
Oxidation behavior of ternary Fe-15Cu-5Al and Fe-85Cu-5Al alloys in pure oxygen at 900 ℃
XIANG Jun-huai (向军淮)1,2,3, NIU Yan (牛 焱)2, DUAN Xian-zhi (段先志)1
1. Jiangxi Key Laboratory of Surface Engineering,
Jiangxi Science and Technology Normal University, Nanchang 330013, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China;
3. Key Laboratory of Ministry of Education for Low Dimensional Materials and Application Technology,
Xiangtan University, Xiangtan 411105, China
Received 28 July 2005; accepted 15 September 2006
Abstract:
The oxidation of two two-phase ternary Fe-Cu-Al alloys containing about 5%(mole fraction) aluminium, one Fe-rich and one Cu-rich, has been studied at 900 ℃ in 1×105 Pa pure oxygen. The Fe-rich Fe-15Cu-5Al alloy presents two quasi-parabolic stages, with a large decrease of the parabolic rate constant after about 50 min. The presence of 5% Al greatly reduces the oxidation rate of this alloy with respect to a binary Fe-Cu alloy of similar composition by forming an external protective Al2O3 layer, which is present near the scale/alloy interface. Due to the high stress-growth effect, this thin Al2O3 layer cannot completely prevent further oxidation of the alloy underneath. On the contrary, the Cu-rich Fe-85Cu-5Al alloy presents a single parabolic stage and forms a thick and porous external scale, coupled to the internal oxidation of Fe and Al. As a result, the oxidation of Cu-rich alloy at 900 ℃ is much faster than that of the Fe-rich alloy.
Key words:
Fe-15Cu-5Al alloy; Fe-85Cu-5Al alloy; ternary alloy; oxidation behavior ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;
1 Introduction
Multicomponent superalloys, often containing more than one phase, are becoming more and more important for practical high-temperature applications[1]. However, the oxidation theory of complex alloys has not yet been established systematically even for the relatively simple ternary systems due to the great complexity of the problem with respect to binary alloys. These include the thermodynamic behavior of the alloys, the nature of the diffusion process in the alloys and scales, the dependence of the solubility and diffusivity of the oxidant in the alloy, the nature and composition of the scales[2-3]. Recent studies of the oxidation of several ternary two-phase systems[4-6] have shown that the third element in the alloys may play a quite different role in the oxidation process.
The thermodynamic stabilities and the growth rates of the oxides of the three components involved in the present ternary Cu-Fe-Al systems are very different. Moreover, the components of the system do not form any intermetallic phase in the composition range examined. Therefore, the Fe-Cu-Al alloys can be considered typical model systems useful for the investigation of the oxidation mechanisms of ternary two-phase alloys. A recent study of the oxidation at 800 ℃ of Fe-15Cu-5Al and Fe-85Cu-5Al [7] showed that the addition of 5% Al significantly reduced the oxidation rate of the Fe-rich alloy by forming an external alumina scale with respect to a binary Fe-Cu alloy of similar composition. Conversely, the Cu-rich alloy Fe-85Cu-5Al oxidized much more rapidly, forming thick and porous external scales, coupled to the internal oxidation of aluminium. The aim of the present work is to examine the different oxidation behaviors of these two alloys at a higher temperature of 900 ℃, to clarify the third element effect in the oxidation process more clearly.
2 Experimental
Two different ternary alloys were prepared by repeatedly melting appropriate amounts of the three pure
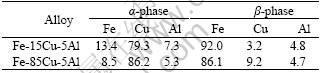
(99.8%) components under a Ti-gettered inert atmosphere using non-consumable tungsten electrodes. The alloy ingots were subsequently annealed for 28 h at 850 ℃ in a pure argon atmosphere to remove residual mechanical stresses and to achieve a better equilibration of the alloy phases. As shown in Fig.1, at room temperature the alloys Fe-15Cu-5Al and Fe-85Cu-5Al are composed of a mixture of two phases, including a Cu-rich solid solution (α-phase, light) and a Fe-rich solid solution (β-phase, dark) (Fig.2). The actual average composition ofαandβ-phase of Fe-15Cu-5Al and Fe-85Cu-5Al are shown in Table 1.
Samples with a size of 12 mm×6 mm×1.2 mm were oxidized at 900 ℃ under 1×105 Pa flowing pure O2. Continuous mass change measurements were carried out by a Cahn thermobalance mod. 2000 for 24 h. The oxidized samples were examined by means of X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray microanalysis attachment to the SEM (EDX).
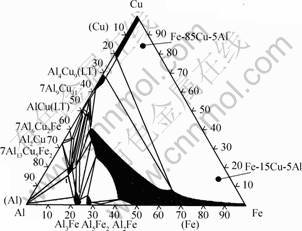
Fig. 1 Phase diagram of ternary Fe-Cu-Al system at 25 ℃
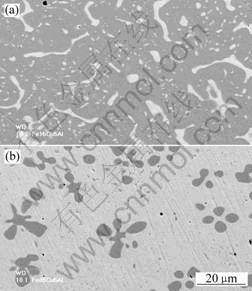
Fig. 2 Microstructures (SEM/BEI) of ternary Fe-15Cu-5Al (a) and Fe-85Cu-5Al (b) alloys at room temperature, respectively
(Light phase: Cu-rich a-phase; Dark phase: Fe-rich b-phase)
Table 1 Actual average composition of a and b phase of Fe-15Cu-5Al and Fe-85Cu-5Al alloys (mole fraction, %)
3 Results
3.1 Scaling kinetics
Fig.3 presents the parabolic plots for oxidation kinetics of Fe-15Cu-5Al (Fig.3(a)) and Fe-85Cu-5Al alloys (Fig.3(b)) at 900 ℃. Approximate values of the parabolic rate constants, calculated from an analysis of the mass-gain data vs the square root of time, are reported in Table 2, where they are compared with those for the air oxidation of pure Cu and pure Fe.
The kinetic curve of Fe-15Cu-5Al at 900 ℃ can be approximately considered composed of two quasi-parabolic stages, which is similar to that at 800 ℃[7]. At both temperatures, the oxidation rates of the first stage are rather small, with the value of kp far below that of the second stage. At 900 ℃, the Fe-rich Fe-15Cu-5Al alloy shows a large decrease of the parabolic rate constant after about 50 min. On the contrary, Fe-85Cu-5Al follows a parabolic behavior to a good approximation at both temperatures. On the whole, Fe-85Cu-5Al corrodes much more slowly than pure iron [8-9], but a little faster than pure Cu[10] at the same temperature. The Cu-rich alloy (Fe-85Cu-5Al) corrodes much more quickly than the Fe-rich alloy (Fe-15Cu-5Al).
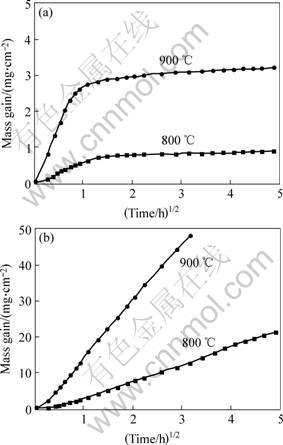
Fig. 3 Parabolic plots for oxidation kinetics of Fe-15Cu-5Al (a) and Fe-85Cu-5Al (b) alloys in 0.1MPa O2 at 800 ℃ and 900 ℃
Table 2 Approximate parabolic rate constants for oxidation of two Fe-Cu-5Al alloys and two pure metals at 900 ℃

3.2 Scale microstructure and composition
Compared with the oxidation at 800 ℃[7], Fe-15Cu-5Al oxidized at 900oC (Fig.4) produces relatively thicker scales (about 5 mm). The outer scales are composed of the oxides of iron, which contain a little aluminium (about 3.3%, mole fraction) and very little copper (less than 1%, mole fraction), possibly in solution. There is a very thin (less than 1 mm) and continuous alumina layer near the alloy/scale interface. The very thin alumina layer is not fully protective due to the formation of flaws, probably as a result of the high stress at 900 ℃, so the oxidation of the alloy may still happen beneath the alumina layer and thus produce new scales. An irregular thin layer of iron oxides is found to be present beneath the dark alumina layer if observed carefully (Fig.4). Although the very thin alumina layer is not fully protective, it does reduce the corrosion rate of the alloy greatly. In addition, the color ofα-phase adjacent to the scales looks much lighter than that far away from the scales. The EDX analysis shows that the content of iron of the former is much lower than that of the latter. The depletion of Fe presumably results from outward diffusion of iron, which is caused by the growth of iron oxides of outer scales.
The scales grown on Fe-85Cu-5Al after 24 h oxidation at 900 ℃ (Fig.5) are very similar to those grown at 800 ℃[7]. The Cu-rich alloy forms a thick and porous external scale composed of an outermost layer of copper oxides and an inner region containing a mixture of copper and Fe-Al oxides, coupled to the internal oxidation of iron and aluminium.
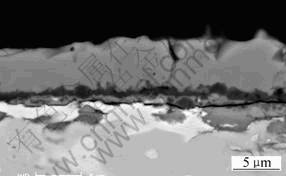
Fig. 4 SEM/BEI micrograph of cross section of Fe-15Cu-5Al oxidized in 0.1 MPa O2 at 900 ℃ for 24 h
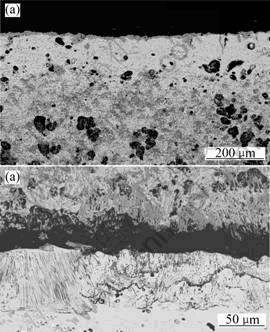
Fig. 5 SEM/BEI micrographs of cross sections of Fe-85Cu-5Al oxidized in 0.1 MPa O2 for 10 h at 900 ℃: (a) General view; (b) Enlarged view of internal oxidation zone
4 Discussion
For Fe-Al alloys, it has been proposed that for a protective behavior at least 8%(mass fraction) aluminium is necessary at 800 ℃, while 10%-12% aluminium is required at 600 ℃. At 1 000 ℃, 5%(mass fraction) aluminium was reported to be sufficient to form an external protective scale[11]. Around 800 ℃, alloys with 2.5%(mass fraction) aluminium (5.1%Al, mole fraction) can form alumina scales which, however, are not fully protective since they may permit the development of nodular growths of bulky iron oxide, associated with a large increase in the oxidation rate[11]. By comparing the oxidation behavior of Fe-5Al binary alloys with that of Fe-15Cu-5Al ternary alloys at 800 ℃, the presence of 15%(mole fraction) Cu in Fe-15Cu-5Al has a beneficial effect since it is actually able to reduce the critical aluminium content needed for the formation of external alumina scales. This is mainly a result of the effects of the presence of copper on the solubility and diffusivity of oxygen in the two-phase Fe+Cu matrix of the region of internal oxidation. And this is also the result of the decrease of the rate of growth of the external scales with respect to the oxidation of pure iron and of an increase of the effective diffusion coefficient of aluminium in the ternary-alloy matrix [7]. The situation is similar at 900 ℃.
In addition, from the previous investigation, it is deduced that the increase of temperature may result in a more protective behavior for Fe-Al binary alloys due to a larger tendency to form alumina layer if aluminium content is fixed near the critical concentration. However, for the ternary Fe-15Cu-5Al alloy, when the temperature increases from 800 to 900 ℃, it shows a less protective behavior. This result shows that the addition of 15%(mole fraction) Cu tends to increase the flaw of the formed alumina layer as the temperature increases. This situation is probably correlated with the higher stress growth effects for the alumina layer at higher temperatures.
The addition of 10%(mole fraction) Fe to Cu-5Al alloy turns the solid-solution binary alloys into a two-phase ternary alloys, which is different from the addition of Ni to Cu-Al. However, as long as the alloys are able to form external scales of copper oxides, as observed in the case of the oxidation of Fe-85Cu-5Al[4], the oxygen pressure prevailing at the alloy/scale interface must correspond to the Cu/Cu2O equilibrium, which is much larger than the pressure for the equilibrium of iron with its oxide. Thus, Fe and Al must be oxidized internally rather than externally. As a consequence, the matrix of the alloy in the outer zone of the region of internal oxidation is composed of pure copper. Therefore, the parameters involved in the internal oxidation of the ternary Cu-rich alloys are substantially those relevant to pure copper[7]. In addition, the large growth rate of the external mixed scales tends to increase the critical Al content[12]. In fact, the addition of Fe to Cu-Al alloy is more effective than the addition of Ni with respect to the increase of critical Al content, as shown by the fact that at 800 ℃ it is more difficult for the Fe-85Cu-5Al alloy to form external alumina scale than the Ni-85Cu-5Al alloy[7,13]. The case should be similar at 900 ℃. A recent study reveals that the critical content to form external alumina scales for Cu-Al alloy oxidized at 900℃ is about 6.8%(mole fraction) Al[14]. Therefore, the limited aluminium content (5%Al, mole fraction) cannot make the Fe-85Cu-5Al ternary alloy form external alumina scales, which is in accordance with what is observed.
5 Conclusions
1) The oxidation behaviors of two two-phase ternary Fe-Cu-5Al alloys at 900 ℃ differ greatly from each other. The presence of 5%(mole fraction) Al greatly reduces the oxidation rate of Fe-15Cu-5Al alloy with respect to a binary Fe-Cu alloy of similar composition by forming an external Al2O3 layer. The addition of 15%(mole fraction) Cu reduces the critical Al content to form Al2O3 layer with respect to binary Fe-Al alloys. On the other hand, the addition of 15%(mole fraction) Cu tends to increase the flaw of the formed alumina layer when the temperature increases from 800 ℃ to 900 ℃.
2) The limited aluminium content (5%, mole fraction) cannot make the Fe-85Cu-5Al ternary alloy form external alumina scales, which results in a very serious corrosion behavior as the pure copper. The addition of 10%(mole fraction) Fe to Cu-5Al tends to increase the critical Al content to form Al2O3 layer with respect to binary Cu-Al alloys.
References
[1] SIMS C T, STOLOFF N S, HAGEL W C. Superalloys II[M]. New York: Wiley, 1987.
[2] NIU Y, GESMUNDO F. An approximate analysis of the external oxidation of ternary alloys forming insoluble oxides. I: high oxidant pressures[J]. Oxid Met, 2001, 56: 517-536.
[3] GESMUNDO F, NIU Y, WANG W. An approximate analysis of the external oxidation of ternary alloys forming insoluble oxides. II: low oxidant pressures[J]. Oxid Met, 2001, 56: 537-549.
[4] WANG S Y, GESMUNDO F, WU W T, NIU Y. A non-classical type of third-element effect in the oxidation of Cu-xCr-2Al alloys at 1173 K[J]. Scripta Materialia, 2006, 54: 1563-1568.
[5] NIU Y, WU Y, GESMUNDO F. The oxidation of three Ni-6Si-xAl alloys in 1 atm O2 at 1 000 ℃ [J]. Corros Sci, 2006, 48: 1-22.
[6] XIANG J H, NIU Y, GESMUNDO F. The oxidation of two ternary Fe-Cu-10 at.%Al alloys in 1atm of pure O2 at 800-900℃[J]. Corros Sci, 2005, 47: 1493-1505.
[7] XIANG J H, NIU Y, GESMUNDO F. The oxidation of two ternary Fe-Cu-5 at.%Al alloys in 1atm of pure O2 at 800 ℃ [J]. Oxid Met, 2004, 61: 403-423.
[8] GESMUNDO F, NIU Y, OQUAB D, ROOS C, PIERAGGI B, VIANI F. The air oxidation of two-phase Fe-Cu alloys at 600-800 ℃[J]. Oxid Met, 1998, 49: 115-146.
[9] BERNARD. L’Oxydation des Metaux[M]. Paris, France: Gauthiers-Villars, 1964.
[10] NIU Y, GESMUNDO F. DOUGLASS D L, VIANI F. The air oxidation of two-phase Cu-Cr alloys at 700-900 ℃ [J]. Oxid Met, 1997, 48: 357-380.
[11] PRESCOTT R, GRAHAM M J. The oxidation of iron-aluminium alloys[J]. Oxid Met, 1992, 38: 73-87.
[12] GESMUNDO F, VIANI F. Transition from internal to external oxidation for binary alloys in the presence of an outer scale[J]. Oxid Met, 1986, 25: 269-282.
[13] NIU Y, XIANG J H, GESMUNDO F. The oxidation of two ternary Ni-Cu-5 at.%Al alloys in 1atm of pure O2 at 800-900 ℃[J]. Oxid Met, 2003, 60: 293-313.
[14] XIANG J H, NIU Y, WU W T. Critical Al content to form external-alumina scales on Cu-Al alloys[J]. Intermetallics, accepted for publication
(Edited by PENG Chao-qun)
Foundation item: Project(KF0610) supported by the Open Project Program of Key Laboratory of Low Dimensional Materials and Application Technology (Xiangtan University), Ministry of Education, China;Project ([2006]245) supported by Science and Technology Research Item of Jiangxi Provincial Department of Education, China
Corresponding author: XIANG Jun-huai; Tel: +86-791-3801423; E-mail: xiangjunhuai@163.com


