ternate electrode system in the solution of l mol/L VOSO4+1.6 mol/L H2SO4. The TPGF electrode with area of 1 cm×1 cm was used as working electrode, Pt foil electrode was severed as counter electrode, and standard calomel electrode (SCE) with Luggin capillary was employed as the reference electrode. The scan rates in cyclic voltammetry were 0.01, 0.03, 0.05 V/s and 0.08 mV/s, respectively. The measurement of AC impedance was processed after five scans of cyclic voltammetry, and the data were fitted by Zsimp Win 3.20 Demo software on CHI660B workstation. The oscillation amplitude was 5 mV, and the frequency ranged from 103 Hz to 106 Hz for AC impedance test at 22 ℃.
3 Results and discussion
3.1 FT-IR analysis
FT-IR spectra of PGF samples are shown in Fig.1. The peaks at 1 654 cm-1 and 3 421 cm-1 are assigned to the stretching vibration of C==O and —OH, respectively. The peak at 1 400 cm-1 is attributed to the curving vibration of —OH. The peaks at 1 049 cm-1 and 1 250 cm-1 correspond to the stretching vibration of C—O. No new absorbing peaks appear on the TPGF sample, while some functional groups present the different absorbing intensity. The character peak of —OH (3 421 cm-1) is obviously broadened and intensified. The peak intensity of C—H (at 2 975 cm-1) decreases with the increase of oxidation degree, and disappears after acid plus heat complex treatment.

Fig.1 FT-IR spectra of PGF with different treating conditions
1—Untreated; 2—Acid treated; 3—Acid plus heat complex treated
The peak intensity of C==O (1 654 cm-1) varies under the treating conditions. The —OH curving vibration peak presented (1 400 cm-1) decreases slightly after treatment. The stretching vibration peaks of C—O at 1 049 and 1 250 cm-1 reduce rapidly with acid treatment or acid plus heat complex treatment.
The FT-IR results indicate that acid plus heat treatment reduces the content of C—O groups, while increases the number of —OH and C==O functional groups on PGF surface. It may be caused by the elimination of impurity of alcohol or ether from the surface of PGF and the formation of —COOH groups on the PGF surface during treatment.
3.2 SEM and BET analysisThe SEM images of PGF and TPGF are shown in Fig.2. It can be seen that the surface of PGF is clean, while the TPGF has some layered solid material clung on the graphite fiber surface, which is supposed to be the products of graphite crystallite corrosion due to acid oxidation. The specific areas of PGF and TPGF obtained by BET single-point adsorption method are 0.31 m2/g and 0.45 m2/g, respectively. The increased specific area is also related with the concentrative sulphuric oxidation process.
3.3 Cyclic voltammogram
The cyclic voltammogram for the PGF electrode without modification obtained in l mol/L VOSO4+ l.6 mol/L H2SO4 solution is shown in Fig.3 (Curve l). Only the anodic (A′) and cathodic (B′) peaks associated with the VO2+/VO2+ couple can be observed in the sweep range from -1.0 to 1.5 V, the anodic (C′) and cathodic (D′) peaks corresponding to the redox V2+/V3+ couple can hardly be observed. Curve 2 in Fig.3 shows the cyclic voltammogram of the TPGF electrode in the acidic vanadium sulphuric solution in the potential range from -1.0 to 1.5 V. The anodic peak (A) at approximately 1.5 V is due to the oxidation of VO2+ to VO2+, whereas the cathodic peak (B) is corresponding to the reduction of VO2+ to VO2+. Further reduction of VO2+ to V3+ occurs at peak (F), the oxidation of V3+ to VO2+ occurring at peak (E). The oxidation and reduction of V2+/V3+ occur at peak (C) and peak (D), respectively.
Though the peak potential separation (Δ Ep) is up to 0.8 V, but the ratio of cathodic peak current (Ipc) to anodic peak current (Ipa) (Ipc/Ipa is about 1.0) for the VO2+/VO2+ couple indicates that the reaction is a quasi reversible process on TPGF electrode. But the peak potential separation (ΔEp) of 0.06 V for the V2+/V3+ couple indicates that the reaction is electrochemically reversible on TPGF electrode. Nevertheless, the smaller ΔEp and the higher peak current of reaction on the electrode surface indicate that the treated electrode materials performance good activity and complete reversibility in the acidic vanadium solution. It can be concluded that the PGF electrode after sulphuric acid dipping and heat treatment exhibits excellent electrochemical activity in the acidic vanadium sulphuric solution, and is suitable to be served as the electrode in VRB.
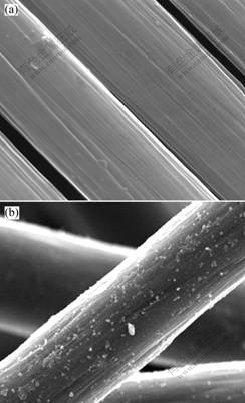
Fig.2 SEM images of different graphite fibers
(a) PGF; (b) TPGF
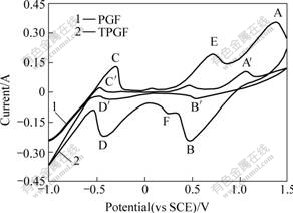
Fig.3 Cyclic voltammograms of PGF and TPGF at scan rate of 3 mV/s
It can be seen from FT-IR analysis that the functional groups of —COOH on the TPGF increase after treatment. The increased activity of TPGF for the vanadium redox reactions can thus be attributed to the increased surface concentration of the functional groups of —COOH induced during activation. The —COOH groups on the electrode surface probably behave as active sites, catalyzing the vanadium species reactions.
The mechanism of catalysis for reactions on the electrode surface can be hypothesized as follows.
In the positive half-battery, the reactions occur as:
![]()
It can be seen from this reaction that the charge and discharge processes at the positive electrode involve the transfer of an oxygen atom, which is likely to be the rate determining step in the overall mechanism. The availability of oxygen groups on the electrode surface would thus be expected to affect overall rate of the reactions. Thus, during charge the following processes occur.
Firstly, VO2+ ions transfer from the bulk solution to the electrode surface and ion-exchange with hydrogen ions of the carboxylic functional groups on the graphite surface, then bonds onto the electrode surface:
![]() (1)
(1)
Secondly, electron transfer occurs from the VO2+ to the electrode along the —C—O—O—V— bond as well as the transfer of one of the oxygen atoms on the C—O—O functional group to the VO2+ forming VO2+:
Finally, the VO2+ exchanges with H+ from solution and diffuses back into the bulk solution:
From the above hypothetical reaction sequence for the charge process involving the V(Ⅳ)V/(Ⅴ) redox couple, it can be seen that electron transfer reaction can be accelerated along the —C—O—O—V— bond, and oxygen transfer from the —COOH functional group will also be easier than directly from H2O. The rate of the overall process would thus be increased leading to reduced activation overpotential.
For the discharge process, the reverse reactions would occur, although the steric hindrance associated with the more bulky VO2+ ion attaching itself to a surface —C—O—O group would make this process more difficult than the charge process.
It can be concluded that acid and heat treatment produces the functional group of —COOH, and then the functional group catalyses the V(Ⅳ)/V(Ⅴ) redox reaction. The improvement in electrode activity is thus supposed to be due to —COOH functional groups produced by acid and heat treatment.
Fig.4 shows a typical series of cyclic voltammo- grams obtained in 1.0 mol/L VOSO4 and 1.6 mol/L H2SO4 solution at different scan rates. As the VO2+/VO2+ reaction is quasi reversible at the TPGF, the peak current Ip is given as follows[19]:
![]() (4)
(4)
where A is the electrode area, cm2; c0 is the bulk concentration of oxidant, mol/L; v is the potential scan rate, V/s; D0 is the diffusion coefficient of the oxidant; α is the charge transfer coefficient; n is the number of transfer electrons involved in the rate-determining step.
When the test temperature is kept at 25 ℃ and the electrode area is 1 cm2, Eqn.(4) reduces to:
![]() (5)
(5)
A plot of Ip vs v1/2 for the cyclic voltammograms from Fig.4 is shown in Fig.5, where Ip is the cathodic peak current of oxidation peaks of VO2+. Assuming αn equal to 0.5[19], the diffusion coefficient of VO2+ calculated from the slope of this straight line is 4.4×10-5 cm2/s. Compared with the value of 1.4?10-6 cm2/s at glassy-carbon electrodes reported by SUN et al[7], the TPGF presents better electrochemical performance for VO2+/VO2+ couple reaction.
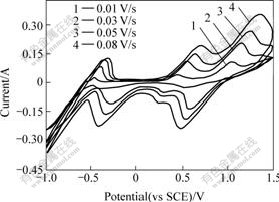

Fig.5 Relationship between anodic peak current Ip of VO2+/VO2+ and scan rate υ1/2
3.4 Effect of sulphuric acid concentration on treated electrode
The CV curves of electrolyte with different H2SO4
concentrations are shown in Fig.6. It can be seen that the redox peaks of the VO2+/VO2+ and V3+/VO2+ couples vary more markedly than the redox peaks of the V2+/V3+ couple in the electrolyte with l mol/L VOSO4 and the concentration of H2SO4 varying from 0.6 mol/L to 2.0 mol/L. When the concentration of H2SO4 reaches 2.0 mol/L, the redox peaks of the VO2+/VO2+ and V3+/VO2+ couples are higher and more distinct than that in the electrolyte with less H+, this can be explained from the reaction as follows:
![]()
![]()
H+ takes part in the reactions, while H+ doesn’t participate in the redox reaction of V2+/V3+couple:

Fig.6 Cyclic voltammograms of electrolyte with different H2SO4 concentrations
![]()
3.5 Effect of VOSO4 concentration
The EIS of the TPGF electrode in the electrolyte with the concentration of VOSO4 varying from 0.2 mol/L to 2.0 mol/L in 1.6 mol/L H2SO4 is shown in Fig.7. The resistance of solution (Rl) and resistane of charge transfer (Rct) as well as the double-layer capacitance (Cd) are fitted and listed in Table 1. The equivalent circuit of graphite felt electrodes at 100 mV is shown in Fig.8. It can be seen that the solution resistance and the double-layer capacitance arise, while the charge transfer resistance decreases with the increase of VOSO4 concentration, but the charge transfer resistance decreases more rapidly than the solution resistance increasing. These experimental results show that TPGF electrode behaves low polarization in the high concentration of VOSO4, which can be beneficial to enhancing the performance of VRB.
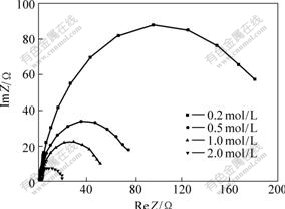
Fig.7 EIS of character with different VOSO4 concentrations
Table 1 Character parameter of electrode with different VOSO4 concentrations

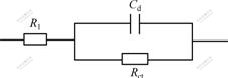
Fig.8 Equivalent circuit of graphite felt electrodes at 100 mV
4 Conclusions
1) The TPGF modified with sulphuric acid and heat treatment exhibits a great improvement in electro- chemical activity in acidic vanadium sulphuric solution, which increases the number of —COOH functional groups on the surface of the PGF, and is suitable to be served as the electrode in VRB.
2) The surface of TPGF is eroded by concentrated sulphuric acid oxidation, and the specific surface area increases from 0.31 m2/g to 0.45 m2/g.
3) The diffusion coefficients of the oxidation for VO2+ at TPGF obtained from the scope of Ip vs v1/2 is 4.4?10-5 cm2/s. Concentration of sulphuric acid effects the activity for VO2+/VO2+ and V3+/VO2+ redox couple due to H+ taking part in these reactions. The TPGF electrode behaves low polarization in the high concentration of VOSO4.
4) The increased activity of TPGF for the vanadium redox reactions can be attributed to the increased surface and the concentration of the functional groups of —COOH. The —COOH groups on the electrode surface probably behave as active sites, catalyzing the vanadium species reactions, which accelerate electron transfer reaction and oxygen transfer.
References[1] SKYLLAS-KAZACOS M, RYCHCIK M, ROBINS R, et al. New all-vanadium redox cell[J]. J Electrochem Soc, 1986, 133(5): 1057-1058.
[2] SUM E, SKYLLAS-KAZACOS M. A study of the V(II)/V(III) redox couple for redox flow cell applications[J]. Journal of Power Sources, 1985, 15(2/3): 179-190.
[3] SUM E, RYCHCIK M, SKYLLAS-KAZACOS M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a reodx battery[J]. Journal of Power Sources, 1985, 16(1): 85-95.
[4] FABJAN C, GACHE J, HARRER B. The vanadium redox-battery: an efficient storage unit for photovoltaic systems[J]. Electrochimica Acta, 2001, 47(5): 825-831.
[5] JOERISSEN L, GARCHE J, FABJAN C. Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems[J]. Journal of Power Sources, 2004, 127(1/2): 98-104.
[6] RYCHCIK M, SKYLLAS-KAZAOCS M. Evaluation of electrode materials for all-vanadium redox flow cell[J]. Journal of Power Sources, 1987, 19(1): 45-54.
[7] SUN B T, SKYLLAS-KAZACOS M. Modification of graphite electrode materials for vanadium redox flow cell applications—thermal treatment[J]. Electrochimca Acta, 1992, 37(7): 1253-1269.
[8] HADDADI-ASL V, KAZACOS M, SKYLLAS-KAZACOS M. Conductive carbon-polypropylene composite electrodes for vanadium redox flow battery[J]. J Appl Electrochem, 1995, 25(1): 29-33.
[9] RYCHCIK M, SKYLLAS-KAZACOS M. Evaluation of electrode materials for all-vanadium redox flow cell[J]. Journal of Power Sources, 1987, 19(1): 45-54.
[10] HUANG Ke-long, WU Qiu-mei, LIU Su-qin. Performance of graphite power-carbon black composite electrodes for the vanadium redox flow battery[J]. Chinese Journal of Power Source, 2004, 28(2): 91-93. (in Chinese)
[11] LI Xiao-gang, HUANG Ke-long, LIU Su-qin. Properties of the current collector of all vanadium redox flow battery[J]. Battery Bimonthly, 2005, 35(2): 93-94. (in Chinese)
[12] WU Qiu-mei, HUANG Ke-long, LIU Su-qin, et al. Study of PAN-graphite felt electrode in the vanadium redox flow battery[J]. Chinese Journal of Power Source, 2005, 29(7): 456-458. (in Chinese)
[13] RYU Y G, PYUN S I, KIM C S, et al. A study on the formation of surface functional groups during oxygen reduction on a platinum-dispersed carbon electrode in an 85% H3PO4 solution at elevated temperature[J]. Carbon, 1998, 36(3): 293-298.
[14] HUANG Ke-long, TAN Ning, LIU Su-qin, et al. Reaction mechanism of V(IV)/V(V) redox couple at graphite felt electrode[J]. The Chinese Journal of Nonferrous Metals, 2006, 14(5): 871-876.(in Chinese)
[15] LI Xu, HORITA K. Electrochemical characterization of carbon black subjected to RF oxygen plasma[J]. Carbon, 2000, 38(1): 133-138.
[16] SANTIAGO M, FORTUNY A, FABREGAT A, et al. Modified activated carbons for catalytic wet air oxidation of phenol[J]. Carbon, 2005, 43(1): 2134-2145.
[17] JUREWICZ K, BABEL K, LKOWSHI A, et al. Ammoxidation of active carbons for improvement of supercapacitor characteristics[J]. Electrochimica Acta, 2003, 48(11): 1491-1498.
[18] JACOBSON N S, CURRY D M. Oxidation microstructure studies of reinforced carbon/carbon[J]. Carbon, 2006, 44(7): 1142-1150.
[19] BARD A J, FAULKNER L R. Electrochemical Methods Fundamentals and Applications [M]. 2nd ed. New York: John Wiley & Sons, 2001.
(Edited by YANG You-ping)
Foundation item: Project (03GKY3015) supported by the Foundation of Hunan Provincial Department of Science and Technology
Received date: 2006-05-21; Accepted date: 2006-07-27
Corresponding author: HUANG Ke-long, Professor; Tel: +86-731-8879850; E-mail: klhuang@mail.csu.edu.cn
- Electrochemical behavior of diverse vanadium ions at modified graphite felt electrode in sulphuric solution


