
J. Cent. South Univ. (2018) 25: 1612-1618
DOI: https://doi.org/10.1007/s11771-018-3853-1
Biodegradation of ethylthionocarbamates by a mixed culture of iron-reducing bacteria enriched from tailings dam sediments
CHEN Shao-hua(陈绍华), SUN Yan(孙燕), XIONG Ling(熊玲)
Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission andMinistry of Education, College of Resources and Environmental Science,South-Central University for Nationalities, Wuhan 430074, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
Ethylthionocarbamates (ETC), which is the most widely used as collectors in the flotation of sulfide, is known to cause serious pollution to soil and groundwater. The potential biodegradation of ETC was evaluated by applying a mixed culture of iron-reducing bacteria (IRB) enriched from tailings dam sediments. The results showed that ETC can be degraded by IRB coupled to Fe(III) reduction, both of which can be increased in the presence of anthraquinone-2,6-disulfonate (AQDS). Moreover, Fe(III)-EDTA was found to be a more favorable terminal electron acceptor compared to α-Fe2O3, e.g., within 30 d, 72% of ETC was degraded when α-Fe2O3+AQDS was applied, while it is 82.67% when Fe(III)-EDTA+AQDS is added. The dynamic models indicated that the kETC degradation was decreased in the order of Fe(III)-EDTA+AQDS>α-Fe2O3+AQDS>Fe(III)-EDTA>α-Fe2O3, with the corresponding maximum biodegradation rates being 2.6, 2.45, 2.4 and 2.0 mg/(L·d), respectively, and positive parallel correlations could be observed between kFe(III) and kETC. These findings demonstrate that IRB has a good application prospect in flotation wastewater.
Key words:
ethylthionocarbamates; biodegradation; iron-reducing bacteria; anthraquinone-2,6-disulfonate;
Cite this article as:
CHEN Shao-hua, SUN Yan, XIONG Ling. Biodegradation of ethylthionocarbamates by a mixed culture of iron-reducing bacteria enriched from tailings dam sediments [J]. Journal of Central South University, 2018, 25(7): 1612–1618.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3853-11 Introduction
Ethylthionocarbamates (ETC), which has been widely used as reagents in sulfide mineral flotation for many decades [1],is highly toxic and very difficult to be degraded naturally. And these flotation reagents that remain in tailings and flotation effluents can cause severe environmental pollution and ecological issues [2–4]. The current flotation wastewater is predominantly controlled using physical and chemical methods. However, these methods have significant disadvantages, such as high energy consumption, high operating cost and may cause secondary pollution, thereby limiting their availability in industry. In contrast, biological strategy has been considered as the most promising and efficient option to address the flotation wastewater as it is simple, stable, cost effective, environmentally friendly properties.
Microbial dissimilatory Fe (III) reduction techniques have been applied in treating metal contaminated land and water [5, 6], oxidation of xenobiotics [7], nutrient release and even the generation of electricity from sediments [8, 9]. Compared to other electron acceptors such as NO3– and SO42–, Fe (III) has much lower toxicity and does not generate secondary contamination on sites [10]. Indeed, some reports have indicated that Fe(III) reduction accounts for up to 65% of the oxidation of organic matter in anaerobic sediments and a variety of subsurface environments [9]. As iron-reducing bacteria (IRB), can utilize Fe(III) as the terminal electron acceptor and oxidize organic compounds especially toxic and refractory organic pollutants [11, 12], much attention has been focused on the process of dissimilatory iron reduction in anaerobic environments [11], and Fe(III) oxide, which can be the most dominant terminal electron acceptor of IRB, is abundant in mine soil and floatation wastewater. In addition, IRB is capable of being readily identified and isolated from tailings dam sediments and mine soil. Therefore, IRB has a great potential in bioremediation of ETC that contaminated in mine soil or floatation wastewater, using Fe(III) as the terminal electron acceptor. In the present investigation, the potential biodegradation of ETC was evaluated by applying a mixed culture of iron-reducing bacteria (IRB) enriched from tailings dam sediments under anaerobic conditions. And the impact of AQDS on the biodegradation of ETC with different forms of Fe(III) were also illustrated.
2 Materials and methods
AQDS (>98%) was purchased from Sigma- Aldrich. ETC was obtained from Zhuzhou Mineral Processing Reagent Plant (Zhuzhou, China). Yeast extract was obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Deionized water was used in the present work. All other reagents were analytical grade and were used as received.
2.1 Microbial source
Anaerobic sludge was obtained from tailing dam located in Huangshi city and used as a source of indigenous IRB.
2.2 Enrichment culture
The following composition of basal medium was used for IRB enrichment: NaHCO3, 2.5 g/L; NH4Cl, 0.25 g/L; CaCl2·H2O, 0.02 g/L; KCl,0.1 g/L; NaH2PO4·H2O, 0.68 g/L; yeast extract,0.05 g/L; a vitamin solution (1%, v/v) and trace elements solution (1%, v/v). It should be noted that trace elements solution contained 30 mg/L of CoCl2·6H2O, 0.15 mg/L of CuCl2, 5.7 mg/L of H3BO3, 20 mg/L of MnCl2·4H2O, 2.5 mg/L of Na2MoO4·2H2O, 1.5 mg/L of NiCl2·2H2O, and 2.1 mg/L of ZnCl2 [13]. In addition, ETC and 25 mmol/L α-Fe2O3 was added into medium as carbon source and electron acceptor,respectively. All media were sterilized by autoclaving for 20 min and cooled to room temperature under a constant stream of 80% N2-20% CO2.Enrichment of IRB was prepared in 500 mL serum bottles containing 100 mL ETC-Fe(III) medium and 100 mL sludge sample, and the serum bottles were sealed with butyl rubber stoppers and aluminum caps. The head space of the bottles was filled with high pure nitrogen gas (99.99%). The final pH of the medium was adjusted to 7.0.
During enrichment, the ETC concentration was gradually increased from 10 to 50 mg/L at a 12-day interval under incubation at 30 °C and 130 r/min. During each step, the mixture was transferred into new media at a portion of 10% as that of inoculum (v/v). Two months later, the IRB culture having capabilities of reducing Fe(III) and degrading ETC were obtained. Standard anaerobic techniques were used throughout and the samples were incubated in an anaerobic chamber with a N2 stream.
2.3 Experimental methods
Experiments were conducted in 500 mL serum bottles filled with 200 mL medium, then enriched bacteria were added to obtain a final concentration of 1.2 g mixed liquid suspended solids (MLSS)/L. Also, different amounts of ETC, α-Fe2O3 or Fe(III)-EDTA were added to each microcosm, and the final concentration was 30 mg/L, 40 mmol/L and 25 mmol/L, respectively. In addition, AQDS were added into the serum bottles to estimate its effect on biodegradation of ETC with different forms of Fe(III). Finally, the solution pH was adjusted to 7.0. All of the bottles were flushed with high purity nitrogen gas for 30 min to maintain an anaerobic condition [14]. The bottles were sealed with butyl rubber stoppers and aluminum caps. Syringes and needles were used for sample collection. In order to estimate abiotic ETC degradation, sterile control experiments were prepared under the same conditions and autoclaved for 20 min at 120 °C prior to the addition of ETC.
All experiments were carried out in a thermostated water bath at 130 r/min and 30 °C for 30 d in the dark. During this period, samples were periodically withdrawn to measure the concentration of ETC and ferrous. All the experiments were conducted in triplicate.
2.4 Analytical methods
A pH meter (ORZ0N818, USA) was employed for pH measurement. ETC concentration was analyzed at 241 nm using a UV-vis spectrophotometer (Shimadzu, Japan).
The concentration of adsorbed Fe(II) was determined by 1,10-phenanthroline colorimetric assay [15, 16] after Fe(II) was extracted from the samples using 0.5 mol/L HCl for 1.5 h while the dissolved Fe(II) was determined by spectrophotometry after filtrating the mineral and sorbed Fe(II) from the aqueous phase [17].
3 Results and discussion
3.1 Biodegradation of ETC under different Fe(III) forms and AQDS
Figure 1(a) shows that the decrease of ETC was negligible over a period of 30 d for the sterile control, indicating that the abiotic process did not play an important role in ETC abatement from the solution. This further suggests that the disappearance of ETC was primarily due to the biological process. ETC was observed to decrease gradually under unamended condition within 15 d and nearly leveled off in the following 15 d, achieving 25.13% degradation. However, when Fe(III) sources were added, an apparently greater degradation of ETC was observed, e.g. 55.6% and 72% were observed for the cases with α-Fe2O3 and α-Fe2O3+AQDS, respectively, probably due to the fact that α-Fe2O3 can act as a semiconductive solid that could enhance the interfacial reactions and electron transfer reaction [18–21]. Although α-Fe2O3 can stimulate the activity of IRB, the solubility of α-Fe2O3 at neutral condition is very low. In addition, numerous researches have demonstrated that IRB did not produce or release electron-shuttling compounds [22], therefore, this amendment cannot fully promote IRB to transfer electrons to the α-Fe2O3 surface [22]. However, AQDS as an electron shuttle is capable of accepting an electron from an Fe(III)-reducing microorganism and transferring it to the Fe(III) oxide surface, following by its regeneration to the oxidized form [9]. Nevertheless, the degradation of ETC at α-Fe2O3+AQDS condition is greater than that of α-Fe2O3 only.
In order to increase the degradation rate of ETC, soluble chelated Fe(III), which is more accessible to Fe(III) reductases [9, 23], rather than Fe(III) oxides was further investigated. Therefore, the effect of Fe(III)-EDTA as the terminal electron acceptor on the biodegradation of ETC was also studied (Figure 1(b)). From Figures 1(a) and (b), it could be concluded that the degradation rate of ETC was greater when AQDS was added into the Fe(III)-EDTA amended system, e.g. 66.67% and 82.67% for Fe(III)-EDTA and Fe(III)-EDTA+ AQDS amended conditions, respectively. The biodegradation of ETC in different amended conditions could be described by the pseudo-first- order kinetics, the biodegradation rate constant(k) were 0.0691, 0.0525, 0.0437 and 0.033 d–1 under Fe(III)-EDTA+AQDS,Fe(III)-EDTA+AQDS Fe(III)-EDTA and α-Fe2O3 amended conditions, respectively, which were 5.8, 4.4, 3.6, 2.8 times greater than the results without amendment. Under these four amended conditions, the k values of ETC were increased as Fe(III)- EDTA+AQDS>α-Fe2O3+ AQDS>Fe(III)-EDTA>α-Fe2O3, the corresponding maximum biodegradation rate was 2.6, 2.45, 2.4 and 2.0 mg/(L·d), respectively.
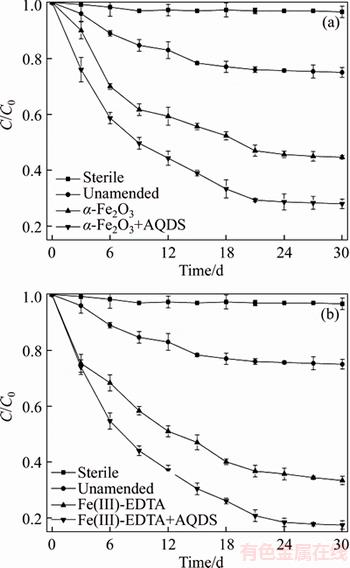
Figure 1 Effect of AQDS and α-Fe2O3 (a), Fe(III)-EDTA (b) on biodegradation of ETC
3.2 Fe(III) reduction under different amended conditions
The dissolved Fe(II) and total Fe(II) production under different amended conditions were investigated, and the results are shown in Figure 2.
Figures 1 and 2 show that Fe(III) reduction occurred when ETC degradation happened, indicating that the ETC degradation was coupled to Fe(III) reduction. However, in the sterile control experiments, no Fe(III) reduction was observed, further suggesting that the reduction of Fe(III) to Fe(II) is a biological transformation rather than a chemical reaction.

Figure 2 Dissolved Fe(II) (a) and total Fe(II) (b) production under different amended conditions
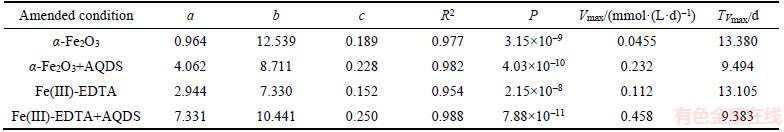
The dissolved Fe(II) concentrations increased to 0.8, 2.4, 3.87, 7.05 mmol/L after 21 d under conditions of α-Fe2O3, Fe(III)-EDTA, α-Fe2O3+ AQDS and Fe(III)-EDTA+AQDS amended, respectively. At the end of incubation, the highest dissolved Fe(II) concentrations were obtained in the presence of Fe(III)-EDTA+AQDS, followed by α-Fe2O3+AQDS, Fe(III)-EDTA and α-Fe2O3, which were 0.92, 2.7, 4.02 and 7.3 mmol/L, respectively, with the corresponding maximum reaction rates (Vmax) of Fe(III) reduction being 0.458, 0.232, 0.112 and 0.0455 mmol/(L·d), respectively (Table 1). From Figure 2(a), dissolved Fe(II) concentrations in the presence of AQDS were significantly enhanced as compared to that in the absence of AQDS, illustrating that AQDS can act as an electron shuttle that would further stimulate the microbial Fe(III) reduction rate [18, 24]. The reason why electron shuttles can accelerate the rate of Fe(III) reduction may be a combination of both processes, with IRB donating electrons to soluble shuttles substances that are more available than insoluble Fe(III) oxides. The microbially reduced intermediates could then transfer electrons to insoluble Fe(III) oxides more readily than IRB [16].
The rate of Fe(III) reduction was relatively lower under the condition of α-Fe2O3 amended, in comparison to Fe(III)-EDTA amended in the absence of AQDS, mainly because the reaction rate of insoluble α-Fe2O3 was limited by the availability of Fe(III) [16], which indicates that Fe(III)-EDTA was a more favorable terminal electron acceptor compared to α-Fe2O3 on the biodegradation of ETC by IRB.
Monitoring the amounts of total Fe(II) produced in the culture under different amended conditions (Figure 2(b)), not only could have underestimated total iron reduction, but could provide direct information that ETC biodegradation was dependent on the presence of Fe(III) oxide. After 30 d of inoculation, the total Fe(II) production with Fe(III)-EDTA amended was significantly larger than that with α-Fe2O3 amended, with the Fe(III) reduction ratios of 22.80% and 10.15%, respectively. In addition, when AQDS was added, a considerably increase in Fe(III) reduction occurred with Fe(III)-EDTA and α-Fe2O3, which ranged from 22.80% to 72.36% and from 10.15% to 49.20%, equivalent to approximately threefold and fivefold higher than that without AQDS, respectively. From Figures 2(a) and (b), we found that under condition of α-Fe2O3, Fe(III)-EDTA, α-Fe2O3+AQDS and Fe(III)-EDTA+AQDS amended, dissolved Fe(II) production only accounts for 22.66%, 29.61%, 32.68% and 40.35% of the total Fe(II) production, respectively, thus it can be seen that sorbed Fe(II) was the predominant form of Fe(II) produced from microbial reduction.
Table 1 Parameters for logistic models of dissolved Fe(II) accumulation under different amended conditions
The rates of Fe(III) reduction were calculated using the average Fe(III) reduction rate (denoted as kFe(III)) under condition of α-Fe2O3, Fe(III)-EDTA, α-Fe2O3+AQDS and Fe(III)-EDTA+AQDS amended, and the calculated values of kFe(III) were 0.135, 0.304, 0.410 and 0.603 mg/(L·d), respectively. The relationship between kFe(III) and biodegradation rate constant of ETC (denoted as kETC) under different amended conditions are shown in Figure 3.
Positive parallel correlations could be observed between kFe(III) and kETC, and higher kFe(III) indicates a higher kETC. This result further confirms that the biodegradation of TEC was coupled to ferric iron reduction.

Figure 3 Relationship between kFe(III) and kETC under different amended conditions
3.3 Effect of AQDS concentrations on biodegradation of ETC
Figure 4 indicates that there is no significant increase of the degradation rate of ETC with increased AQDS concentrations from 100 to 400 μmol/L under condition of Fe(III)-EDTA amended (the data of α-Fe2O3 amended was similar to Fe(III)-EDTA amended and not shown), mainly because only low concentrations of AQDS could continue increasing Fe(III)-oxide reduction through multiple reduction-oxidation cycles.
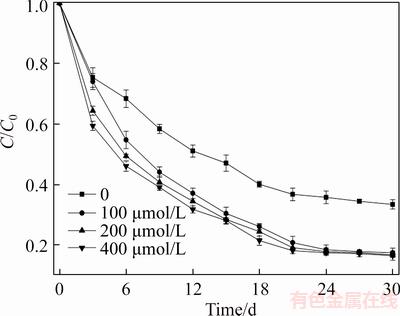
Figure 4 Effect of AQDS concentrations on biodegradation of ETC
3.4 Dynamics models
The logistic models were employed to simulate the relationship between dissolved Fe(II) accumulation and incubation time under different amended conditions [11]. And the logistic models can be expressed as Eq. (1):
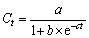 (1)
(1)
where Ct is the dissolved Fe(II) concentration at time t, a is the theoretical maximum concentration of dissolved Fe(II), b and c are constants. The reaction rate is expressed as Eq. (2):
 (2)
(2)
where the initial derivative of V is calculated based on Eq. (3):
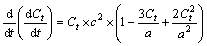 (3)
(3)
When the initial derivative of V is zero, then Ct can be calculated and is equal to 0.5 which can then be used in Eq. (2) to obtain the maximum reaction rate (Vmax) of Fe(III) reduction, i.e., 0.25ac. The incubation time to reach the Vmax is TVmax, which can be calculated from the logistic formula and is equal to
Table 1 shows that logistic model can accurately describe the accumulation and incubation time of dissolved Fe(II) under various amended conditions. In the logistic model, the biological meaning of a is the maximum accumulative concentration of dissolved Fe(II).Under the condition of α-Fe2O3, Fe(III)-EDTA, α-Fe2O3+AQDS and Fe(III)-EDTA+AQDS, the maximum concentrations of dissolved Fe(II) were 0.964, 2.944, 4.062 and 7.331 mmol/L, respectively, and the corresponding accumulative concentrations of dissolved Fe(II) were 0.92, 2.7, 4.02 and 7.3 mmol/L at the end of the incubation period, accounting for 95.44%, 91.71%, 98.97% and 99.58%, respectively. Additionally, we can see from Table 1 that AQDS addition increased kFe(III) in the α-Fe2O3 and Fe(III)-EDTA amended, these results confirm previous conclusion from Figure 1 and Figure 3. The large values of a and Vmax, and the low values of TVmax indicated that bacteria reduced Fe(III)-EDTA more rapidly than reduced α-Fe2O3.
4 Conclusions
1) IRB was capable of degrading ETC using Fe(III) as an electron acceptor, with the kFe(III) and kFe(III) being accelerated significantly by the addition of AQDS.
2) Compared to α-Fe2O3, Fe(III)-EDTA was found to be a more favorable terminal electron acceptor to degrade ETC by IRB.
3) Under four amended treatment conditions, logistic model can accurately describe the dissolved Fe(II) accumulation and incubation time, and the kETC were in the order of Fe(III)- EDTA+AQDS>α-Fe2O3+AQDS>Fe(III)-EDTA>α-Fe2O3, with the corresponding maximum biodegradation rates being 2.6, 2.45, 2.4 and 2.0 mg/(L·d), respectively.
References
[1] CHEN Shao-hua, DU Dong-yun, WU Yi-xue, XING Rui-rui. Degradation of ethylthionocarbamate by activated sludge coupled with interior microelectrolysis [J]. Desalination and Water Treatment, 2016, 57(45): 21437–21443. DOI: 10.1080/19443994.2015.1119743.
[2] CHEN Shao-hua, DU Dong-yun. Degradation of n-butyl xanthate using fly ash as heterogeneous Fentonlike catalyst [J]. Journal of Central South University, 2014, 21(4): 1448–1452. DOI: 10.1007/s11771-014-2084-3.
[3] CUI Kui-xin, HE Yue-hui, JIN Sheng-ming. Enhanced UV-visible response of bismuth subcarbonate nanowires for degradation of xanthate and photocatalytic reaction mechanism [J]. Chemosphere, 2016, 149: 245–253. DOI: 10.1016/j.chemosphere.2016.01.111.
[4] CHEN Xing-hua, HU Yue-hua, PENG Hong, CAO Xue-feng. Degradation of ethyl xanthate in flotation residues by hydrogen peroxide [J]. Journal of Central South University, 2015, 22(2): 495–501. DOI: 10.1007/ s11771-015-2548-0.
[5] ESTHER J, SUKLA L B, PRADHAN N, PANDA S. Fe (III) reduction strategies of dissimilatory iron reducing bacteria [J]. Korean Journal of Chemical Engineering, 2015, 32(1): 1–14. DOI: 10.1007/s11814-014-0286-x.
[6] PENG Lai, LIU Yi-wen, GAO Shu-hong, DAI Xiao-hu, NI Bing-Jie. Assessing chromate reduction by dissimilatory iron reducing bacteria using mathematical modeling [J]. Chemosphere, 2015, 139: 334–339. DOI: 10.1016/ j.chemosphere.2015.06.090.
[7] LOVLEY D R, ANDERSON R T. Influence of dissimilatory metal reduction on fate of organic and metal contaminants in the subsurface [J]. Hydrogeology Journal, 2000, 8(1): 77–88. http://works.bepress.com/derek_lovley/231.
[8] BOND D R, HOLMES D E, TENDER L M, LOVLEY D R. Electrode-reducing microorganisms that harvest energy from marine sediments [J]. Science, 2002, 295: 483–485. DOI: 10.1126/science.1066771.
[9] WILKINS M J, LIVENS F R, VAUGHAN D J, LLOYD J R. The impact of Fe(III)-reducing bacteria on uranium mobility [J]. Biogeochemistry, 2006, 78(2): 125–150. DOI: 10.1007/ s10533-005-3655-z.
[10] LI Zhi-ling,SUZUKI D,ZHANG Chun-fang,YANG Su-yin, NAN Jun, YOSHIDA N, WANG Ai-jie, KATAYAMA A. Anaerobic 4-chlorophenol mineralization in an enriched culture under iron-reducing conditions [J]. Journal of Bioscience and Bioengineering, 2014, 118(5): 529–532. DOI: 10.1016/j.jbiosc.2014.04.007.
[11] HE Jiang-zhou, QU Dong. Dissimilatory Fe(III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids [J]. Journal of Environmental Sciences, 2008, 20(9): 1103–1108.
[12] JIN Xin, WANG Fang, GU Cheng-gang, YANG Xing-lun, KENGARA F O,BIAN Yong-rong, SONG Yang, JIANG Xin. The interactive biotic and abiotic processes of DDT transformation under dissimilatory iron-reducing conditions [J]. Chemosphere, 2015, 138: 18–24. DOI: 10.1016/j. chemosphere.2015.05.020.
[13] DOU Jun-fen, LIU Xiang, DING Ai-zhong. Anaerobic degradation of naphthalene by the mixed bacteria under nitrate reducing conditions [J]. Journal of Hazardous Materials, 2009, 165(1–3): 325–331. DOI: 10.1016/j.jhazmat. 2008.10.002.
[14] FENG Chun-hua, YUE Xian-jun, LI Fang-bai, WEI Chao-hai. Bio-current as an indicator for biogenic Fe(II) generation driven by dissimilatory iron reducing bacteria [J]. Biosensors and Bioelectronics, 2013, 39: 51–56. DOI: 10.1016/j.bios.2012.06.037.
[15] LIU Tong-xu, LI Xiao-min, LI Fang-bai, ZHANG Wei, CHEN Man-jia, ZHOU Shun-gui. Reduction of iron oxides by Klebsiella pneumoniae L17: Kinetics and surface properties [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 379(1–3): 143–150. DOI: 10.1016/j.colsurfa. 2010. 11.061.
[16] XU Yan, HE Yan, FENG Xiao-li, LIANG Lu-yi, XU Jian-ming, BROOKES P C, WU Jian-jun. Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z [J]. Science of the Total Environment, 2014, 473–474: 215–223. DOI: 10. 1016/ j.scitotenv.2013.12.022.
[17] LI Fang-bai, LI Xiao-min, ZHOU Shun-gui, ZHUANG Li, CAO Fang, HUANG De-yin, XU Wei, LIU Tong-xu, FENG Chun-hua. Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide [J]. Environmental Pollution, 2010, 158: 1733– 1740. DOI:10.1016 /j.envpol.2009.11.020.
[18] ZHU Zhen-ke, TAO Liang, LI Fang-bai. 2-nitrophenol reduction promoted by S. putrefaciens 200 and biogenic ferrous iron: The role of different size-fractions of dissolvedorganic matter [J]. Journal of Hazardous Material, 2014, 279(3): 436–443. DOI: 10.1016/j.jhazmat. 2014.07.030.
[19] CAO Fang, LIU Tong-xu, WU Chun-yuan, LI Fang-bai, LI Xiao-min, YU Huan-yun, TONG Hui, CHEN Man-jia. Enhanced biotransformation of DDTs by an iron-and humic-reducing bacteria aeromonashydrophila HS01 upon addition of goethite and anthraquinone-2,6- disulphonicdisodium salt (AQDS) [J]. Journal of Agricultural and Food Chemistry, 2012, 60(45): 11238– 11244. DOI: 10.1021/jf303610w.
[20] LUAN Fu-bo, BURGOS W D, XIE Li, ZHOU Qi. Bioreduction of nitrobenzene, naturalorganic matter, and hematite by Shewanella putrefaciens CN32 [J]. Environmental Science & Technology, 2010, 44(1): 184–190. DOI: 10.1021/ es901585z@proofing.
[21] NEUMANN A, OLSON T L, SCHERER M M. Spectroscopic evidence for Fe(II)-Fe(III)electron transfer at clay mineral edge and basal sites [J]. Environmental Science & Technology, 2013, 47(13): 6969–6977. DOI: 10.1021/ es304744v.
[22] SHEN Wei-rong, CHEN Hong, PAN Shan-shan. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms [J]. Bioresource Technology, 2008, 99(7): 2483–2487. DOI: 10.1016/j.biortech.2007. 04.054.
[23] LOVLEY D R, WOODWARD J C. Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction [J]. Chemical Geology, 1996, 132(1–4): 19–24. DOI: https://doi.org/10.1016/S0009-2541(96)00037-X.
[24] LI Xiao-min, LIU Liang, LIU Tong-xu, YUAN Tian, ZHANG Wei, LI Fang-bai, ZHOU Shun-gui, LI Yong-tao. Electron transfer capacity dependence of quinone-mediated Fe(III) reduction and current generation by Klebsiella pneumoniae L17 [J]. Chemosphere, 2013, 92(2): 218–224. DOI: 10.1016/j.chemosphere. 2013. 01.098.
(Edited by HE Yun-bin)
中文导读
尾矿库底泥沉积物中异化铁还原混合菌群降解乙硫氨酯
摘要:乙硫氨酯是一种广泛使用的硫化矿捕收剂,其大量使用给土壤和水体造成了严重的污染。本文研究了尾矿库底泥沉积物中的异化铁还原混合菌群对乙硫氨酯的降解能力。结果表明:异化铁还原混合菌群可以有效地降解乙硫氨酯,并耦联着铁的还原,蒽醌-2,6-二磺酸钠的加入可以有效提高乙硫氨酯的降解速率和铁的还原速率。相对赤铁矿而言,EDTA络合铁是更好的电子受体,例如,在异化铁还原混合菌群降解乙硫氨酯的过程中,加入赤铁矿和蒽醌-2,6-二磺酸钠时,30 d的乙硫氨酯的去除率为72%,而加入EDTA络合铁和蒽醌-2,6-二磺酸钠时,30 d的去除率为82.67%。在加入EDTA络合铁和蒽醌-2,6-二磺酸钠、赤铁矿和蒽醌-2,6-二磺酸钠、EDTA络合铁和赤铁矿条件下,乙硫氨酯的最大生物降解速率分别为2.6, 2.45, 2.4 和2.0 mg/(L·d),铁的还原速率和乙硫氨酯的降解速率常数呈现很好的正相关性。研究表明异化铁还原菌在浮选废水处理方面具有良好的应用前景。
关键词:乙硫氨酯;生物降解;异化铁还原菌;蒽醌-2,6-二磺酸钠
Foundation item: Project(51708561) supported by the National Natural Science Foundation of China; Projects(CZP17097, CZW15037) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2017-02-16; Accepted date: 2017-04-05
Corresponding author: CHEN Shao-hua, PhD, Lecturer; Tel: +86-27–67843918; E-mail:csh0743@163.com; ORCID: 0000-0001- 8500-2635
Abstract: Ethylthionocarbamates (ETC), which is the most widely used as collectors in the flotation of sulfide, is known to cause serious pollution to soil and groundwater. The potential biodegradation of ETC was evaluated by applying a mixed culture of iron-reducing bacteria (IRB) enriched from tailings dam sediments. The results showed that ETC can be degraded by IRB coupled to Fe(III) reduction, both of which can be increased in the presence of anthraquinone-2,6-disulfonate (AQDS). Moreover, Fe(III)-EDTA was found to be a more favorable terminal electron acceptor compared to α-Fe2O3, e.g., within 30 d, 72% of ETC was degraded when α-Fe2O3+AQDS was applied, while it is 82.67% when Fe(III)-EDTA+AQDS is added. The dynamic models indicated that the kETC degradation was decreased in the order of Fe(III)-EDTA+AQDS>α-Fe2O3+AQDS>Fe(III)-EDTA>α-Fe2O3, with the corresponding maximum biodegradation rates being 2.6, 2.45, 2.4 and 2.0 mg/(L·d), respectively, and positive parallel correlations could be observed between kFe(III) and kETC. These findings demonstrate that IRB has a good application prospect in flotation wastewater.


