Trans. Nonferrous Met. Soc. China 25(2015) 471-476
Sol-gel preparation, corrosion resistance and hydrophilicity of Ta-containing TiO2 films on Ti6Al4V alloy
Tao FU1, Jian-min SUN1, Zafer ALAJMI1, Feng WU2
1. Key Laboratory of Biomedical Information Engineering of Ministry of Education,
School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, China;
2. School of Medicine, Xi’an Jiaotong University, Xi’an 710061, China
Received 28 March 2014; accepted 9 September 2014
Abstract:
Ta-containing TiO2 films with Ta contents of 5%, 20%, 33% (mole fraction) were sol-gel coated on the surface roughened Ti6Al4V alloy by dip coating method for biomedical applications. The Ta-TiO2 films on 1.5 mol/L NaOH-HCl pretreated substrate are adherent, but there are cracks for the sample with 33% Ta. X-ray photoelectron spectroscopy results show that Ti and Ta exist as TiO2 and Ta2O5 in the film, and Al element is not detectable. X-ray diffraction and Raman scattering analyses reveal that the addition of Ta decreases crystallization of the films. Potentiodynamic polarization test in a Ca-free Hank’s balanced solution demonstrates that the coating samples markedly improve the corrosion resistance compared with the polished sample. The addition of Ta impedes UV light-induced hydrophilic conversion of the coating samples. The sample with 20% Ta has enough film integrity and hydrophilic conversion rate, and is expected to possess good biological properties.
Key words:
titanium; TiO2; sol-gel; tantalum; corrosion; hydrophilicity;
1 Introduction
Titania films are widely studied as a material for surface modification of titanium alloys and other biomedical metals due to their high corrosion resistance, good biocompatibility, photo-induced hydrophilicity, antibacterial property, etc [1-3]. Among the methods to prepare TiO2 films, sol-gel coating has advantages of the independence of substrate shape, good control of coating composition, thickness and topography, the ease in dopant introduction, and the relative ease of processing.
The studies on tantalum and tantala as biomaterials have drawn much attention in recent decades. Endothelial cells grow much better on tantalum, tantalum oxide, and Ta-containing TiO films [4,5]. Tantalum and its oxide films show bioactivity and excellent cyto- compatibility to osteoblast cells [6]. Ta-containing TiO2 (Ta-TiO2) films have better blood compatibility than TiO2 film [7,8]. The NiTi alloy implanted with Ta has much lower Ni release and better cyto-compatibility [9]. The corrosion resistant Ta-TiO2 film and pure Ta coating can provide good protection for the metallic substrates [10,11].
With the excellent mechanical properties and low density, Ti6Al4V alloy has been used to manufacture various kinds of medical implants especially in orthopedic fields [12,13]. However, corrosion of the alloy and the possible release of toxic and carcinogenetic Al and V ions are a concerned problem for Ti6Al4V implants that will service in human body for a long period of time. In this work, Ta-containing TiO2 films on Ti6Al4V alloy are prepared by dip coating method, and the NaOH-HCl pretreatment [2] is also used to improve structural integrity of the Ta-TiO2 films. Microstructure, chemical composition, corrosion resistance and hydrophilicity of the Ta-TiO2 film coated Ti6Al4V alloy samples are investigated.
2 Experimental
Ti6Al4V alloy plates (dimensions 10 mm x 15 mm x 2 mm) were polished with SiC papers down to grits 1200, ultrasonically cleaned in acetone, ethanol, deionized (DI) water in sequence, and dried in air. The plates were then treated in 1.5 mol/L NaOH solution at 60 °C for 24 h.
The alkali treated Ti6Al4V samples were rinsed in DI water several times, and soaked in dilute HCl solution (pH ~4) at room temperature for 12 h. The evacuated samples were rinsed in DI water again and dried at 200 °C for several hours before sol-gel coating.
The TiO2 sol was prepared with tetrabutyl-orthotitanate, diethanolamine (DEA), water and ethanol, with the molar ratio of 1: 1: 1: 26.5 [2]. For the sol, 1 mmol Ti ions corresponded to 1.73 g or ~2 mL solution. The Ta2O5 sol was prepared by adding 1 mmol DEA and 1 mmol tantalum ethoxide into 26.5 mmol ethanol, with 1.68 g or ~2 mL solution obtained. The two sols were mixed with different mass ratios to obtain Ta-TiO2 sols with the Ta/(Ti+Ta) mole fractions of 5%, 20% and 33% (samples TC5, TC20, TC33). The sols were stirred for several minutes and aged at 0 °C for 24 h.
TiO2 and Ta-TiO2 films on the NaOH-HCl treated Ti6Al4V plates were prepared by dip coating method with a withdrawal speed of 2 mm/s. The gel coatings were dried at room temperature for 30 min and at 80 °C for 30 min. The dipping-drying process was repeated to increase the film thickness. The dried samples were then calcined in a programmed electric furnace at 400 °C for 10 min and at 500 °C for 10 min (temperature ramping rate 10 °C/min).
Surface morphology of the samples was observed with scanning electron microscope (SEM, FEI Quanta 600F). Elemental composition and chemical bonding state of the samples were examined with X-ray photoelectron spectroscope (XPS, Al Ka, VG K-Alpha). Phase structure of the samples was analyzed with X-ray diffraction (XRD, Cu Ka, Rigaku D/MAX-2400) and Raman scattering (Horiba HR 800, 633 nm).
Corrosion resistance of the Ti6Al4V samples was evaluated by potentiodynamic polarization test with an electrochemical workstation (Model CS150, Corrtest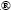 ) under ambient conditions. The electrolyte was a Ca-free Hank’s balanced salt solution (HBSS, NaCl 8.00 g/L, KCl 0.40 g/L, NaHCO3 0.34 g/L, KH2PO4 0.06 g/L, Na2HPO4·12H2O 0.12 g/L). A platinum electrode was used as counter electrode, and the saturated calomel electrode (SCE) was reference electrode. Contact angle of the samples was measured by injecting 5 mL DI water on the sample surface with a contact angle goniometer (JY-82) under ambient conditions. Some samples were subjected to UV illumination treatment (wave length centered at 253 nm) to increase the surface hydrophilicity.
) under ambient conditions. The electrolyte was a Ca-free Hank’s balanced salt solution (HBSS, NaCl 8.00 g/L, KCl 0.40 g/L, NaHCO3 0.34 g/L, KH2PO4 0.06 g/L, Na2HPO4·12H2O 0.12 g/L). A platinum electrode was used as counter electrode, and the saturated calomel electrode (SCE) was reference electrode. Contact angle of the samples was measured by injecting 5 mL DI water on the sample surface with a contact angle goniometer (JY-82) under ambient conditions. Some samples were subjected to UV illumination treatment (wave length centered at 253 nm) to increase the surface hydrophilicity.
3 Results and discussion
3.1 SEM, XPS, XRD and Raman analyses
Surface morphologies of the prepared Ti6Al4V samples are shown in Fig. 1. The 1.5 mol/L NaOH-HCl treated sample has a porous surface, with the pore size of 160-240 nm. The alkali treated titanium surface is composed of porous titanate hydrogel, and it can be converted to titania network by proton exchange during soaking in HCl solution. The pore size is near to the thickness of two-layered TiO2 film (~250 nm). Thus, this sample was selected for sol-gel coating to utilize the balancing effect of the porous surface on volume shrinkage of the gel films during the drying and calcination processes [2].

Fig. 1 SEM surface images of 1.5 mol/L NaOH-HCl treated Ti6Al4V sample (a), coating samples TC0 (b), TC20 (c) and TC33 (d)
The films of coating samples TC0, TC5 and TC20 are adherent and crack-free (Figs. 1(b), (c)), but cracks are observed for sample TC33 (Fig. 1(d)). Two possible reasons are responsible for the deteriorated structural integrity of TC33 film. First, the sol has high content of tantalum ethoxide, which is prone to hydrolysis during coating preparation. Second, the addition of tantalum ethoxide may increase viscosity of the sol, resulting in larger film thickness and more cracks of the film. It is reported that NiTi alloy implanted with Ta (peak concentration 20%) has enhanced corrosion resistance and better cyto-compatibility [9,14]. Thus, sample TC20 with enough Ta content and structural integrity is expected to have good biological properties.
Chemical composition of samples TC0 and TC33 was analyzed by XPS, with the Ti 2p, Ta 4f and O 1s spectra shown in Fig. 2. Titanium on the surface presents mainly in the form of TiO2, with the peaks located at binding energies of 464.3 eV (Ti 2p1/2) and 458.5 eV (Ti 2p3/2). The Al signal is undetectable (not shown), revealing the full coverage of the alloy substrate. In Ta 4f spectrum, the peaks at 28.0 eV (4f5/2) and 26.1 eV (4f7/2) indicate that Ta is in the form of Ta2O5. The O 1s spectrum can be deconvoluted into three peaks. The subpeak 1 (P1) at 529.9 eV is attributed to TiO2, the subpeak 2 (P2) at ~530.7 eV is associated with Ti2O3 and Ta2O5 [2,15], and the weak subpeak 3 (P3) at 532.1 eV is related to hydroxyl groups. The hydroxyl groups on the surface will increase the hydrophilicity of the coating samples.
Phase structure of the Ti6Al4V samples is analyzed by XRD (Fig. 3). For sample TC0, anatase diffraction peaks are present. The diffraction comes from the sol-gel TiO2 film as well as the NaOH-HCl and heat treated substrate, for weak anatase peak is also detected (2q=25.3°) when the NaOH-HCl treated sample has been subjected to similar heat treatment (Fig. 3(a)). The anatase peak at 2q=25.3° is weak for sample TC20, and it nearly disappears for sample TC33. The addition of Ta seems to have suppressed crystallization of the TiO2 films. This agrees with the report that Ta2O5 hindered the crystallization and crystal growth of sol-gel derived TiO2 powder [16].
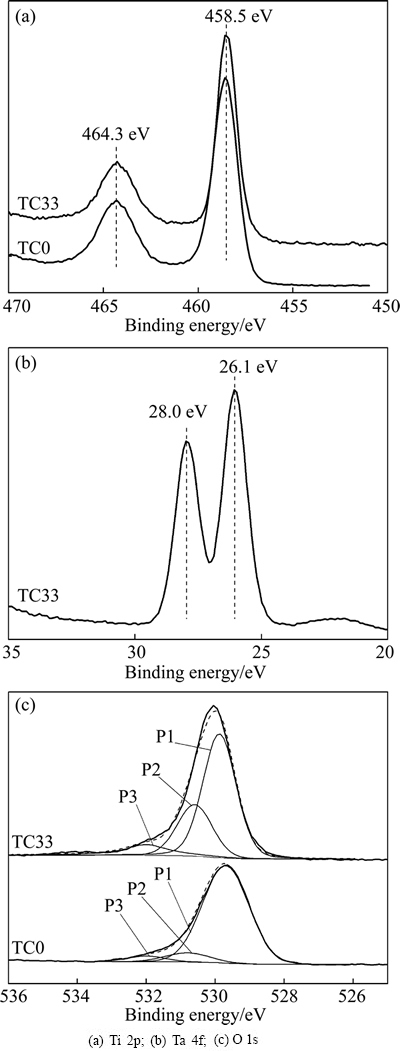
Fig. 2 XPS spectra of coating samples
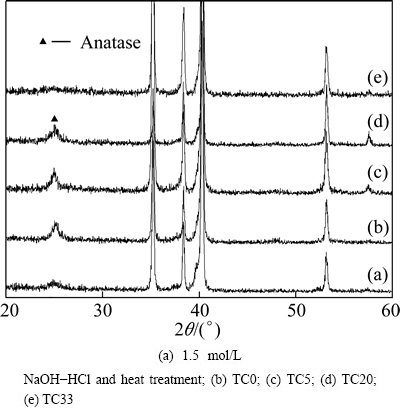
Fig. 3 XRD patterns of Ti6Al4V samples
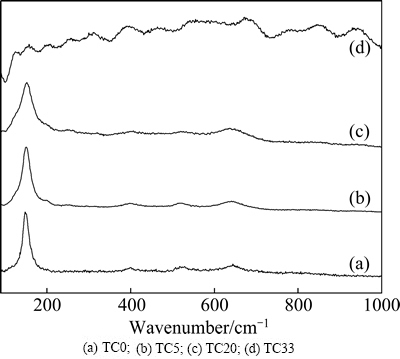
Fig. 4 Normalized Raman spectra of coating samples
Phase structure of the coated Ti6Al4V samples is further examined by Raman scattering analysis (Fig. 4). The peaks at 150, 198, 400, 521 and 640 cm-1 are ascribed to anatase TiO2 [17]. The characteristic peak at 150 cm-1 becomes wider with the increase of Ta content, and the FWHM (full width at half maximum) value is 18, 24, 33 cm-1 for sample TC0, TC5, TC20, respectively. Anatase peaks are not present for sample TC33, and the spectrum is likely random. Thus, the Raman spectra also reveal that the addition of Ta has decreased crystallization of the TiO2 films.
3.2 Corrosion and hydrophilicity tests
Corrosion resistance of the Ti6Al4V samples is evaluated by potentiodynamic polarization test in the Ca-free HBSS. It can be seen from the polarization curves (Fig. 5) that the polished sample has lower corrosion potential (φcorr) and smaller corrosion current density (Jcorr) than the coated samples (Table 1). For the polished sample, a stable but very large passive current density (Jpass=1.968 mA/cm2) and a high pitting potential (φpit=1.36 V) are obtained after a long range of anodic polarization (Table 1). During the anodic polarization, the dissolution of Al and V elements leads to the increase of anodic current, and the current becomes stable when a resistant titania film is formed on the sample surface. The free corrosion potential of Ti alloys in vivo lies in the range of 450-550 mV (vs SHE) (or 206-306 mV (vs SCE), [18]). The polarization of the polished sample is instable in this potential range, suggesting that its corrosion resistance is not sufficiently high for the implant applications.
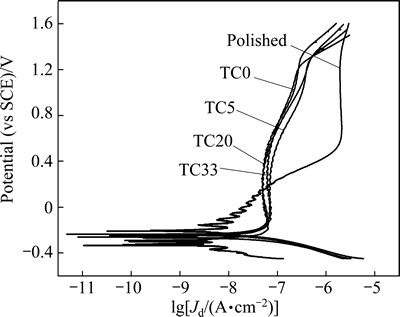
Fig. 5 Potentiodynamic polarization plots of polished and coated Ti6Al4V samples
Table 1 Corrosion test data of Ti6Al4V samples
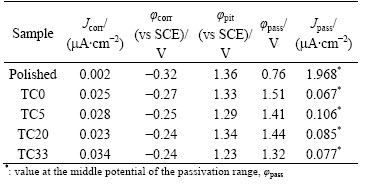
The four coating samples have similar polarization behavior, and their corrosion data are very close (Table 1). For sol-gel Ta-TiO2 films on surface roughened NiTi alloy, the passive current density of the samples decreases with increasing the content of Ta, even by nearly 2 orders of magnitude [19]. This is related to the increased electric resistivity and the enlarged film thickness by the addition of Ta [10,20]. For the present Ti6Al4V samples, the roughened surface layer with residual Al and V may play crucial roles in corrosion behavior of the samples. The related study will be carried out by the polarization and the immersion corrosion tests in the simulated body fluid. Anyway, the four coating samples have much longer passivation ranges (φpass) and much lower passive current densities (Jpass) compared with the polished one. The Ta-TiO2 films have effectively improved the corrosion resistance of Ti6Al4V alloy, which is beneficial for its biocompatibility.
The hydrophilic surface is beneficial for biological properties of materials, e.g., hemo-compatibility and bone-bonding behavior [21,22]. Water contact angles of the coated Ti6Al4V samples are shown in Fig. 6. For sample TC0, the contact angle decreases drastically from 80° to ~10° after 1 h UV illumination, illustrating the photo-induced hydrophilicity of TiO2 film [23]. Samples TC5 and TC20 have contact angles <20° after 1 h illumination. For sample TC33, the contact angle is still above 20° after 3 h illumination treatment. The addition of Ta has impeded the hydrophilic conversion of TiO2 films, especially for sample TC33, which is likely related to the decreased film crystallization. It is reported that the amorphous component in TiO2 films prepared by electron beam evaporation makes their UV-induced hydrophilicity somewhat inferior to the well-crystallized anatase TiO2 film [24]. The hydrophilic conversion of sample TC20 is only slightly influenced by the addition of Ta, and this sample is expected to possess good biological properties.
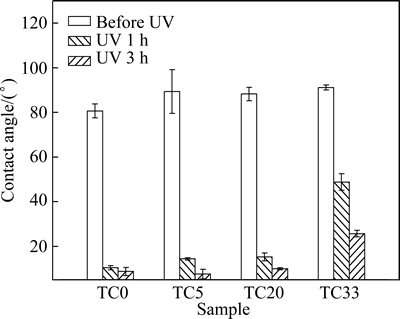
Fig. 6 Contact angles of coated Ti6Al4V samples at different UV irradiation time
4 Conclusions
Ta-containing TiO2 films with the Ta contents of 5%, 20% and 33% were sol-gel coated on surface roughened Ti6Al4V alloy by dip coating method. The Ta-TiO2 films on 1.5 mol/L NaOH-HCl pretreated substrate are adherent, but there are cracks for sample TC33. Ti and Ta exist as TiO2 and Ta2O5 in the films, and Al element is not detectable. The addition of Ta decreases crystallization of the TiO2 films. The coating samples markedly improve corrosion resistance compared with the polished one. The addition of Ta impedes UV light-induced hydrophilic conversion of the coating samples. Sample TC20 has enough film integrity and hydrophilic conversion rate, and is expected to possess good biological properties.
References
[1] KARPAGAVALLI R, ZHOU A H, CHELLAMUTHU P, NGUYEN K. Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V [J]. Journal of Biomedical Materials Research A, 2007, 83: 1087-1095.
[2] FU T, LIU B G, ZHOU Y M, WU X M. Sol-gel titania coating on NiTi alloy with a porous titania film as interlayer [J]. Journal of Sol-Gel Science & Technology, 2011, 58: 307-311.
[3] LIANG Cheng-hao, GUO Liang, CHEN Wan, LIU Jing-xiao. Electrochemical behavior of SUS316L stainless steel after surface modification [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(2): 398-401.
[4] LENG Y X, CHEN J Y, YANG P, SUN H, WANG J, HUANG N. The biocompatibility of the tantalum and tantalum oxide films synthesized by pulse metal vacuum arc source deposition [J]. Nuclear Instruments & Methods in Physics Research Section B, 2006, 242(1-2): 30-32.
[5] CHEN J Y, LENG Y X, ZHANG X, YANG P, SUN H, WANG J, ZHAO A S, CHU P K, HUANG N. Effect of tantalum content of titanium oxide film fabricated by magnetron sputtering on the behavior of cultured human umbilical vein endothelial cells (HUVEC) [J]. Nuclear Instruments & Methods in Physics Research Section B, 2006, 242(1-2): 26-29.
[6] MENG F H, LI Z H, LIU X Y. Synthesis of tantalum thin films on titanium by plasma immersion ion implantation and deposition [J]. Surface & Coatings Technology, 2013, 229: 205-209.
[7] YANG W M, LIU Y W, ZHANG Q, LENG Y X, ZHOU H F, YANG P, CHEN J Y, HUANG N. Biomedical response of tantalum oxide films deposited by DC reactive unbalanced magnetron sputtering [J]. Surface & Coatings Technology, 2007, 201: 8062-8065.
[8] CHEN J Y, LENG Y X, TIAN X B, WANG L P, HUANG N, CHU P K, YANG P. Antithrombogenic investigation of surface energy and optical bandgap and hemo-compatibility mechanism of Ti(Ta5+)O2 thin films [J]. Biomaterials, 2002, 23: 2545-2552.
[9] ZHAO T T, YANG R X, ZHONG C, LI Y, XIANG Y. Effective inhibition of nickel release by tantalum-implanted TiNi alloy and its cyto-compatibility evaluation in vitro [J]. Journal of Materials Science, 2011, 46: 2529-2535.
[10] LEVESQUE A, BOUTEVILLE A, de BAYNAST H, LAVEISSIERE B. Evaluation of corrosion behaviour of tantalum coating obtained by low pressure chemical vapor deposition using electrochemical polarization [J]. Journal de Physique IV, 2002, 12(PR4): 69-74.
[11] ZHOU Y, LI M, CHENG Y, ZHENG Y F, XI T F, WEI S C. Tantalum coated NiTi alloy by PIIID for biomedical application [J]. Surface & Coatings Technology, 2013, 228: s2-s6.
[12] HOFFMANN B, FELDMANN M, ZIEGLER G. Sol-gel and precursor-derived coatings with cover function on medical alloys [J]. Journal of Materials Chemistry, 2007, 17: 4034-4040.
[13] ADVINCULA M C, PETERSEN D, RAHEMTULLA F, ADVINCULA R, LEMONS J E. Surface analysis and biocorrosion properties of nanostructured surface sol-gel coatings on Ti6Al4V titanium alloy implants [J]. Journal of Biomedical Materials Research B, 2007, 80: 107-120.
[14] LI Y, ZHAO T T, WEI S B, XIANG Y, CHEN H. Effect of Ta2O5/TiO2 thin film on mechanical properties, corrosion and cell behavior of the NiTi alloy implanted with tantalum [J]. Materials Science and Engineering C, 2010, 30(8): 1227-1235.
[15] PEARCE S J, CHARLTON M D B, HILTUNEN J, PUUSTINEN J, LAPPALAINEN J, WILKINSON J S. Structural characteristics and optical properties of plasma assisted reactive magnetron sputtered dielectric thin films for planar waveguiding applications [J]. Surface & Coatings Technology, 2012, 206: 4930-4939.
[16] MOHAMMADI M R, FRAY D J, SADRNEZHAAD S K, MOHAMMADI A. A simple particulate sol-gel route to synthesize nanostructural TiO2-Ta2O5 binary oxides and their characteristics [J]. Materials Science and Engineering B, 2007, 142: 16-27.
[17] CHANG H, HUANG P J. Thermo-Raman studies on anatase and rutile [J]. Journal of Raman Spectroscopy, 1998, 29(2): 97-102.
[18] HOAR T P, MEARS D C. Corrosion-resistant alloys in chloride solutions—Materials for surgical implants [J]. Proceedings of the Royal Society of London Series A—Mathematical and Physical Sciences, 1966, 294: 486-510.
[19] DONG B H, WU F, ALAJMI Z, ZHANG C, FU T, GE Y. Sol-gel derived Ta-containing TiO2 films on surface roughened NiTi alloy [J]. Rare Metals, 2014, 33(1): 21-27.
[20] LIU J X, CHEN J H, YANG D Z, WANG W Q, WANG Y N, CAI Y J. Characterization of TiO2/Ta2O5 films synthesized by ion beam on NiTi alloy for biomedical applications [J]. Journal of Materials Science & Technology, 2001, 17: s35-s39.
[21] CHUN Y, LEVI D S, MOHANCHANDRA K P, CARMAN G P. Superhydrophilic surface treatment for thin film NiTi vascular applications [J]. Materials Science and Engineering C, 2009, 29: 2436-2441.
[22] UENO T, YAMADA M, SUZUKI T, MINAMIKAWA H, SATO N, HORI N, TAKEUCHI K, HATTORI M, OGAWA T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model [J]. Biomaterials, 2010, 31: 1546-1557.
[23] FENG X J, ZHAI J, JIANG L. The fabrication and switchable superhydro-phobicity of TiO2 nanorod films [J]. Angewandte Chemie—International Edition, 2005, 44(32): 5115-5118.
[24] YANG T S, SHIU C B, WONG M S. Structure and hydrophilicity of titanium oxide films prepared by electron beam evaporation [J]. Surface Science, 2004, 548(1-3): 75-82.
Ti6Al4V合金表面含钽TiO2薄膜的溶胶-凝胶法制备、耐蚀性和亲水性
付 涛1,孙见敏1,Zafer ALAJMI1, 吴 锋2
1. 西安交通大学 生命科学与技术学院,生物医学信息工程教育部重点实验室,西安 710049;
2. 西安交通大学 医学院,西安710061
摘 要:配制含5%、20%和33%(摩尔分数)钽的二氧化钛溶胶,采用浸提法在表面粗化的Ti6Al4V合金表面涂覆含钽TiO2溶胶-凝胶薄膜。经过1.5 mol/L NaOH-HCl预处理基体表面的含钽TiO2薄膜附着较好,但含33%Ta的试样出现了裂纹。X射线光电子谱分析表明,钛和钽在薄膜中以TiO2、Ta2O5形式存在,未检测到铝元素。X射线衍射和拉曼散射分析表明,钽的加入降低了膜层的结晶性。在无钙Hank’s平衡盐液中的动电位极化实验表明,与抛光试样相比,涂层试样的耐蚀性显著改善。钽的加入对涂层试样的紫外光致亲水性转变有阻碍作用。含20% Ta的试样具有足够的膜层完整性和亲水性转化速率,可望具有较好的生物学性能。
关键词:钛;二氧化钛;溶胶-凝胶;钽;腐蚀;亲水性
(Edited by Xiang-qun LI)
Foundation item: Project (xjj2011096) supported by the Fundamental Research Fund for the Central Universities, China; Projects (50901058, 51374174) supported by the National Natural Science Foundation of China
Corresponding author: Tao FU; Tel: +86-29-82669021; E-mail: taofu@mail.xjtu.edu.cn
DOI: 10.1016/S1003-6326(15)63626-3
Abstract: Ta-containing TiO2 films with Ta contents of 5%, 20%, 33% (mole fraction) were sol-gel coated on the surface roughened Ti6Al4V alloy by dip coating method for biomedical applications. The Ta-TiO2 films on 1.5 mol/L NaOH-HCl pretreated substrate are adherent, but there are cracks for the sample with 33% Ta. X-ray photoelectron spectroscopy results show that Ti and Ta exist as TiO2 and Ta2O5 in the film, and Al element is not detectable. X-ray diffraction and Raman scattering analyses reveal that the addition of Ta decreases crystallization of the films. Potentiodynamic polarization test in a Ca-free Hank’s balanced solution demonstrates that the coating samples markedly improve the corrosion resistance compared with the polished sample. The addition of Ta impedes UV light-induced hydrophilic conversion of the coating samples. The sample with 20% Ta has enough film integrity and hydrophilic conversion rate, and is expected to possess good biological properties.


