
Preparation and characterization of icariin/PHBV drug delivery coatings on anodic oxidized titanium
DAI Yao1, LIU Hai-rong1, XIA Lei-lei1, ZHOU Zheng2
1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. School of Biology, Hunan University, Changsha 410082, China
Received 23 September 2010; accepted 5 January 2011
Abstract:
A composite material was fabricated by applying a biodegradable drug delivery coating, consisting of poly (3-hydroxyburyrate-co-3-hydroxyvalerate) (PHBV) and icariin, to an anodic oxidized titanium plate. The coating was prepared by evaporating chloroform solution containing PHBV and icariin on the titanium plate under vacuum condition. Icariin/PHBV coated titanium plates significantly enhance the proliferation of MG-63 cells compared with the PHBV coated and anodic oxidized ones. Increased icariin contained in the coating displays an elevated influence on cell proliferation. The results show that icariin gradually releases from the coating to cells mainly through the phospholipid-based cellular membrane instead of the culture medium. The overall results suggest that the novel icariin/PHBV coating can be used to enhance the bioactivity of titanium based orthopedic implants.
Key words:
poly(3-hydroxyburyrate-co-3-hydroxyvalerate)(PHBV); icariin; drug delivery coating; titanium;
1 Introduction
Titanium and titanium alloys are frequently used in biomedical devices and components such as orthopedic implants or prostheses because of their excellent mechanical properties, corrosion resistance and good biocompatibility [1]. After implantation in vivo, the surface of titanium can form directly a structural and functional connection with bone tissue, which is known as osseointegration [2]. However, it takes several months to complete a successful osseointegration [3]. To reduce this osseointegration period in bone replacement therapy, various surface modification technologies, such as mechanical methods, chemical methods and physical methods, have been carried out [4].
Traditional Chinese medicines (TCM) have been used in China to treat bone fractures and promote bone regeneration for thousands of years [5]. Studies have demonstrated that icariin (extract of epimedium) promotes the proliferation [6], differentiation [7], mineralization [8] and gene expression [9-10] of osteoblast cells. Normal medicine delivery in the body requires circulatory system. However, inevitably injured local capillary vessels during artificial bone transplant operation inhibit the drug delivery process and a drug delivery coating combined with orthopedic implant may overcome this barrier.
At the implant-tissue interface, the drug delivery coating affects directly the cellular response of osteoblast cells. The ideal materials for this drug delivery coating are biodegradable materials with good biocompatibility. It has been revealed that the biodegradability and biocompatibility of PHBV meet requirements of the drug delivery coating [11-16]. Additionally, the rate of drug release can be manipulated via adjusting the PHBV degradation rate slightly by varying the copolymer composition [1].
In the present study, a novel icariin containing PHBV coating was designed to modify the surface of the anodic oxidization treated titanium plate. To analyze the influence of the icariin/PHBV coating on osteoblast cells, the morphology, adhesion and proliferation of osteoblast-like MG-63 cells were examined.
2 Experimental
2.1 Anodic oxidation (AO)
After the scratches of titanium plates (10 mm×10 mm×2 mm) were polished by 400-grits SiC sand paper, a further polishing was carried out by using the acid etching solution, which contained HNO3 and HF (volume ratio of 1:1). Then, samples were ultrasonically cleaned with double distilled water (ddH2O) and ethanol. Finally, samples were dried in air.
A sample, used as an anode, and two cathodic titanium plates were immersed into electrolyte solution containing NaF (0.138 mol/L) and H3PO4 (0.5 mol/L). Then they were applied a constant voltage of 10 V for 20 min at 40 °C. All prepared samples were ultrasonically cleaned with ddH2O and dried in air.
2.2 Preparation of icariin/PHBV coating
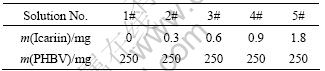
After a series of icariin (Zelang Medical Technology, China) dimethyl sulfoxide (DMSO) solutions were prepared, 600 μL icariin solutions with a series of concentration were added into 6 mL PHBV (5% hydroxyvalerate (HV) in mole fraction and Mw of 300 kDa, Tianan Biology, China) chloroform solution, respectively. Anodic oxidized titanium specimens were immerged and ultrasonically treated for 5 min in the icariin/PHBV- containing solution. The excess solution on the surface of titanium plates was gently removed with a glass rod. Finally, specimens were dried in a vacuum drying oven at room temperature for 2 h to obtain a tightly adhered icariin/PHBV coating. In this study, 5 solutions with different concentrations (1#-5#) were used to generate 5 different coatings (1#-5#), respectively. The mass of components contained in 5 solutions (total volume 6.6 mL) was listed in Table 1.
Table 1 Ingredients of solutions used to prepare icariin/PHBV coatings
2.3 Surface analysis
The surface morphologies were observed in a field emission scanning electron microscope (FESEM, JSM6700F, JEOL, Japan) and an environment scanning electron microscope (ESEM, Quanta 200, FEI, USA).
2.4 Contact angle measurement
After approximately 10 μL distilled water was gently dropped onto the surface of samples, water contact angle was measured with pendant drop method at 20 °C by a contact angle meter (SL200B, KINO Industry, China).
2.5 Cell culture
Human osteosarcoma cell line, MG-63, which was obtained from the cell bank of China (Shanghai), was cultured with RPMI 1640 (Thermo) medium supplemented with fetal bovine serum (Thermo, volume ratio of 1:10) at 37 °C in a humidified incubator (Thermo) with an atmosphere of 5% CO2.
2.6 Cell adhesion
In wells of the 24-well plate (Greiner bio-one), 1×105 MG-63 cells were seeded to every sample. Six hours later, samples were collected for cells fixation. After all specimens were pre-fixed with 3% glutaraldehyde for 30 min, the dehydration process was carried out by combining a gradient ethanol/ddH2O dehydration and a gradient hexamethyldisilazane/ethanol dehydration. The morphologies of cells were observed by the environment scanning electron microscope.
2.7 Cell proliferation
The culture medium was removed from all sample wells after 1, 3, 5 and 7 d of culture, meanwhile 1 mL alamarBlue containing medium, which was prepared by following the manufacture’s instruction, was added. The plate was then further incubated in a humidified incubator for 6 h. 100 μL diluted solution from each well was transferred to an ELISA plate. The absorbance of collected samples at both 570 nm and 630 nm wavelengths were detected by ELISA Reader (Thermo). The percent of reduced alamarBlue was calculated according to the manufacture’s instruction. The alamarBlue-containing media in all samples were replaced with fresh normal culture media soon after the detection.
2.8 Dissolution products of icariin/PHBV coating (sample 5#)
After 10 samples with icariin/PHBV coating 5# were incubated with 10 mL RPMI 1640 (Thermo) medium at 37 °C for 24 h, the medium containing dissolution products was collected and replaced with fresh medium. The dissolution products collected at day 1, 3, 5, 7 were named 1D, 3D, 5D and 7D. Then those collected media were used to prepare the culture media by adding fetal bovine serum (Thermo, volume ratio of 1:10). The normal culture medium was used as the control sample. The proliferation of MG-63 cells was examined by using those media to culture cells. The alamarBlue assay was employed to detect cell proliferation as well.
3 Results
3.1 Surface morphologies
The morphologies of samples obtained by different treatments were analyzed by FESEM or ESEM. The representative FESEM micrographs of the polished and AO treated titanium surfaces are shown in Fig. 1. At the low magnification (Figs. 1(a) and (b)), the surfaces of polished and AO treated titanium plate look like the same, but at the high magnification it displays that the surface of AO treated titanium plate is covered with ordered nanotubes (Fig. 1(c)).
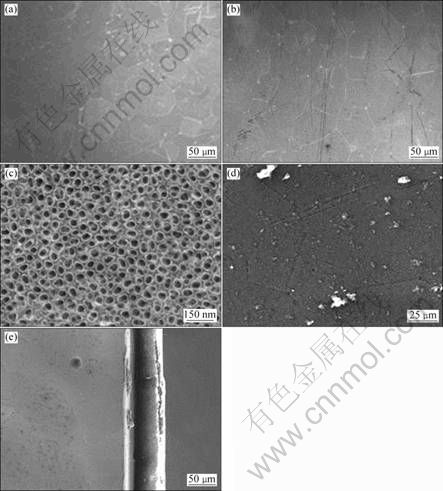
Fig. 1 FESEM morphologies of polished titanium surface (a), AO treated titanium surface (b, c), ESEM morphologies of front view (d) and side view (e) of icariin/PHBV coated sample 5#
The icariin/PHBV coating separates from the polished titanium plate after vacuum evaporation, but the icariin/PHBV coating remains tightly attached with AO treated titanium plate after it has been sterilized with autoclave at 120 °C.
Surface topology of the icariin/PHBV coating was examined by ESEM. The results exhibit that the surface of this icariin/PHBV coating is decorated with some granules (Fig. 1(d)). The side view (Fig. 1(e)) displays that the thickness of the icariin/PHBV coating is around 70 μm.
3.2 Surface hydrophilicity
Surface hydrophilicity of the icariin/PHBV coating was examined by measuring contact angle to water (Fig. 2). There is no difference between the contact angle of the PHBV coating (1#) and the icariin/PHBV coating (5#), indicating that the contact angle is not influenced by the icariin content in this coating. Both coatings exhibit more hydrophobic surfaces compared with the surface of AO treated titanium plate.
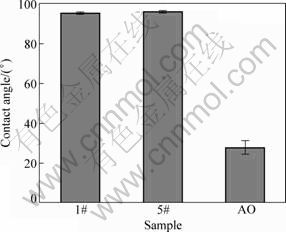
Fig. 2 Contact angles of PHBV coating (1#), icariin/PHBV coating (5#) and AO treated titanium surface
3.3 Cell morphologies
The MG-63 cell line was used to investigate the bioactivity of the icariin/PHBV coating. Figure 3 exhibits images of MG-63 cells after 6 h and 7 d of culture, respectively. Figures 3(a) and (b) demonstrate morphologies of MG-63 cells in the cell adhesion stage. Cells on the surface of AO treated titanium (Fig. 3(a)) exhibit the typical morphology of polygon as another study [17]. Moreover, MG-63 cells on the icariin/PHBV coating display a healthy and active appearance (Figs. 3(b), (c), and (d)), since they are elongated, flatted and widespread. According to morphologies of MG-63 cells after 7 d of culture (Figs. 3(c) and (d)), lots of filopodia between the neighboring cells have been observed, demonstrating that the spreading and proliferation of cells are excellent.

Fig. 3 ESEM morphologies of cells cultured on AO treated titanium plate for 6 h (a) and on sample 5# for 6 h (b) and 7 d (c, d)
3.4 Cell proliferation
The percent of reduced alamarBlue lineally reflects the cell number within the linear range of this method. The results of all samples, detected by the alamarBlue assay, are shown in Fig. 4. After 1 d of culture, MG-63 cell numbers of different samples vary predominantly, resulting from the difference of cell seeding efficiency. Three days later, the cell numbers of samples 2#, 3#, 4# and 5# are higher than the other samples because of positive influence of icariin on cell proliferation. It has been confirmed that PHBV displays no significant influence on cell proliferation by comparing the reduced alamarBlue values of sample AO and sample 1#. Based on Fig. 4, after 3, 5, and 7 d of culture respectively, sample 5# shows the highest alamarBlue reduction value, illustrating that the highest icariin content of the coating results in the greatest effect on cell proliferation. Increased icariin content of the coating shows an enhanced influence on cell proliferation by comparing the alamarBlue reduction values of samples 2#, 3#, 4# and 5# detected following 3, 5, and 7 d of culture, respectively. The results demonstrate that the icariin/PHBV coating significantly promotes the proliferation of MG-63 cells.
To investigate the way of icariin transmission, whether through the culture medium, the cellular membrane or even both, culture media containing dissolution products from sample 5# were prepared as described in section 2.8.
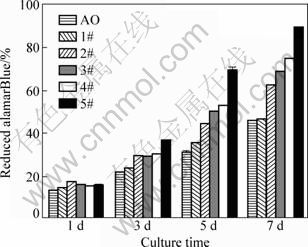
Fig. 4 Percent of reduced alamarBlue of samples (cells cultured on different samples)
According to Fig. 5, media containing dissolution products only slightly enhance cell proliferation. It suggests that only a small amount of icariin dissolved into the medium from this coating. Hence, the icariin/PHBV coatings mainly deliver icariin locally through the cellular membrane and dramatically influence cell proliferation of attached cells.

Fig. 5 Percent of reduced alamarBlue of samples (cells cultured by media containing dissolution products of coating)
4 Discussion
Titanium-based orthopedic implants are commercial produced and widely used for clinical treatments, but those biomaterials lack affinity to human bone tissue [4]. Therefore, an additional coating, like a hydroxyapatite coating by plasma spraying [18], is required to enhance the affinity of titanium-based biomaterials to osteoblast cells. However, the high temperature during the plasma spraying process reduces the bioactivity of the HA coating.
Surface properties of implants play a pivotal role in cell adhesion [19-24]. In this study, a novel icariin/PHBV layer coated titanium plates were prepared at room temperature. After 6 h of culture, seeded MG-63 cells on the icariin/PHBV coating are elongated, flatted and widespread (Fig. 3(b)), suggesting that this coating is suitable for cell attachment. Following MG-63 cells adhesion, the stimulation of cell proliferation has also been confirmed by the alamarBlue assay (Fig. 4). Due to the highest content of icariin in the icariin/PHBV coating (5#), the proliferation of MG-63 cells dramatically elevates when they are cultured on this coating (Fig. 4). To test the way of icariin delivering to cells, a series of experiments were carried out. Since cell proliferation only is slightly influenced by media containing released ingredients from the icariin/PHBV coating (Fig. 5), it is clear that icariin transmits predominantly from the coating to cells through the cellular membrane instead of the culture medium.
The icariin/PHBV coating, easily manufactured, significantly improves the affinity of titanium to osteoblast cells, implying that commercial usage of this icariin/PHBV coating would be accepted in the future.
5 Conclusions
1) The icariin/PHBV coating with around 70 μm in thickness tightly attaches to the surface of anodic oxidation treated titanium.
2) The icariin/PHBV coated titanium plates significantly enhance the proliferation of MG-63 cells compared with the PHBV coated and anodic oxidized ones. Increased icariin in the coating displays an increased influence on cell proliferation.
3) The icariin/PHBV coating gradually delivers icariin to adhere cells mainly through the cellular membrane instead of the culture medium.
References
[1] RANTER B D, HOFFMAN A S, SCHOEN F J, LEMONS J E. Biomaterials science: An introduction to materials in medicine [M]. 2nd ed. London: Elsevier Academic Press, 2004.
[2] BRANEMARK P I. Osseointegration and its experimental background [J]. The Journal of Prosthetic Dentistry, 1983, 50: 399-410.
[3] DAS K, BOSE S, BANDYOPADHYAY A. Surface modifications and cell–materials interactions with anodized Ti [J]. Acta Biomaterialia, 2007, 3: 573-585.
[4] LIU Xuan-yong, CHU P K, DING Chuan-xian. Surface modification of titanium, titanium alloys and related materials for biomedical applications [J]. Materials Science and Engineering R, 2004, 47: 49-121.
[5] WONG R W K, RABIE A B M. Traditional Chinese medicines and bone formation—A review [J]. Journal of Oral and Maxillofacial Surgery, 2006, 64: 828-837.
[6] MENG F H. LI Y B, XIONG Z L, JIANG Z M, LI F M. Osteoblastic proliferative activity of epimedium brevicornum maxim [J]. Phytomedicine, 2005, 12: 189-193.
[7] CHEN Ke-ming, GE Bao-feng, MA Hui-ping, LIU Xing-yan, BA Meng-hai, MA Li-ping, LV Ming-bo. Effects of icariin on the osteogenic differentiation of bone marrow mysenchymal stem cells in vitro [J]. Chinese Journal of Osteoporosis, 2008, 14(9): 642-645. (in Chinese)
[8] XIAO Qiang-bing, ZOU Ji. An Experimental study on type I collagen expression of icariin injection on rat osteoblasts in vitro [J]. Chinese Journal of Traditional Medical Traumatology & Orthopedics, 2007, 15(9): 36-38. (in Chinese)
[9] HSIEH T P, SHEU S Y, SUN J S, CHEN M H, LIU M H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression [J]. Phytomedicine, 2010, 17: 414-423.
[10] ZHAO Ji-yuan, OHBA S, SHINKAI M, CHUNG U, NAGAMUNE T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner [J]. Biochemical and Biophysical Research Communications, 2008, 369: 444-448.
[11] LI Hai-yan, CHANG Jiang. Fabrication and characterization of bioactive wollastonite/PHBV composite scaffolds [J]. Biomaterials, 2004, 25: 5473-5480.
[12] LI Hai-yan, CHANG Jiang. In vitro degradation of porous degradable and bioactive PHBV/wollastonite composite scaffolds [J]. Polymer Degradation and Stability, 2005, 87: 301-307.
[13] WANG Yang, BIAN Yu-zhu, WU Qiong, CHEN Guo-qiang. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits [J]. Biomaterials, 2008, 29: 2858-2868.
[14] JI Yan, LI Xiao-tao, CHEN Guo-qiang. Interactions between a poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes [J]. Biomaterials, 2008, 29: 3807-3814.
[15] XIAO Xiao-qiang, ZHAO Yan, CHEN Guo-qiang. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells [J]. Biomaterials, 2007, 28: 3608-3616.
[16] WANG Ya-wu, YANG Fei, WU Qiong, CHENG Yin-chung, YU P H F, CHEN Jin-chun, CHENG Guo-qiang. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast [J]. Biomaterials, 2005, 26: 755-761.
[17] NARAYANANA R, LEE H J, KWON T Y, KIM K H. Anodic TiO2 nanotubes from stirred baths: Hydroxyapatite growth & osteoblast responses [J]. Materials Chemistry and Physics, 2011, 125: 510-517.
[18] XUE Wei-chang, TAO Shun-yan, LIU Xuan-yong, ZHENG Xue-bing, DING Chuan-xian. In vivo evaluation of plasma sprayed hydroxyapatite coatings having different crystallinity [J]. Biomaterials, 2004, 25: 415-421.
[19] ZHU Xiao-long, CHEN Jun, SCHEIDELER L, REICHIL R, JUERGEN G G. Effects of topography and composition of titanium surface oxides on osteoblast responses [J]. Biomaterials, 2004, 25: 4087-4103.
[20] BOYAN B D, HUMMERT T W, DEAN D D, SCHWARTZ Z. Role of material surfaces in regulating bone and cartilage cell Response [J]. Biomaterials, 1996, 17: 137-146.
[21] HATANO K, INOUE H, KOJO T, MATSUNAGA T, TSUJISAWA T, UCHIYANA C, UCHIDA Y. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene [J]. Bone, 1999, 25(4): 439-445.
[22] LAMPIN M, WAROCQUIER C R, LEGRIS C, DEGRANGE M, SIGOT-LUIZARD M F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration [J]. Journal of Biomedical Materials Research, 1997, 36(1): 99-108.
[23] ZHAO B H, LEE I S, HAN I H. Effects of surface morphology on human osteosarcoma cell response [J]. Current Applied Physics, 2007, 7(S1): e6-e10.
[24] DEGASNE I, BASLE M F, DEMAIS V, HURE G, LESOURD M, GROLLEAU B, MERCIER L, CHAPPARD D. Effects of roughness, fibronectin and vitronectin on attachment, spreading and proliferation of human osteoblast-like cells (Saos-2) on titanium surface [J]. Calcified Tissue International, 1999, 64: 499-507.
阳极氧化钛表面淫羊藿苷/PHBV药物缓释涂层的制备与表征
戴 瑶1, 刘海蓉1, 夏磊磊1, 周 征2
1. 湖南大学 材料科学与工程学院,长沙 410082;
2. 湖南大学 生物学院,长沙 410082
摘 要:以聚3-羟基丁酸酯-co-3-羟基戊酸酯(PHBV)和中药淫羊藿苷的三氯甲烷溶液为原料,通过真空干燥使溶剂挥发,在阳极氧化钛表面制备可降解的药物缓释涂层。体外细胞实验表明,相比于阳极氧化钛和PHBV涂层,含淫羊藿苷PHBV涂层能够显著地促进成骨细胞MG-63在其表面增殖,并且随着涂层中淫羊藿苷含量的增加,促进细胞增殖的作用越明显。淫羊藿苷主要通过细胞膜吸收而不是通过溶解在培养液中的方式发挥作用。结果表明,此药物缓释涂层能够有效增强钛植入材料的生物活性。
关键词:聚3-羟基丁酸酯-co-3-羟基戊酸酯(PHBV);淫羊藿苷;药物缓释涂层;钛
(Edited by YANG Hua)
Foundation item: Project (2010DFA32270) supported by International Science & Technology Cooperation Program of China; Project (2010) supported by Scientific Research Foundation for the Returned Oversea Scholars of Ministry of Education of China
Corresponding author: LIU Hai-rong; Tel: +86-731-88821727; E-mail: liuhairong@hnu.edu.cn
DOI: 10.1016/S1003-6326(11)61035-2
Abstract: A composite material was fabricated by applying a biodegradable drug delivery coating, consisting of poly (3-hydroxyburyrate-co-3-hydroxyvalerate) (PHBV) and icariin, to an anodic oxidized titanium plate. The coating was prepared by evaporating chloroform solution containing PHBV and icariin on the titanium plate under vacuum condition. Icariin/PHBV coated titanium plates significantly enhance the proliferation of MG-63 cells compared with the PHBV coated and anodic oxidized ones. Increased icariin contained in the coating displays an elevated influence on cell proliferation. The results show that icariin gradually releases from the coating to cells mainly through the phospholipid-based cellular membrane instead of the culture medium. The overall results suggest that the novel icariin/PHBV coating can be used to enhance the bioactivity of titanium based orthopedic implants.


